Core strength | Nature Catalysis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

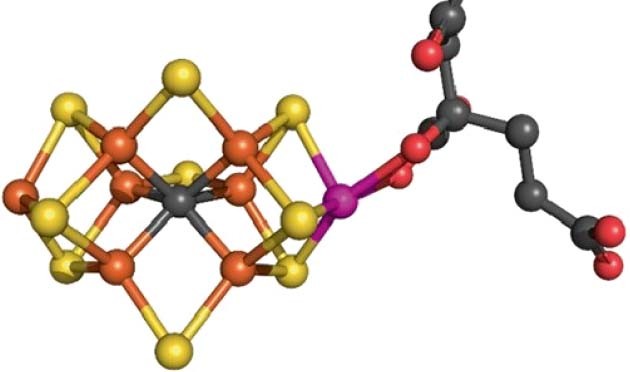
Access through your institution Buy or subscribe One leading hypothesis involves dynamic carbon coordination in the Fe–C bonds, which weaken or break to allow the binding of substrates and
intermediates at the Fe atoms. The other proposes a structural role for the carbon atom based on the similarity of the CFe6 motif to that of cementite, the component of carbon steels which
endows toughness. In this collaborative study, carefully targeted 13C isotopic labelling is performed on wild-type and variant (α-Ala70- and α-Ile70-substituted) MoFe protein. Electron
nuclear double resonance (ENDOR) spectroscopy is performed on these enriched species in their ground states as well as after activation with various substrates and inhibitors (N2, propargyl
alcohol (an alkyne) and CO). Notably, the net 13C hyperfine coupling measured is near-zero in all cases, and seemingly invariant to the environment. Density functional theory (DFT)
calculations indicate that the individual contributions of each Fe–C bond to spin transfer are large. Near-zero overall spin of the CFe6 core can therefore only be achieved if there are
three spin-up and three spin-down Fe atoms coordinated to the carbon atom in all these various states. This is inconsistent with hemilabile coordination and a dynamic functional role of the
carbon atom. These findings instead lend support to the idea of an important structural role for this unique carbon atom, holding the FeMo-cofactor together and providing stability as it
undergoes the catalytic cycle. Researchers may need to look elsewhere, such as to the Fe–S bonds, to explain the dynamic coordination behaviour in the nitrogenase active site. This is a
preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value
online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Nature Catalysis
https://www.nature.com/natcatal Benjamin Martindale Authors * Benjamin Martindale View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR
Correspondence to Benjamin Martindale. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Martindale, B. Core strength. _Nat Catal_ 5, 843 (2022).
https://doi.org/10.1038/s41929-022-00866-4 Download citation * Published: 19 October 2022 * Issue Date: October 2022 * DOI: https://doi.org/10.1038/s41929-022-00866-4 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative
