Local recurrence and radionecrosis after single-isocenter multiple targets stereotactic radiotherapy for brain metastases
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Stereotactic radiotherapy (SRT) is frequently used to treat brain metastases (BMs). The single-isocenter for multiple targets (SIMT) technique allows for faster treatment of large
numbers of BMs, but may adversely affect planning target volume (PTV) coverage due to possible increased positioning uncertainties with an increased isocenter to tumor distance (ITD). This
study aims to investigate the association of ITD with local recurrence (LR) and radionecrosis (RN). Patients treated with SRT using a single isocenter for multiple BMs were retrospectively
analyzed. Previous cranial radiotherapy and inability to undergo MR imaging were exclusion criteria. Patients were irradiated using a Versa HD LINAC with 6 MV flattening filter-free (FFF)
energy and a 6D robotic couch. A non-coplanar volumetric modulated arc technique was used and plans were delivered using 6MV FFF energy. Associations between potential risk factors and LR/RN
were investigated with Cox regression analyses. Seventy-five patients with a total of 357 BMs were included. Median survival after SRT was nine months. LR occurred in 7 (9%) patients and 10
(13%) had RN. After 18 months, LR-free survival was 89% and RN-free survival was 85%, respectively. ITD was not significantly associated with LR and RN. GTV was significantly associated
with both LR (HR 1.10, 95% CI 1.02–1.17, _P_ 0.0079) and RN (HR 1.09, 95% CI 1.01–1.17, _P_ 0.020). LINAC-based SIMT SRT is a safe and effective treatment modality for patients with multiple
BMs. We found no increased risk of LR or RN for BMs located further away from the isocenter. SIMILAR CONTENT BEING VIEWED BY OTHERS RESPONSE OF TREATMENT-NAIVE BRAIN METASTASES TO
STEREOTACTIC RADIOSURGERY Article Open access 02 May 2024 HISTOPATHOLOGY AND SURGICAL OUTCOME OF SYMPTOMATIC TREATMENT-RELATED CHANGES AFTER GAMMA KNIFE RADIOSURGERY IN PATIENTS WITH BRAIN
METASTASES Article Open access 22 February 2022 STEREOTACTIC IRRADIATION OF NON-SMALL CELL LUNG CANCER BRAIN METASTASES: EVALUATION OF LOCAL AND CEREBRAL CONTROL IN A LARGE SERIES Article
Open access 08 July 2020 INTRODUCTION Stereotactic radiotherapy (SRT) is a commonly chosen treatment for patients with one or more brain metastases (BMs), either as sole treatment or in
combination with neurosurgery. In patients with multiple BMs, whole brain radiotherapy (WBRT) used to be the standard of care. However, a shift in treatment strategy from WBRT to SRT has
been observed in these patients, because WBRT is associated with an increased risk of neurotoxicity without survival benefit1,2. Previous research has shown that in patients with metastases
with limited volume, the overall survival and adverse events were similar for patients with 2–4 and for those with 5–10 BMs3. However, one limitation of SRT for a large number of BMs has
been the lengthy treatment time (20 + minutes per lesion, > 1 h for ≥ 3 lesions) due to the need to treat each lesion with a unique radiation isocenter (the point where the radiation
beams converge)4. This makes SRT less practical for the treatment of more than three BMs at the same time. Linear accelerator (LINAC)-based SRT enables the use of the single-isocenter for
multiple targets (SIMT) technique4,5. With SIMT SRT, multiple lesions are treated simultaneously with the same setup based on a single isocenter. The average overall treatment time in this
SIMT SRT is less than 25 min for multiple BMs6. Additionally, hypofractionated SRT becomes more feasible in practice as an alternative to one-fraction SRT due to these shorter treatment
times. Hypofractionated SRT might reduce local tumor recurrence (LR) and radionecrosis (RN) rates, particularly in larger metastases7. The largest potential drawback of SIMT SRT is the
potential risk of planning target volume (PTV) displacement when the isocenter-to-tumor distance (ITD) is longer. Target volumes with longer ITDs are more sensitive to rotational errors and
this may therefore lead to reduced PTV coverage. These errors are caused by residual patient movement during treatment and mechanical limitations in accuracy of couch movement. Collimator
and gantry rotations can also add to rotational uncertainty in SIMT SRT. While small rotational errors would not be relevant in a single-isocenter for single targets technique, these errors
could have a substantial effect on PTV coverage in SIMT SRT. Reduced PTV coverage has consequently been associated with a longer ITD, especially in smaller BMs8. In theory, this makes it
more likely that the tumor receives a reduced radiation dose while the surrounding healthy brain tissue is overdosed9. Decreased tumor coverage could lead to higher rates of LR, while
increased radiation dose in healthy brain tissue could increase the likelihood of RN, although clinical evidence has not been reported. The impact of the SIMT technique on local outcomes
such as LR and RN have not been extensively investigated. The purpose of this study was to evaluate the effect of ITD on LR and RN. METHODS AND MATERIALS ETHICS The Medical Ethics Committee
Leiden The Hague Delft gave approval for this study (reference number N24.032) and granted a waiver of informed consent, because it exclusively required retrospectively collected data. All
methods were performed in accordance with the relevant guidelines and regulations. PATIENT SELECTION All adult patients who were treated with SIMT SRT between November 2020 and April 2023
for two or more BMs at our hospital were identified and evaluated for inclusion. Patients were excluded if they had any contraindications for MR imaging or had previously undergone cranial
radiotherapy. Patients were allowed to undergo a neurosurgical resection shortly prior to SRT, but the resected metastases were excluded from the per lesion analyses. TREATMENT PLANNING
Treatment planning for BMs was based on a CT scan with a thermoplastic mask and cerebral 3D MR imaging scans (voxel size 0.9 × 0.9 × 0.9 mm3). All SIMT SRT plans were made using a treatment
planning software (RayStation®, RaySearch Laboratories, Stockholm, Sweden). Patients were irradiated with 6 MV flattening-filter free (FFF) energy of a Versa HD (Elekta, Stockholm, Sweden)
LINAC machine including a 6D robotic couch (Hexapod couch, Elekta, Stockholm, Sweden). All patients were treated using a non-coplanar volumetric modulated arc (VMAT) technique using six arcs
divided over three couch angles. Radiation oncologists delineated the gross tumor volumes (GTVs) based on T1-weighted MR imaging scans. PTVs were generated by creating a 1 mm margin around
the GTV. No margin around the GTV was utilized in BMs in the brainstem. This GTV-PTV margin was used for all BMs based on online Cone Beam CT position verification and a 6D Hexapod couch
correction for translations and rotations10,11. 6D couch correction could be performed before and during the treatment if needed. An additional Cone Beam CT was utilized after the
correction. Q-fix masks (Q-fix, Avondale, USA) with a bite plate were used to reduce rotations. An action level of 1 mm (vector) and 1° was used for position verification. The action level
was applied during the Cone Beam CT with a 0° couch angle. Patients were repositioned when the threshold was violated. In each plan, 99% of the PTV had to be covered by 100% of the
prescribed dose. Small BMs (10 cm3 or smaller) received 21 Gy in one fraction, while BMs > 10 cm3 received 18 Gy in one fraction or 25.5 Gy in three fractions. BMs close to or in the
brainstem were treated with 24 Gy in three fractions. Four patients were treated with 35 Gy in five fractions as part of an ongoing prospective trial12. TREATMENT OUTCOMES After SRT,
three-monthly follow-up MR imaging scans were performed, with increased intervals after at least one year. The Response Assessment in Neuro-oncology Brain Metastases (RANO) criteria were
used to define progression13. LR and RN were evaluated on MR imaging with gadolinium-enhanced T1-weighted images, T2-weighted images, a diffusion weighted series, and (optionally) a
perfusion series. The primary outcome measures were LR and RN. The distinction between LR and RN was made by evaluating RN-specific findings such as absence of perfusion, edema, and an
increased volume followed by loss of volume. This process also included the interpretation of the multidisciplinary tumor board. When available, histology of resected lesions was also
utilized to distinguish between LR and RN. FDG-PET-scans were not routinely used to make the distinction between LR and RN. Regional recurrence was defined as new intracranial solid tumors
outside of the original PTVs. Leptomeningeal disease was diagnosed with cerebrospinal fluid analysis and/or MR imaging. The ITD was calculated in the treatment planning software from the
isocenter of the plan to the center of the BM. Data on patient and tumor characteristics were collected from the treatment planning software or electronic patient dossiers. These included
age, sex, primary tumor histology, number of treated BMs, tumor location, GTV, PTV, presence of extracranial disease, Karnofsky performance status, systemic therapies, treatment dose and
fractionation, Paddick conformity index, and Paddick gradient index14,15. STATISTICS Overall survival was determined from the first day of SRT until the date of death or the last follow-up.
Kaplan–Meier analysis was used to estimate survival rates. Univariable and multivariable Cox regression analyses were performed to evaluate the associations between independent variables and
the rates of LR and RN in all BMs. A nomogram was constructed based on the multivariable Cox regression analyses. Internal validation of the nomogram was performed by calculating the
concordance index (C-index) for a random sample of the study population. A _P_ value of < 0.05 (two-sided) was considered statistically significant. All statistical analyses were
performed using the software R version 4.3.1. After univariable Cox regression analyses, variables with a _P_ value < 0.06 (borderline significant) were included in the multivariable Cox
regression analyses to address potential confounding factors. RESULTS Seventy-five patients with a total of 450 BMs (median 4 per patient) were included. In five patients, not every BM was
treated with SRT, so only the irradiated BMs were included. The median number of irradiated BMs per patient was 3 (range 2–18). In 21 patients (28%), one or two of the largest BMs had been
resected; these resected BMs were excluded from the analyses. This left a total of 357 BMs included for per lesion data analyses. The median follow-up time was 8.4 months (range 0.5–30.3).
The median age during SRT was 67 years (range 31–83). The majority of patients had a primary tumor in the lungs. BMs were symptomatic in 75% of patients. A majority of patients received an
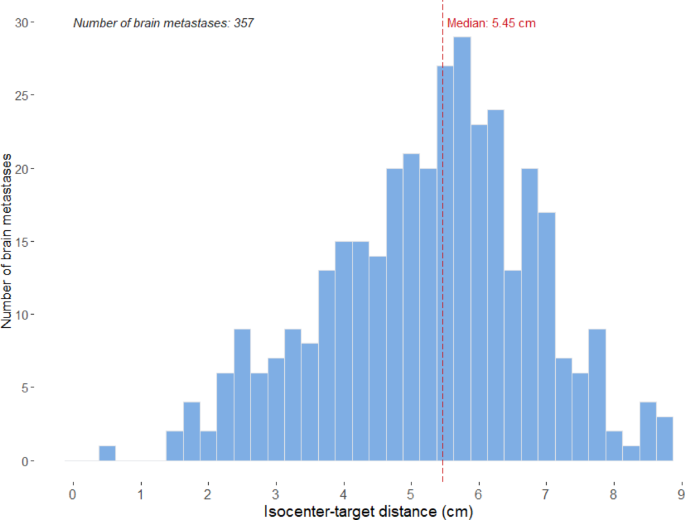
SRT schedule of either a single fraction (21 Gy) or three fractions (8.5 Gy up to a total dose of 25.5 Gy). Detailed patient and tumor characteristics can be found in Table 1. Median ITD was
5.5 cm (range 0.5–8.7) (Fig. 1). LR was found in 12 out of 357 BMs (3%) in 7 out of 75 patients (9%). The LR-free survival rate per BM was 96% at 6 months, 93% at 12 months, and 89% at 18
months (Fig. 2A). Median time from SRT to LR was 7 months (range 3–13 months). Treatment for LR consisted of surgery, re-SRT, or was not initiated due to regional or extracranial progression
leading to a decision for best supportive care. Symptomatic RN was found in 12 out of 357 BMs (3%) in 10 out of 75 patients (13%). The symptomatic RN-free survival rate per BM was 98% at 6
months, 94% at 12 months, and 85% at 18 months (Fig. 2B). Median time from SRT to symptomatic RN was 8 months. RN was treated with dexamethasone in all cases. One patient with RN received
treatment with the anti-VEGF monoclonal antibody bevacizumab. Median survival after SRT was 9 months (interquartile range; IQR: 4.4–20.3). Regional recurrence was found in 29 out of 75
patients (39%), while leptomeningeal disease occurred in 7 patients (9%). ITD was not significantly associated with LR or RN in any analysis. GTV was significantly associated with LR (HR
1.10, 95% CI 1.02–1.17, _P_ 0.0079) and with symptomatic RN (HR 1.09, 95% CI 1.01–1.17, _P_ 0.020) in separate uni- and multivariable Cox regression analyses (Table 2). Lung adenocarcinoma
as a primary tumor was associated with higher rates of LR compared to other primary tumors (HR 5.48, 95% CI 1.13–26.7, _P_ 0.035). No significant difference in LR or RN was found between
single and multifraction SRT (Table 2, Fig. 2). Finally, no association between concurrent immunotherapy treatment and other systemic treatment or no systemic treatment was found with LR or
symptomatic RN. A clinical nomogram based on the multivariable Cox regression analysis was created for LR (see Additional File 1). In the internal validation, the C-index of the LR nomogram
was 0.799 (95% CI 0.622–0.976), which indicates adequate discrimination by the model. DISCUSSION LINAC-based SIMT SRT is a novel treatment technique which makes SRT for a large number of BMs
patient-friendly and practically feasible within 30 min. Treatment times are reduced compared to conventional single-isocenter for single targets SRT, Gamma Knife, and CyberKnife6. In this
report, we found no increased risk of LR or RN for BMs located further away from the isocenter. Therefore, this work further supports the use of SIMT SRT for a large number of BMs in
clinical practice. This is to our knowledge the second study to report on the impact of ITD on clinical outcomes. The first study by Kraft et al. also found no association between ITD and
LR16. They used a TrueBeam or Edge LINAC (Varian Medical Systems, Palo Alto, USA) to treat patients and they reported local control rates of 94% of BMs after twelve months, similar to the
93% found in this study, where patients were treated with a Versa HD LINAC. Another group, Faccenda et al., also reported a 94% local control rate after twelve months and found no
significant differences between the differences in target coverage and the distance from the isocenter17. This group also found no significant correlation between target coverage and local
recurrence18. Several studies have reported on the safety and efficacy of the SIMT SRT technique for patients with multiple BMs. Alongi et al. reported no acute or subacute toxicities19.
Minniti et al. reported that neurocognitive function did not significantly decline after SIMT SRT while maintaining adequate local tumor control20. Additionally, Kim et al. prospectively
analyzed a cohort of SIMT SRT patients and reported low rates of LR and RN21. No large randomized trials have yet been performed for comparisons with other treatment modalities. Our results
largely support the earlier findings. As BMs which are located further away from the isocenter are not more likely to progress or to develop RN, the largest potential downside of SIMT SRT
has been addressed. This is especially relevant because a recent study reported that patients treated with LINAC-based SIMT SRT had similar overall survival and lower incidence of RN
compared to Gamma Knife treatment, suggesting that SIMT SRT is at least comparable to Gamma Knife in terms of clinical outcomes22. In this study, a PTV margin of 1 mm was utilized in all
patients except for those close to the brainstem. This is largely based on the report of Kirkpatrick et al. who found no significant difference in terms of local control between 1 and 3 mm
GTV-PTV margins for BMs in patients with 1–3 BMs23. Furthermore, Badloe et al. found that PTV-margin reduction from 2 to 0 mm did not reduce local control rates and concluded that margin
reduction is safe in LINAC-based SRT for solitary brain metastases24. The current results of local control rates (89% after 18 months) indicate that margin reduction to 1 mm in patients with
multiple BM (up to 18 metastases) is indeed appropriate when a stable thermoplastic mask is used in combination with 6D couch, which corrects for rotational errors. Lung adenocarcinoma as a
primary tumor, compared to other primary tumors, was associated with higher rates of LR. This may be attributed to relatively high survival rates observed in patients with BMs from these
primary tumors, as opposed to patients with, for instance, SCLC and colorectal cancer25,26,27. Due to the longer survival times, adverse events such as LR are more likely to occur. All
patients in the cohort were treated after immunotherapy had become widely available, which was commonly used in NSCLC adenocarcinoma patients. Our results additionally showed no significant
associations between immunotherapy treatment and symptomatic RN. Existing literature is inconclusive on this topic, as several studies have found a link between immunotherapy and RN,
particularly in melanoma BMs, but other studies are unable to reproduce this association28,29,30,31,32,33. The inhomogeneity of data, namely factors such as different immunotherapy types,
different primary tumor types, and the different time intervals between immunotherapy treatment and SRT, makes it challenging to give a clear answer to the question if immunotherapy in
combination with SRT increases RN rates. The Dutch national guideline for BMs currently allows continuation of immunotherapy during SRT34. The current study had some limitations. Firstly,
selection and information biases are potential concerns due to its retrospective design. Secondly, the number of patients was limited as a result of the relative novelty of SIMT SRT, which
may limit the ability to detect statistical associations (type II error). Finally, the distinction between LR and RN on MR imaging is notoriously difficult without histological confirmation
which was not available in the majority of cases35,36,37. Thirdly, the Cone Beam imaging with a 0° couch can potentially result in misalignment at nonzero couch angles for non-coplanar
irradiation38. Brainlab ExacTrac (Brainlab AG, Munich, Germany) or a surface-guided radiation therapy has been used to mitigate couch walkout of Elekta VersaHD that otherwise would not be
detected by a 0° couch Cone Beam CT39. Finally, it was not possible to do an adequate analysis of neurocognitive outcomes due to missing data. Despite these limitations, this work supports
the existing literature suggesting that SIMT SRT is safe and effective16,17,19,20,21. Our results did not show an association between ITD and LR or RN. SIMT SRT enables much shorter
treatment times for patients with a large number of BMs, addressing a former disadvantage of SRT compared to WBRT. With the lower risk of neurotoxicity and similar survival rates compared to
WBRT, SRT should be considered in the majority of patients regardless of number of metastases1,2. CONCLUSION With single-isocenter LINAC-based SRT for multiple brain metastases, we found no
correlation between the distance of the isocenter and the incidence of radionecrosis or local recurrence. Single-isocenter SRT using a 6D robotic couch is a safe and effective treatment
option. DATA AVAILABILITY The datasets supporting the conclusions of the article are stored in an institutional repository and are available from the corresponding author on reasonable
request. ABBREVIATIONS * BM(s): Brain metastasis/metastases * C-index: Concordance index * FFF: Flattening filter-free * GTV: Gross tumor volume * ITD: Isocenter-to-tumor distance * LINAC:
Linear accelerator * LR: Local recurrence * MRI: Magnetic resonance imaging * NSCLC: Non-small cell lung cancer * PTV: Planning target volume * RN: Radionecrosis * SCLC: Small cell lung
cancer * SIMT: Single-isocenter for multiple targets * SRT: Stereotactic radiotherapy * WBRT: Whole brain radiotherapy REFERENCES * Brown, P. D. et al. Effect of radiosurgery alone versus
radiosurgery with whole brain radiation therapy on cognitive function in patients with 1–3 brain metastases a randomized clinical trial. _JAMA_ 316(4), 401–409.
https://doi.org/10.1001/jama.2016.9839.Effect (2016). Article PubMed PubMed Central Google Scholar * Kocher, M. et al. Adjuvant whole-brain radiotherapy versus observation after
radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. _J. Clin. Oncol._ 29(2), 134–141. https://doi.org/10.1200/JCO.2010.30.1655
(2011). Article PubMed Google Scholar * Yamamoto, M. et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective
observational study. _Lancet Oncol._ 15(4), 387–395. https://doi.org/10.1016/S1470-2045(14)70061-0 (2014). Article PubMed Google Scholar * Clark, G. M., Popple, R. A., Young, P. E. &
Fiveash, J. B. Feasibility of single-isocenter volumetric modulated arc radiosurgery for treatment of multiple brain metastases. _Int. J. Radiat. Oncol. Biol. Phys._ 76(1), 296–302.
https://doi.org/10.1016/j.ijrobp.2009.05.029 (2010). Article PubMed Google Scholar * Clark, G. M. et al. Plan quality and treatment planning technique for single isocenter cranial
radiosurgery with volumetric modulated arc therapy. _Pract. Radiat. Oncol._ 2(4), 306–313. https://doi.org/10.1016/j.prro.2011.12.003 (2012). Article PubMed Google Scholar * Thomas, E. M.
et al. Comparison of plan quality and delivery time between volumetric arc therapy (RapidArc) and Gamma Knife radiosurgery for multiple cranial metastases. _Neurosurgery_ 75(4), 409–417.
https://doi.org/10.1227/NEU.0000000000000448 (2014). Article PubMed Google Scholar * Chon, H., Yoon, K. J., Lee, D., Kwon, D. H. & Cho, Y. H. Single-fraction versus hypofractionated
stereotactic radiosurgery for medium-sized brain metastases of 25–3 cm. _J. Neuro-Oncol._ 145(1), 49–56. https://doi.org/10.1007/s11060-019-03265-1 (2019). Article Google Scholar * Roper,
J., Chanyavanich, V., Betzel, G., Switchenko, J. & Dhabaan, A. Single-isocenter multiple-target stereotactic radiosurgery: Risk of compromised coverage. _Int. J. Radiat. Oncol. Biol.
Phys._ 93(3), 540–546. https://doi.org/10.1016/j.ijrobp.2015.07.2262 (2015). Article PubMed PubMed Central Google Scholar * Palmiero, A. N., Critchfield, L., St Clair, W., Randall, M.
& Pokhrel, D. Single-isocenter volumetric modulated arc therapy (VMAT) radiosurgery for multiple brain metastases: Potential loss of target(s) coverage due to isocenter misalignment.
_Cureus_ 12(10), e11267. https://doi.org/10.7759/cureus.11267 (2020). Article PubMed PubMed Central Google Scholar * Buitelaar-Gallé, M. et al. EP-2339: Evaluation of a new mask system
for stereotactic radiotherapy in brain lesions. _Radiother. Oncol._ 127, S1290–S1291. https://doi.org/10.1016/s0167-8140(18)32648-3 (2018). Article Google Scholar * van Santvoort, J.,
Wiggenraad, R. & Bos, P. Positioning accuracy in stereotactic radiotherapy using a mask system with added vacuum mouth piece and stereoscopic X-ray positioning. _Int. J. Radiat. Oncol.
Biol. Phys._ 72(1), 261–267. https://doi.org/10.1016/j.ijrobp.2008.05.006 (2008). Article PubMed Google Scholar * Crouzen, J. A. et al. SAFESTEREO: Phase II randomized trial to compare
stereotactic radiosurgery with fractionated stereotactic radiosurgery for brain metastases. _BMC Cancer_ 23(1), 273. https://doi.org/10.1186/s12885-023-10761-1 (2023). Article CAS PubMed
PubMed Central Google Scholar * Lin, N. U. et al. Response assessment criteria for brain metastases: Proposal from the RANO group. _Lancet Oncol._ 16(6), e270–e278.
https://doi.org/10.1016/S1470-2045(15)70057-4 (2015). Article PubMed Google Scholar * Paddick, I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. _Tech.
Note. J. Neurosurg._ 93(Suppl 3), 219–222. https://doi.org/10.3171/jns.2000.93.supplement (2000). Article Google Scholar * Paddick, I. & Lippitz, B. A simple dose gradient measurement
tool to complement the conformity index. _J. Neurosurg._ 105(Suppl), 194–201. https://doi.org/10.3171/sup.2006.105.7.194 (2006). Article PubMed Google Scholar * Kraft, J. et al. Distance
to Isocenter is not associated with an increased risk for local failure in LINAC-based single-isocenter stereotactic radiosurgery (SRS) or radiotherapy (SRT) for multiple brain metastases.
_Radiother. Oncol._ https://doi.org/10.1016/j.radonc.2021.03.022 (2021). Article PubMed Google Scholar * Faccenda, V. et al. Single-isocenter linac-based radiosurgery for brain metastases
with coplanar arcs: A dosimetric and clinical analysis. _Cancers (Basel)_ https://doi.org/10.3390/cancers15184496 (2023). Article PubMed Google Scholar * Faccenda, V. et al. Dosimetric
and clinical prognostic factors in single-isocenter linac-based stereotactic radiotherapy for brain metastases. _Cancers (Basel)_ https://doi.org/10.3390/cancers16183243 (2024). Article
PubMed Google Scholar * Alongi, F. et al. First experience and clinical results using a new non-coplanar mono-isocenter technique (HyperArc) for Linac-based VMAT radiosurgery in brain
metastases. _J. Cancer Res. Clin. Oncol._ 145(1), 193–200. https://doi.org/10.1007/s00432-018-2781-7 (2019). Article CAS PubMed Google Scholar * Minniti, G. et al. Neurological outcome
and memory performance in patients with 10 or more brain metastases treated with frameless linear accelerator (LINAC)-based stereotactic radiosurgery. _J. Neurooncol._ 148(1), 47–55.
https://doi.org/10.1007/s11060-020-03442-7 (2020). Article PubMed Google Scholar * Kim, G. J. et al. Outcomes in patients With 4–10 Brain metastases treated with dose-adapted
single-isocenter multitarget stereotactic radiosurgery: A prospective study. _Adv. Radiat. Oncol._ https://doi.org/10.1016/j.adro.2021.100760 (2021). Article PubMed PubMed Central Google
Scholar * Sebastian, N. T. et al. Linear accelerator-based radiosurgery is associated with lower incidence of radionecrosis compared with gamma knife for treatment of multiple brain
metastases. _Radiother Oncol._ 147, 136–143. https://doi.org/10.1016/j.radonc.2020.03.024 (2020). Article CAS PubMed Google Scholar * Kirkpatrick, J. P. et al. Defining the optimal
planning target volume in image-guided stereotactic radiosurgery of brain metastases: Results of a randomized trial. _Int. J. Radiat. Oncol. Biol. Phys._ 91(1), 100–108.
https://doi.org/10.1016/j.ijrobp.2014.09.004 (2015). Article PubMed Google Scholar * Badloe, J. et al. Impact of PTV margin reduction (2 mm to 0 mm) on pseudoprogression in stereotactic
radiotherapy of solitary brain metastases. _Tech. Innov. Pat. Support Radiat. Oncol._ 17, 40–47. https://doi.org/10.1016/j.tipsro.2021.02.008 (2021). Article Google Scholar * Sperduto, P.
W. et al. Survival in patients with brain metastases: Summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. _J. Clin.
Oncol._ 38(32), 3773–3784. https://doi.org/10.1200/JCO.20.01255 (2020). Article CAS PubMed PubMed Central Google Scholar * Sperduto, P. W. et al. Graded prognostic assessment (GPA) for
patients with lung cancer and brain metastases: initial report of the small cell lung cancer GPA and update of the non-small cell lung cancer GPA including the effect of programmed death
ligand 1 and other prognostic factors. _Int. J. Radiat. Oncol. Biol. Phys._ 114(1), 60–74. https://doi.org/10.1016/j.ijrobp.2022.03.020 (2022). Article PubMed PubMed Central Google
Scholar * Crouzen, J. A. et al. External validation of the lung-molGPA to predict survival in patients treated with stereotactic radiotherapy for brain metastases of non-small cell lung
cancer. _Radiother. Oncol._ 198, 110405. https://doi.org/10.1016/j.radonc.2024.110405 (2024). Article PubMed Google Scholar * Martin, A. M. et al. Immunotherapy and symptomatic radiation
necrosis in patients with brain metastases treated with stereotactic radiation. _JAMA Oncol._ 4(8), 1123–1124. https://doi.org/10.1001/jamaoncol.2017.3993 (2018). Article PubMed PubMed
Central Google Scholar * Kim, P. H. et al. Immune checkpoint inhibitor therapy may increase the incidence of treatment-related necrosis after stereotactic radiosurgery for brain
metastases: A systematic review and meta-analysis. _Eur Radiol._ 31(6), 4114–4129. https://doi.org/10.1007/s00330-020-07514-0 (2021). Article CAS PubMed Google Scholar * Lehrer, E. J. et
al. Radiation necrosis in renal cell carcinoma brain metastases treated with checkpoint inhibitors and radiosurgery: An international multicenter study. _Cancer_ 128(7), 1429–1438.
https://doi.org/10.1002/cncr.34087 (2022). Article CAS PubMed Google Scholar * Rahman, R. et al. The impact of timing of immunotherapy with cranial irradiation in melanoma patients with
brain metastases: Intracranial progression, survival and toxicity. _J. Neurooncol._ 138(2), 299–306. https://doi.org/10.1007/s11060-018-2795-7 (2018). Article PubMed Google Scholar * Leu,
J. et al. Time interval from diagnosis to treatment of brain metastases with stereotactic radiosurgery is not associated with radionecrosis or local failure. _Front Oncol._ 13, 1132777.
https://doi.org/10.3389/fonc.2023.1132777 (2023). Article CAS PubMed PubMed Central Google Scholar * Anvari, A., Sasanpour, P. & Kheradmardi, M. R. Radiotherapy and immunotherapy in
melanoma brain metastases. _Hematol. Oncol. Stem Cell Ther._ 16(1), 1–20. https://doi.org/10.1016/j.hemonc.2021.11.001 (2023). Article CAS PubMed Google Scholar * Hilkens, N. A. et al.
Herziene richtlijn ‘Hersenmetastasen’. _Ned. Tijdschr. Geneeskd._ 164, 1–4 (2020). Google Scholar * Milano, M. T. et al. Single- and multifraction stereotactic radiosurgery dose/volume
tolerances of the brain. _Int. J. Radiat. Oncol. Biol. Phys._ https://doi.org/10.1016/j.ijrobp.2020.08.013 (2020). Article PubMed PubMed Central Google Scholar * Le Rhun, E., Dhermain,
F., Vogin, G., Reyns, N. & Metellus, P. Radionecrosis after stereotactic radiotherapy for brain metastases. _Expert Rev. Neurother._ 16(8), 903–914.
https://doi.org/10.1080/14737175.2016.1184572 (2016). Article CAS PubMed Google Scholar * Chao, S. T. et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis.
_Int. J. Radiat. Oncol. Biol. Phys._ 87(3), 449–457. https://doi.org/10.1016/j.ijrobp.2013.05.015 (2013). Article PubMed Google Scholar * Benedict, S. H. et al. Stereotactic body
radiation therapy: The report of AAPM task group 101. _Med . Phys._ 37(8), 4078–4101. https://doi.org/10.1118/1.3438081 (2010). Article PubMed Google Scholar * Walter, Y. A., Hubbard, A.
N., Durham, P. F. & Wu, H. T. Five-year evaluation of linear accelerator-based SRS platform isocentricity. _J. Appl. Clin. Med. Phys._ 26(4), e14597. https://doi.org/10.1002/acm2.14597
(2025). Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank Marc de Goede and Xander Staal for their help with collecting the treatment planning data. FUNDING This
work was supported by the Jacobusstichting (the Hague, the Netherlands). They were not involved in the conceptualization, design, data collection, analysis, decision to publish, or
preparation of the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Radiotherapy, Haaglanden Medical Center, Lijnbaan 32, 2512 VA, The Hague, The Netherlands J. A.
Crouzen, J. D. Zindler, M. E. Mast, M. C. Versluis, M. Hashimzadah, M. Kiderlen & N. C. M. G. van der Voort van Zyp * Department of Radiotherapy, HollandPTC, Huismansingel 4, 2629 JH,
Delft, The Netherlands J. D. Zindler * Department of Medical Physics, Haaglanden Medical Center, Lijnbaan 32, 2512 VA, The Hague, The Netherlands J. J. E. Kleijnen & A. L. Petoukhova *
Department of Neurosurgery, Haaglanden Medical Center, Lijnbaan 32, 2512 VA, The Hague, The Netherlands M. L. D. Broekman * Department of Neurosurgery, Leiden University Medical Center,
Albinusdreef 2, 2333 ZA, Leiden, The Netherlands M. L. D. Broekman * Department of Cell and Chemical Biology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA, Leiden, The
Netherlands M. L. D. Broekman Authors * J. A. Crouzen View author publications You can also search for this author inPubMed Google Scholar * J. D. Zindler View author publications You can
also search for this author inPubMed Google Scholar * M. E. Mast View author publications You can also search for this author inPubMed Google Scholar * J. J. E. Kleijnen View author
publications You can also search for this author inPubMed Google Scholar * M. C. Versluis View author publications You can also search for this author inPubMed Google Scholar * M.
Hashimzadah View author publications You can also search for this author inPubMed Google Scholar * M. Kiderlen View author publications You can also search for this author inPubMed Google
Scholar * N. C. M. G. van der Voort van Zyp View author publications You can also search for this author inPubMed Google Scholar * M. L. D. Broekman View author publications You can also
search for this author inPubMed Google Scholar * A. L. Petoukhova View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.C., J.Z., M.M., and
A.P. came up with the concept and methodology of the study. J.C. wrote the main manuscript text along with J.Z, M.M. and A.P. J.C. prepared all figures. J.C. and M.M. did the formal data
analysis. All authors reviewed and edited the manuscript. CORRESPONDING AUTHOR Correspondence to J. D. Zindler. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. ETHICS APPROVAL The Medical Ethics Committee Leiden The Hague Delft gave approval for this study (reference number N24.032) and granted a waiver of informed consent, because it
exclusively required retrospectively collected data. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL Below is the link to the electronic supplementary material. SUPPLEMENTARY MATERIAL 1 RIGHTS AND PERMISSIONS OPEN ACCESS This
article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction
in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the
licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article
are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Crouzen, J.A., Zindler, J.D., Mast, M.E. _et al._ Local
recurrence and radionecrosis after single-isocenter multiple targets stereotactic radiotherapy for brain metastases. _Sci Rep_ 15, 15722 (2025). https://doi.org/10.1038/s41598-025-01034-x
Download citation * Received: 14 January 2025 * Accepted: 02 May 2025 * Published: 05 May 2025 * DOI: https://doi.org/10.1038/s41598-025-01034-x SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative KEYWORDS * Brain metastases * Single-isocenter multiple targets technique * Stereotactic radiotherapy * Radionecrosis * Local recurrence
