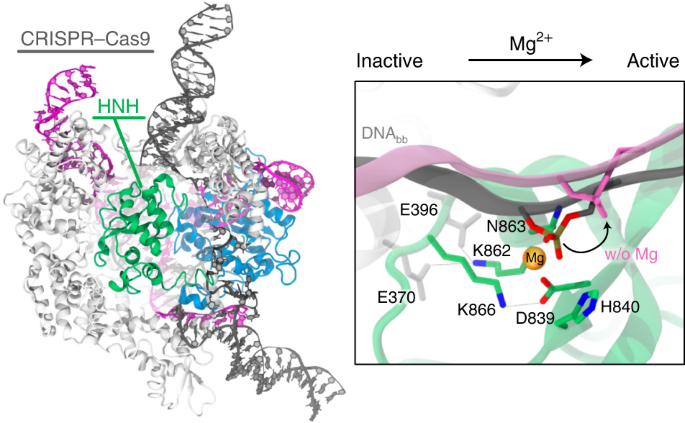
Principles of target dna cleavage and the role of mg2+ in the catalysis of crispr–cas9
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT At the core of the CRISPR–Cas9 genome-editing technology, the endonuclease Cas9 introduces site-specific breaks in DNA. However, precise mechanistic information to ameliorate Cas9
function is still missing. Here, multimicrosecond molecular dynamics, free energy and multiscale simulations are combined with solution NMR and DNA cleavage experiments to resolve the
catalytic mechanism of target DNA cleavage. We show that the conformation of an active HNH nuclease is tightly dependent on the catalytic Mg2+, unveiling its cardinal structural role. This
activated Mg2+-bound HNH is consistently described through molecular simulations, nuclear magnetic resonance (NMR) and DNA cleavage assays, revealing also that the protonation state of the
catalytic H840 is strongly affected by active site mutations. Finally, ab initio quantum mechanics (density functional theory)/molecular mechanics simulations and metadynamics establish the
catalytic mechanism, showing that the catalysis is activated by H840 and completed by K866, thus rationalizing DNA cleavage experiments. This information is critical to enhancing the
enzymatic function of CRISPR–Cas9 towards improved genome editing. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution
ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time
Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS AN ALPHA-HELICAL LID GUIDES THE TARGET DNA TOWARD CATALYSIS IN CRISPR-CAS12A Article Open
access 17 February 2024 MECHANISMS FOR TARGET RECOGNITION AND CLEAVAGE BY THE CAS12I RNA-GUIDED ENDONUCLEASE Article 07 September 2020 MECHANISTIC INSIGHTS INTO THE R-LOOP FORMATION AND
CLEAVAGE IN CRISPR-CAS12I1 Article Open access 09 June 2021 DATA AVAILABILITY Atomic coordinates of the optimized computational models are available in figshare with the identifier
https://doi.org/10.6084/m9.figshare.19158080. NMR resonance assignments for the HNH nuclease are available in the BMRB entry 27949. All other data are available from the authors upon
reasonable request. Source data are provided with this paper. REFERENCES * Doudna, J. A. & Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9.
_Science_ 346, 1258096 (2014). Article PubMed Google Scholar * Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. _Science_ 337, 816–821
(2012). CAS PubMed PubMed Central Google Scholar * Jinek, M. et al. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. _Science_ 343, 1247997 (2014). Article
PubMed PubMed Central Google Scholar * Nishimasu, H. et al. Crystal structure of Cas9 in complex with guide RNA and target DNA. _Cell_ 156, 935–949 (2014). Article CAS PubMed PubMed
Central Google Scholar * Casalino, L., Nierzwicki, Ł., Jinek, M. & Palermo, G. Catalytic mechanism of non-target DNA cleavage in CRISPR-Cas9 revealed by ab Initio molecular dynamics.
_ACS Catal._ 10, 13596–13605 (2020). Article CAS PubMed PubMed Central Google Scholar * Palermo, G., Miao, Y., Walker, R. C., Jinek, M. & McCammon, J. A. CRISPR-Cas9 conformational
activation as elucidated from enhanced molecular simulations. _Proc. Natl Acad. Sci. USA_ 114, 7260–7265 (2017). Article CAS PubMed PubMed Central Google Scholar * Dagdas, Y. S., Chen,
J. S., Sternberg, S. H., Doudna, J. A. & Yildiz, A. A conformational checkpoint between DNA binding and cleavage by CRISPR-Cas9. _Sci. Adv._ 3, eaao0027 (2017). Article PubMed PubMed
Central Google Scholar * Sternberg, S. H., LaFrance, B., Kaplan, M. & Doudna, J. A. Conformational control of DNA target cleavage by CRISPR–Cas9. _Nature_ 527, 110–113 (2015). Article
CAS PubMed PubMed Central Google Scholar * Biertümpfel, C., Yang, W. & Suck, D. Crystal structure of T4 endonuclease VII resolving a Holliday junction. _Nature_ 449, 616–620
(2007). Article PubMed Google Scholar * Zuo, Z. & Liu, J. Structure and dynamics of Cas9 HNH domain catalytic state. _Sci. Rep._ 7, 17271 (2017). Article PubMed PubMed Central
Google Scholar * Anders, C., Niewoehner, O., Duerst, A. & Jinek, M. Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. _Nature_ 513, 569–573 (2014).
Article CAS PubMed PubMed Central Google Scholar * Jiang, F. et al. Structures of a CRISPR-Cas9 R-loop complex primed for DNA cleavage. _Science_ 351, 867–871 (2016). Article CAS
PubMed PubMed Central Google Scholar * Huai, G. et al. Structural insights into DNA cleavage activation of CRISPR-Cas9 system. _Nat. Commun._ 8, 1375 (2017). Article PubMed PubMed
Central Google Scholar * Zhu, X. et al. Cryo-EM structures reveal coordinated domain motions that govern DNA cleavage by Cas9. _Nat. Struct. Mol. Biol._ 26, 679–685 (2019). Article CAS
PubMed PubMed Central Google Scholar * Bravo, J. P. K. et al. Structural basis for mismatch surveillance by CRISPR–Cas9. _Nature_ 603, 343–347 (2022). Article CAS PubMed PubMed Central
Google Scholar * Pacesa, M. et al. R-loop formation and conformational activation mechanisms of Cas9. _Nature_ 609, 191–96 (2022). Article CAS PubMed PubMed Central Google Scholar *
Zuo, Z. et al. Structural and functional insights into the bona fide catalytic state of _Streptococcus pyogenes_ Cas9 HNH nuclease domain. _eLife_ 8, e46500 (2019). Article PubMed PubMed
Central Google Scholar * Yoon, H., Zhao, L. N. & Warshel, A. Exploring the catalytic mechanism of Cas9 using information inferred from endonuclease VII. _ACS Catal._ 9, 1329–1336
(2019). Article CAS PubMed Google Scholar * Zhao, L. N., Mondal, D. & Warshel, A. Exploring alternative catalytic mechanisms of the Cas9 HNH domain. _Proteins_ 88, 260–264 (2019).
Article PubMed Google Scholar * Kästner, J. Umbrella sampling. _WIREs Comput. Mol. Sci._ 1, 932–942 (2011). Article Google Scholar * East, K. W. et al. Allosteric motions of the
CRISPR–Cas9 HNH nuclease probed by NMR and molecular dynamics. _J. Am. Chem. Soc._ 142, 1348–1358 (2020). Article CAS PubMed PubMed Central Google Scholar * Nierzwicki, Ł. et al.
Enhanced specificity mutations perturb allosteric signaling in the CRISPR-Cas9 HNH endonuclease. _eLife_ 10, e73601 (2021). Article CAS PubMed PubMed Central Google Scholar * Swails, J.
M. & Roitberg, A. E. Enhancing conformation and protonation state sampling of hen egg white lysozyme using pH replica exchange molecular dynamics. _J. Chem. Theory Comput._ 8, 4393–4404
(2012). Article CAS PubMed Google Scholar * Hansen, A. L. & Kay, L. E. Measurement of histidine p_K_a values and tautomer populations in invisible protein states. _Proc. Natl Acad.
Sci. USA_ 111, 1705–1712 (2014). Article Google Scholar * Shimahara, H. et al. Tautomerism of histidine 64 associated with proton transfer in catalysis of carbonic anhydrase. _J. Biol.
Chem._ 282, 9646–9656 (2007). Article CAS PubMed Google Scholar * Brunk, E. et al. Pushing frontiers of first-principles based computer simulations of chemical and biological systems.
_Chimia (Aarau)_ 65, 667–671 (2011). Article CAS Google Scholar * Carter, E. A., Ciccotti, G., Hynes, J. T. & Kapral, R. Constrained reaction coordinate dynamics for the simulation of
rare events. _Chem. Phys. Lett._ 156, 472–477 (1989). Article CAS Google Scholar * Laio, A., VandeVondele, J. & Rothlisberger, U. A Hamiltonian electrostatic coupling scheme for
hybrid Car–Parrinello molecular dynamics simulations. _J. Chem. Phys._ 116, 6941–6947 (2002). Article CAS Google Scholar * Becke, A. D. Density-functional exchange-energy approximation
with correct asymptotic behavior. _Phys. Rev. A_ 38, 3098–3100 (1988). Article CAS Google Scholar * Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti
correlation-energy formula into a functional of the electron density. _Phys. Rev. B_ 37, 785–789 (1988). Article CAS Google Scholar * Dürr, S. L. et al. The role of conserved residues in
the DEDDh motif: the proton-transfer mechanism of HIV-1 RNase H. _ACS Catal._ 11, 7915–7927 (2021). Article Google Scholar * Casalino, L., Palermo, G., Rothlisberger, U. & Magistrato,
A. Who activates the nucleophile in ribozyme catalysis? An answer from the splicing mechanism of group II introns. _J. Am. Chem. Soc._ 138, 10374–10377 (2016). Article CAS PubMed Google
Scholar * Borišek, J. & Magistrato, A. All-atom simulations decrypt the molecular terms of RNA catalysis in the exon-ligation step of the spliceosome. _ACS Catal._ 10, 5328–5334 (2020).
Article Google Scholar * Palermo, G. et al. Catalytic metal ions and enzymatic processing of DNA and RNA. _Acc. Chem. Res._ 48, 220–228 (2015). Article CAS PubMed Google Scholar *
Gong, S., Yu, H. H., Johnson, K. A. & Taylor, D. W. DNA unwinding is the primary determinant of CRISPR-Cas9 activity. _Cell Rep._ 22, 359–371 (2018). Article CAS PubMed Google Scholar
* Laio, A. & Parrinello, M. Escaping free-energy minima. _Proc. Natl Acad. Sci. USA_ 99, 12562–12566 (2002). Article CAS PubMed PubMed Central Google Scholar * Cisneros, G. A. et
al. Reaction mechanism of the ε subunit of _E. coli_ DNA polymerase III: insights into active site metal coordination and catalytically significant residues. _J. Am. Chem. Soc._ 131,
1550–1556 (2009). Article CAS PubMed PubMed Central Google Scholar * Wang, Y. et al. Real-time observation of cas9 postcatalytic domain motions. _Proc. Natl Acad. Sci. USA_ 118,
e2010650118 (2021). Article CAS PubMed Google Scholar * Palermo, G. Structure and dynamics of the CRISPR–Cas9 catalytic complex. _J. Chem. Inf. Model._ 59, 2394–2406 (2019). Article CAS
PubMed Google Scholar * Galburt, E. A. & Stoddard, B. L. Catalytic mechanisms of restriction and homing endonucleases. _Biochemistry_ 41, 13851–13860 (2002). Article CAS PubMed
Google Scholar * Perez, A. et al. Refinement of the AMBER force field for nucleic acids: improving the description of α/γ conformers. _Biophys. J._ 92, 3817–3829 (2007). Article CAS
PubMed PubMed Central Google Scholar * Banas, P. et al. Performance of molecular mechanics force fields for RNA simulations: stability of UUCG and GNRA hairpins. _J. Chem. Theor. Comput._
6, 3836–3849 (2010). Article CAS Google Scholar * Zgarbova, M. et al. Refinement of the Cornell et al. nucleic acids force field based on reference quantum chemical calculations of
glycosidic torsion profiles. _J. Chem. Theory Comput._ 7, 2886–2902 (2011). Article CAS PubMed PubMed Central Google Scholar * Li, P., Roberts, B. P., Chakravorty, D. K. & Merz, K.
M. Rational design of particle mesh Ewald compatible Lennard-Jones parameters for +2 metal cations in explicit solvent. _J. Chem. Theory Comput._ 9, 2733–2748 (2013). Article CAS PubMed
PubMed Central Google Scholar * Berendsen, H. J. C., Postma, J. P. M., van Gunsteren, W. F., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. _J. Chem.
Phys._ 81, 3684–3690 (1984). CAS Google Scholar * Case, D. A. et al. AMBER 2020 (Univ. of California, San Francisco, 2020). * Parrinello, M., Andreoni, W. & Curioni, A. CPMD (IBM
Corporation and Max-Planck Institute, 2000). * Hoover, W. G. Canonical dynamics: equilibrium phase-space distributions. _Phys. Rev. A_ 31, 1695–1697 (1985). Article CAS Google Scholar *
Nosé, S. An extension of the canonical ensemble molecular dynamics method. _Mol. Phys._ 57, 187–191 (1986). Article Google Scholar * Car, R. & Parrinello, M. Unified approach for
molecular dynamics and density-functional theory. _Phys. Rev. Lett._ 55, 2471–2474 (1985). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This material is based
on work supported by the National Institute of Health (grant no. R01GM141329, to G.P.) and the National Science Foundation (grant no. CHE-1905374, to G.P.). G.P.L. is supported by the
National Science Foundation (grant no. MCB-2143760). This work was also supported in part by the National Institute of Health (grant no. R01GM136815 to G.P. and G.P.L.). M.J. acknowledges
support from the Swiss National Science Foundation (31003A_182567). M.J. is an International Research Scholar of the Howard Hughes Medical Institute and Vallee Scholar of the Bert L & N
Kuggie Vallee Foundation. Computer time for MD has been awarded by XSEDE under grant no. TG-MCB160059 and by NERSC under grant no. M3807 (to G.P.). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Bioengineering, University of California Riverside, Riverside, CA, USA Łukasz Nierzwicki, Rohaine V. Hsu, Mohd Ahsan, Pablo R. Arantes & Giulia Palermo *
Department of Molecular Biology, Cell Biology and Biochemistry, Brown University, Providence, RI, USA Kyle W. East, Erin Skeens & George P. Lisi * Department of Biochemistry, University
of Zürich, Zurich, Switzerland Jonas M. Binz, Martin Pacesa & Martin Jinek * Department of Chemistry, University of California Riverside, Riverside, CA, USA Giulia Palermo Authors *
Łukasz Nierzwicki View author publications You can also search for this author inPubMed Google Scholar * Kyle W. East View author publications You can also search for this author inPubMed
Google Scholar * Jonas M. Binz View author publications You can also search for this author inPubMed Google Scholar * Rohaine V. Hsu View author publications You can also search for this
author inPubMed Google Scholar * Mohd Ahsan View author publications You can also search for this author inPubMed Google Scholar * Pablo R. Arantes View author publications You can also
search for this author inPubMed Google Scholar * Erin Skeens View author publications You can also search for this author inPubMed Google Scholar * Martin Pacesa View author publications You
can also search for this author inPubMed Google Scholar * Martin Jinek View author publications You can also search for this author inPubMed Google Scholar * George P. Lisi View author
publications You can also search for this author inPubMed Google Scholar * Giulia Palermo View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
L.N. performed molecular simulations and analysed data. K.W.E. and E.S. performed NMR experiments. J.M.B. and M.P. performed DNA cleavage experiments. P.R.A., R.V.H. and M.A. performed
molecular simulations. M.J. supervised DNA cleavage experiments. G.P.L. supervised NMR experiments. G.P. conceived this research, supervised computational studies and wrote the manuscript,
with critical input from all authors. CORRESPONDING AUTHORS Correspondence to George P. Lisi or Giulia Palermo. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Catalysis_ thanks Quanjiang Ji, Priyadarshi Satpati, Jeong-Yong Suh and the other, anonymous, reviewer(s) for their contribution to the
peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Methods, Discussion, Figs. 1–29 and Tables 1 and 2. REPORTING SUMMARY SOURCE DATA SOURCE DATA FIG. 3 Unprocessed gel
pictures for the In vitro cleavage kinetics of Cas9 HNH mutants on a double-stranded DNA on-target substrate. RIGHTS AND PERMISSIONS Springer Nature or its licensor holds exclusive rights to
this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the
terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Nierzwicki, Ł., East, K.W., Binz, J.M. _et al._ Principles of target DNA
cleavage and the role of Mg2+ in the catalysis of CRISPR–Cas9. _Nat Catal_ 5, 912–922 (2022). https://doi.org/10.1038/s41929-022-00848-6 Download citation * Received: 06 February 2022 *
Accepted: 25 August 2022 * Published: 06 October 2022 * Issue Date: October 2022 * DOI: https://doi.org/10.1038/s41929-022-00848-6 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
