Boom-bust population dynamics increase diversity in evolving competitive communities
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The processes and mechanisms underlying the origin and maintenance of biological diversity have long been of central importance in ecology and evolution. The competitive exclusion
principle states that the number of coexisting species is limited by the number of resources, or by the species’ similarity in resource use. Natural systems such as the extreme diversity of
unicellular life in the oceans provide counter examples. It is known that mathematical models incorporating population fluctuations can lead to violations of the exclusion principle. Here we
use simple eco-evolutionary models to show that a certain type of population dynamics, boom-bust dynamics, can allow for the evolution of much larger amounts of diversity than would be
expected with stable equilibrium dynamics. Boom-bust dynamics are characterized by long periods of almost exponential growth (boom) and a subsequent population crash due to competition
(bust). When such ecological dynamics are incorporated into an evolutionary model that allows for adaptive diversification in continuous phenotype spaces, desynchronization of the boom-bust
cycles of coexisting species can lead to the maintenance of high levels of diversity. SIMILAR CONTENT BEING VIEWED BY OTHERS LIFE HISTORY COMPLEMENTARITY AND THE MAINTENANCE OF BIODIVERSITY
Article 07 June 2023 UNIVERSAL SCALING OF EXTINCTION TIME IN STOCHASTIC EVOLUTIONARY DYNAMICS Article Open access 27 December 2022 THE EVOLUTION OF BIOGEOCHEMICAL RECYCLING BY
PERSISTENCE-BASED SELECTION Article Open access 04 March 2022 INTRODUCTION The amazing diversity of life has sustained the debate about the origins and limits of biodiversity. While random,
selectively neutral processes are thought by some to play an important role, e.g., in ecosystem dynamics1 and in molecular evolution, it seems that a majority of researchers would agree that
non-neutral ecological interactions—competition, predation, mutualism—are central to understanding diversity, with competition having received the most attention. Coexistence between
competing species requires that intraspecific competition is strong enough relative to interspecific competition. This is captured by the concept of limiting similarity: to coexist,
populations must be sufficiently different in their resource use. If populations use the same resource in the same way, they cannot coexist, a phenomenon known as the competitive exclusion
principle2. The exclusion principle has faced challenges from many empirical counter examples, in which the number of coexisting and ecologically interacting species was significantly higher
than the number of limiting resources. The best known such example is the Paradox of the Plankton3, which is based on a comparison between the relatively small number of biochemical
resources essential for plankton growth, and the number of known coexisting plankton species, which is orders of magnitude larger. Different theoretical explanations for conditions that
circumvent the exclusion principle have been proposed, and it is known that fluctuating population sizes can lead to violations2,4,5,6. With fluctuating population sizes, the storage
effect7,8, as well as relative non-linearities2 can lead to coexistence of more competitor species than essential resource species, e.g., because of cyclical dominance between competitors9.
Most of these examples involve models with a finite number of distinct resources, but coexistence due to fluctuating population dynamics has also been shown in models with continuous niches.
With continuous, externally imposed (seasonal) periodic cycles in population sizes, time essentially becomes an additional niche dimension along which populations can segregate and
coexist10. In an evolutionary context, it has been shown that limiting similarity in a continuous niche space used by an evolving community whose member species are undergoing externally
forced population fluctuations, larger amplitude fluctuations lead to more diversity, and hence effectively to smaller limiting similarity11. Most previous models used in his context have
assumed that the population fluctuations are externally imposed, and that there is a finite number of distinct resources. Here we investigate the questions of evolving diversity and limiting
similarity in a setting where population fluctuations are not externally imposed, but are instead due to competitive interactions within and between the evolving species. In addition, we
address the question of diversity in continuous phenotypes spaces, corresponding to continuously varying resource use. Rather than the 1-dimensional phenotype spaces that are usually assumed
with continuous resource distributions11,12,13, our phenotype spaces are potentially high-dimensional. We use the models of14,15,16,17,18, which are extensions of classical competition
models to high-dimensional continuous phenotype spaces. In previous work, we assumed that the underlying ecological dynamics have a stable equilibrium (the carrying capacity). However, by
using difference equations rather than differential equations to describe ecological dynamics, it is straightforward to extend these models to allow for complicated ecological dynamics,
which are by definition endogenously generated (i.e., the population fluctuations are a result of the competitive interactions). We show that for certain types of endogenously generated
fluctuations, which we term “boom-bust” dynamics, the amount of diversity that evolves and is maintained at evolutionary steady state can be much larger than the diversity maintained without
ecological fluctuations. Boom-bust dynamics consist of long periods of (near-) exponential growth followed by a deep crash, in such a way that the boom-bust cycles of different species
become spontaneously desynchronized. The amount of excess diversity enabled by boom-bust dynamics increases with the dimension of phenotype space, so that species can be much more tightly
packed in high-dimensional spaces, corresponding to a much smaller limiting similarity necessary for coexistence in high-dimensional niche spaces. Apart from asynchronous boom-bust dynamics,
essentially all other types of complex fluctuations, including asynchronous chaotic dynamics not exhibiting the boom-bust fluctuations, do not increase the diversity at evolutionary steady.
Our models thus provide a specific and robust mechanism for the evolutionary origin and maintenance of highly diverse competitive communities. RESULTS To accommodate various types of
ecological dynamics, we consider ecological models given by difference equations, and hence set in discrete time. The basic ecological model we use is a difference equation19,20,21 that
links population densities _N_ of two consecutive generations _t_ and _t_ + 1, $$N(t+1)=F(N(t))=N(t)\frac{\lambda }{1+aN{(t)}^{\beta }}.$$ (1) where _λ_ > 0 is the per capita number of
offspring, and _a_ > 0 and _β_ > 0 are parameters describing the effect of competition. For _λ_ < 1, _N_(_t_) converges to 0 for any initial condition _N_(0) > 0, and hence
extinction is the only possible outcome. We therefore assume _λ_ > 1 in what follows. In that case, model (1) has a non-zero equilibrium at _K_ = ((_λ_−1)/_a_)1/_β_, which is the carrying
capacity of the population. It is then convenient to write (1) as: $$N(t+1)=N(t)\frac{\lambda }{1+(\lambda -1){(N(t)/K)}^{\beta }},$$ (2) as this makes it easy to formulate the model in
terms of continuous phenotypes (see below). Model (2) was shown to fit well a wide range of data20, and for _β_ = 1 can be derived from the logistic differential equation by integration over
a finite time interval22. The model given by (1) and (2) is phenomenological in nature. Its basic dynamic properties are briefly described in the first section of Methods. While other
simple discrete-time models can be derived from first principles, this does not appear to be the case for model (1) if _β_ > 123. Rather, this model should be viewed as a heuristic model
that can exhibit a wide array of dynamic regimes, including the boom-bust regime that will be of paramount importance in this paper (see below). Because the difference Eq. (2) has three
rather than two parameters, it can exhibit certain dynamical properties that better known difference equations, such as the Ricker equation20,24, do not have. For example, for small values
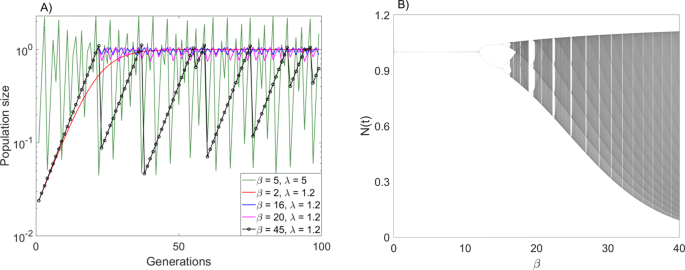
of _λ_, model (2) can exhibit highly chaotic dynamics (as e.g., measured by the Lyapunov exponent) despite the fluctuations in population size being (arbitrarily) small (Fig. 1).
Importantly, for small values of _λ_, and for large enough _β_, model (2) exhibits “boom-bust” dynamics (Fig. 1), in which long periods of near-exponential growth (due to small _λ_) are
followed by deep crashes (due to high _β_) once the population size is above _K_ for the first time after the exponential phase. This cycle repeats itself qualitatively, but the dynamics is
in fact chaotic and exhibits sensitive dependence on initial conditions, because the population size after the crash, and hence the length of the subsequent exponential phase, is different
in each cycle. We note that such boom-bust dynamics cannot be observed in the Ricker model. Some of the possible dynamic regimes of model (2) and transitions between them are shown in Fig.
1. We note that there are in principle many different models that can exhibit boom-bust dynamics (including models set in continuous time, see Discussion section). We chose model (2) as a
generic model with boom-bust dynamics for certain parameter regions, viz. for _λ_-values close to 1 and large enough _β_. Rather than being interested in the likelihood of a particular model
exhibiting boom-bust dynamics, we are interested in the consequences of such dynamics for the evolution of diversity. Therefore, while pointing out the contrast to the consequences of other
types of ecological dynamics, such as cyclic or “regular” chaotic dynamics, delineating the different regions in parameter space generating the different types of dynamics is not relevant
for our purposes. We now consider a generalization of Eq. (2) that includes competition between _S_ phenotypically monomorphic populations. Each population is characterized by its phenotype
\({x}_{s}=({x}_{s}^{1},...,{x}_{s}^{d})\in {{\mathbb{R}}}^{d}\), _s_ = 1, . . . , _S_, where _d_ is the dimension of phenotype space (which is assumed to be Euclidean _d_-space). The
population size of phenotype _x__s_ is denoted by _N__s_. The ecological dynamics of all _S_ clusters are determined by the competition kernel _α_(_x__s_, _x__r_), which measures the
competitive impact of phenotype _x__r_ on phenotype _x__s_, and the carrying capacity _K_(_x__s_), which is the equilibrium population size of phenotype _x__s_ in the absence of any other
phenotypes. (The competition kernel and the carrying capacity are functions \(\alpha :{{\mathbb{R}}}^{d}\times {{\mathbb{R}}}^{d}\to {\mathbb{R}}\) and \(K:{{\mathbb{R}}}^{d}\to
{\mathbb{R}}\), respectively.) The discrete time dynamics of each phenotype in the competitive community is then given by $${N}_{s}(t+1) ={N}_{s}(t) \\ \quad \times \frac{\lambda
}{1+(\lambda -1){\left[\mathop{\sum }\nolimits_{p = 1}^{S}{N}_{p}(t)\alpha ({x}_{s},{x}_{p})/K({x}_{s})\right]}^{\beta }},$$ (3) _s_ = 1, . . . , _S_. The sum in the denominator on the right
hand side of (3) is the effective population size experienced by phenotype _x__s_. Equation (3) is a discrete time analog of the continuous-time many-species logistic competition model in
multidimensional phenotype space that was used in several previous articles16,17,18. In contrast to the continuous time models used previously, in the discrete time model populations can
undergo ecological fluctuations and sudden collapses not only after a population itself exceeds the carrying capacity, but also when the cumulative competition from other phenotypes is
strong enough (i.e., when the effective population size is above _K_). For simplicity, and following15,16,17, we used the following functions for the competition kernel and the carrying
capacity, $$\alpha (x,y)=\exp \left[-\mathop{\sum }\limits_{i=1}^{d}\frac{{({x}^{i}-{y}^{i})}^{2}}{2{\sigma }_{\alpha }^{2}}\right],\ \\ K(x)={K}_{0}\exp \left[-\frac{\mathop{\sum
}\nolimits_{i = 1}^{d}{({x}^{i})}^{4}}{4{\sigma }_{K}^{4}}\right].$$ (4) Thus, competition is symmetric and strongest between phenotypes that are similar, and the carrying capacity has a
unique maximum _K_0 at 0. We note that in general, using Gaussian forms for both the competition kernel and the carrying capacity can result in structural instabilities25. In the second
section of Methods, we describe the numerical procedures for simulating the evolutionary process resulting from (3). Typically, simulations start with a single ancestral phenotype, which
changes due to mutations and can undergo repeated diversification events due to frequency-dependent competition. In particular, for _σ__α_ < _σ__K_ in (4), the continuous time analog of
the model presented above undergoes adaptive diversification and radiation into a steady state species distribution13,17,26, see also27,28. For example, if we set _σ__α_ = 0.5 and _σ__K_ = 1
in (4), then for _β_ = 1 system (3) is equivalent to the corresponding continuous time system22, and in a 2-dimensional phenotype space undergoes diversification into a stable community of
16 coexisting phenotypic clusters (species) with approximately constant population sizes. This is illustrated in Fig. 2A and the corresponding video. As long as _β_ = 1, the observed
diversification is independent of _λ_ and _K_0. The main purpose of this paper is to explore the effect of increasing _β_ to values >1, which eventually makes the local dynamics (2)
unstable. As the exponent _β_ is increased, stationary populations lose stability and, similarly to the single-species model shown in Fig. 1, the ecological dynamics of populations in an
evolving community become first periodic and then chaotic (see below for specific examples of non-equilibrium dynamics). For low intrinsic growth rates _λ_ this has profound effects on the
amount of diversity in the system, as illustrated in Fig. 2 (and accompanying videos of various diversification scenarios corresponding to Fig. 2 can be found here:
figshare.com/s/f2d8ecf480fa372319e1). For such _λ_-values, increasing _β_ in the local dynamics (2) has the effect of eventually inducing pronounced boom-bust population dynamics (cf. Fig.
1). In an evolving community, increasing _β_ induces boom-bust dynamics in each of the phenotypes present in the community, with each phenotypic cluster (species) undergoing multiple
generations of (near-)exponential population growth punctuated by deep crashes. These ecological dynamics unfold in such a way that the dynamics of neighboring clusters of phenotypes are
desynchronized, i.e., such that crashes and subsequent exponential growth phases occur at different time points. With such desynchronized boom-bust ecological dynamics, evolution can
generate a drastic increase in diversity compared to that evolving in ecologically stable communities (Fig. 2). Increased diversity due to boom-bust ecological dynamics typically occurs as
long as the ecological conditions for adaptive diversification due to frequency-dependent competition are met (i.e., as long as _σ__α_ < _σ__K_ in (4)). While the amount of diversity
depends on the exact values of _σ__α_ < _σ__K_, significantly more diverse communities tend to evolve with boom-bust dynamics than with stable equilibrium ecological dynamics. Figure 3
shows the number of species coexisting at the evolutionary saturation state as a function of the parameter _β_ for different values of _λ_. The figure illustrates that _λ_ has to be small
enough for a substantial increase in diversity to be observed for high _β_. Indeed, in the local model (2) boom-bust dynamics can only be observed for small _λ_, and it is exactly these
kinds of population dynamics that allow for increased diversity. For larger _λ_, increasing _β_ results in more “traditional” forms of chaotic dynamics with irregular, high-frequency
oscillations of increasing amplitude. Such local dynamics also lead to chaotic ecological dynamics in populations comprising an evolving community, but they do not generate an increase in
the diversity that can evolve and be maintained. In general, as _β_ is increased to very high values, the model becomes less relevant biologically: the population crashes become very severe,
which results in extinctions that are frequent enough for diversity not to be able to evolve anymore (see below). Figure 4 illustrates the desynchronized boom-bust dynamics in an artificial
community of 25 species, with each species represented by a single phenotype, and such that the phenotypes are arranged on a regular grid in phenotype space (see inset in Fig. 4). This
community corresponds to the case indicated by the right-most square with more than 1 species in Fig. 3, in which _β_ is large enough for the diversity to increase to 25 coexisting species,
rather than the 16 that would evolve for lower _β_. Figure 4 shows the population dynamics of a subset of 5 phenotypes arranged on a line in the grid, resulting from simulating the
ecological dynamics (3) of the whole community of 25 phenotypes. For each phenotype, the dynamics exhibits boom-bust cycles, and neighboring cycles are desynchronized. It is worth noting
that the increased diversity seen for higher _β_-values occupies approximately the same phenotypic range as the lower diversity for lower _β_-values (Fig. 2). This implies that with higher
diversity, the different species are more closely packed in phenotype space, and hence that, generally speaking, conditions of limiting similarity are relaxed in the boom-bust dynamic
regime. Because of lower thresholds for limiting similarity, i.e., denser packing, the increase in diversity at evolutionary stationary state due to boom-bust ecological dynamics becomes
more pronounced with higher dimensions of phenotype space, as illustrated in Fig. 5. Relaxed limiting similarity conditions require a decrease in competitive pressure that species in
neighboring regions of phenotype space exert on each other. Such a decrease can be achieved if neighboring populations are fluctuating in opposite phases, as shown for an artificial example
in Fig. 4. Figure 6 illustrates that in full simulations of the evolutionary process, neighboring species indeed generally exhibit such an anti-correlation for high _β_-values. Essentially,
the anti-correlation between populations of neighboring species stems from the asynchrony of their boom-bust cycles. In the first section of the Supplementary Material, we show that such
desynchronization is expected to emerge spontaneously from an arbitrary small initial difference between populations: in a simple idealized configuration of two competing species with
boom-bust dynamics, their population sizes converge to a state of complete anti-synchronization. For smaller values of _β_ or larger values of _λ_, for which populations do not undergo
boom-bust dynamics this anti-correlation is not seen (Fig. 6). The explanation for higher diversity based on anti-correlated boom-bust cycles of phenotypically close species suggests that to
make this mechanism work, these cycles should be of sufficient length. This means that the population crashes should be sufficiently severe (large _β_), and the intrinsic growth rate _λ_
should be sufficiently small. Essentially, the exponential phase should be long enough for robust desynchronization. This effect cannot be achieved with high intrinsic growth parameters _λ_
(Fig. 3): the increase in diversity is noticeably diminished for _λ_ = 1.6, and is absent for larger _λ_. In particular, the type of chaotic population fluctuations induced by high _β_ for
_λ_-values that are significantly larger than 1 do not lead to increased diversity, because for larger _λ_, complex dynamics are not of the boom-bust type. To confirm that the boom-bust
cycles, rather than chaoticity or other features of the population dynamics defined by Eq. (3), are the essential mechanism for the observed increase in diversity, we stripped the model (3)
from all other features except its ability to run boom-bust cycles: We assumed that each phenotypic population grows exponentially with an exponent _λ_ until the effective density
experienced by a given phenotype, i.e., the cumulative competitive effect of all phenotypes, given by the term \(\mathop{\sum }\nolimits_{p = 1}^{S}{N}_{p}(t)\alpha ({x}_{s},{x}_{p})\) in
denominator of (3), becomes greater than the carrying capacity of that phenotype. When that happened, the population of that phenotype was reduced to a small fraction of its population size,
simulating a severe crash. The mutation and merging procedures were implemented as in the original model. This modified model shows qualitatively very similar results (not shown) and
exhibits significant increases in diversity at a level very similar to the original model, as long as _λ_-values are close to 1, so that the exponential phase starting from low densities is
long and slow, and as long as the population crashes are severe enough. This confirms that the key for the evolution of higher diversity is the existence of pronounced boom-bust dynamics for
all phenotypic populations. An interesting question concerns the effect of the frequency and size of mutations on diversity. These were assumed to be _μ_ = 0.1 and Δ_x_ = 10−2 for the
results presented so far (note that it is really only the product of these two parameters that matters). A reduction in _μ_ and/or Δ_x_ slows down evolution in general and diversification in
particular. This effect is illustrated in Fig. S.3A, where we show the number of species vs. time for four different mutation frequencies. Smaller mutation rates result in longer times to
reach the equilibrium level of diversity. There is, however, another, less direct effect of mutation rate on diversity. For any non-zero extinction threshold and even moderate _β_, there is
a small but finite probability that all phenotypes of a well-developed cluster, and hence the corresponding species, go extinct during a particularly severe bust. The extinct cluster can
eventually get replaced by newly arising mutants, but the time it takes mutations to undergo a sufficient number of phenotypic steps to reach the vacated spot in phenotype space depends on
_μ_ and Δ_x_. For any given mutation rate and size, these processes may equilibrate at different levels of diversity. In particular, lower extinction thresholds (making extinction less
likely) lead to higher levels of diversity at saturation. This is illustrated in Fig. S.3B. In general, diversity decreases drastically for very high _β_-values and eventually the system is
reduced to just a single phenotypic cluster. This occurs because with large _β_-values, the crashes due to the effective density experienced being higher than the carrying capacity become
progressively more severe and can bring all phenotypes comprising one species below the minimum population threshold, thus rendering the species extinct. Even though diversification is still
favored by selection, the rate of species extinction for high _β_-values is too high for diversity to evolve. The very dynamic regime of this “competition” between extinction and
diversification is illustrated in Fig. S.2. DISCUSSION We propose a possible explanation for the emergence and persistence of large amounts of diversity based on competition models for
evolving communities with fluctuating population dynamics. When these fluctuations are in the boom-bust regime, in which long periods of exponential growth are followed by deep population
crashes, diversity in continuous phenotype spaces evolves well beyond what is expected based on limiting similarity with stationary ecological dynamics. The key mechanism that results in
higher diversity is the spontaneous desynchronization of boom-bust cycles between phenotypically similar species, which essentially reduces interspecific competitive impacts and allows for
much denser packing of species in niche space. Population fluctuations have long been considered as a potential mechanism leading to violations of the competitive exclusion principle. For
the most part, past studies have either assumed a fixed set of resources2,9,29, or they have assumed externally imposed fluctuations10,11. In such models, the mechanism of ecological
fluctuations causing an increase in diversity can be viewed as a form of the temporal storage effect7,8, which intuitively corresponds to temporal segregation in niche space10. In fact,
there have also been models showing that population fluctuations can decrease diversity in an evolutionary context30, but these models appear to allow for jack-of-all-trades mutations on a
finite set of resources, which can increase rather than decrease the amount of interspecific competition in the system. Our models extend previous models for the emergence and maintenance of
diversity under stable equilibrium ecological dynamics13,17,26,27,28. They differ from earlier models such as27,28 in key aspects: they consider evolution in high-dimensional phenotypes
that characterize continuously variable and multivariate niche use, and persistent ecological fluctuations are intrinsically generated by overcompensating competition. Desynchronized
boom-bust cycles provide a robust mechanism for a substantial increase in the diversity that can evolve and be maintained in such models, an effect that increases with increasing dimension
of phenotype space. We note that this latter result is not obvious, as with higher phenotypic dimensions the number of phenotypically similar species (nearest neighbours in phenotype space)
increases linearly with the dimension, which may be expected to make desynchronization of neighboring boom-bust cycles more difficult due to denser phenotype packing. This mechanism of
“diversification in time” is similar to those previously reported10,29: time acts as additional niche space, and separation along this niche space can alleviate interspecific competition. In
the language of31, boom-bust desynchronization effectively increases the “environmental dimension”, which is a determinant of the amount of diversity that can be sustained. Again, this is
akin to the temporal storage effect7,8, although the latter is mostly invoked for externally generated population fluctuations. The longer the boom-bust cycles, the more temporal separation
between similar species is possible. If the population crashes in the boom-bust regime become too severe, they produce frequent extinctions, which eventually leads to a net negative effect
on diversity. In our models, higher diversity can only be observed in the presence of pronounced boom-bust cycles, but not with other types of population fluctuations, such as periodic or
chaotic dynamics with high-frequency oscillations. From a modeling perspective, it is worth noting that more standard and more widely used discrete-time models, such as the Ricker equation
or the discrete-time logistic model, even in their chaotic regimes cannot exhibit the type of chaotic boom-bust dynamics that model (2) exhibits for low intrinsic growth rates _λ_ and large
(overcompensating) _β_. This reiterates old cautionary notes about the judicious use of discrete maps for modeling ecological dynamics21. Discrete-time models have proved to be very useful
for many different purposes in ecology and evolution at least since Ricker’s s famous stock and recruitment paper24. However, we note that boom-bust dynamics can also be generated using
continuous-time models. To illustrate this, consider a continuous-time analogue of the modified model introduced at the end of the Results section. This continuous-time model has two phases,
representing slow exponential growth and fast exponential decline in continuous time. In the first phase, long and slow exponential growth occurs from low densities for each phenotypic
population, while keeping track of the effective density experienced by each phenotype, i.e., of the weighted sum overall phenotypic population sizes, with weights given by the competition
kernel (this corresponds to the sum in the denominator of Eq. (3)). Once the effective density of a given phenotype reaches the carrying capacity of that phenotype, there is a very fast
exponential decline until the phenotype reaches a small fraction of the population size it had before the decline, which corresponds to a severe population crash. Simulations of this simple
boom-bust model in continuous time (results not shown) produce qualitatively identical results: the amount of diversity that emerges and is maintained evolutionarily is much larger than the
diversity that would evolve with stable equilibrium dynamics (as e.g., reported using a continuous-time logistic model in17). This again underscores the generality of the effects of
boom-bust ecological dynamics on diversity. We speculate that the mechanisms and results reported here are not limited to competition models, but could also be manifest in communities with
other ecological interactions, e.g., in communities with crowding effects32, or in communities containing both predators and prey33. Whenever the population dynamics exhibit patterns of
rapid growth interspersed by crashes (as may e.g., be expected in many predator-prey systems), temporal desynchronization can occur spontaneously and thus lead to increased diversity. Such
effects were shown in33, who reported that “kill-the-winner” mechanism, in which predation generates crashed in the most abundant consumer species, can generate increased levels of
diversity. These mechanism differ from the ones reported here in that they are extrinsic to the crashing consumer species (and it is difficult to compare those system to baseline systems
with stable ecological dynamics). There is some empirical support for the effect of boom-bust cycles on ecosystem diversity. For example, such patterns were observed in carefully staged
long-running experiments with several plankton species6, and the experimental data showed that out-phase oscillations in predator-prey cycles of zooplankton and phytoplankton were important
for the maintenance of diversity in this system34. Predation from pathogens have also been reported to induce algal boom-bust cycles35. Generally, boom-bust cycles appear to be common in
many marine ecosystems, which are known to be very diverse. For example, it has been suggested to call echinoderms a “boom-bust” phylum36, and recent work shows that in polar plankton
communities, which constitute an important ecosystem in the global ocean, phytoplankton dynamics are often categorized by “boom-bust” cycles37. It is interesting to put our results in the
context of observations of “neutral evolution”. For example38, report neutral taxonomic distributions during early metazoan diversification into relatively empty niche space. In our models,
such expansions could be classified as the boom stage, and according to our model assumption would then indeed occur essentially unabated and in the absence of competitive effects. The
actual selection only occurs during the bust stage with populations of less adapted species crashing earlier and deeper. Such an application of the model (3) would be rather speculative
however, as the unrestricted exponential growth phase would have to last for a very long time and would in any case represent a simplified and unrealistic assumption for such scenarios. We
also note that38 consider neutrality based on taxonomic data, not on functional data, whereas our model only considers functional phenotypic data. It has been noted that the distinction
between taxonomic and functional data is very important in many microbial ecosystems39, and in particular that functional data can be decidedly non-random even when taxonomic distributions
look random. Overall, we think that our results provide a useful evolutionary perspective for thinking about diversity in natural ecosystems. Boom-bust population fluctuations are a robust,
intuitively appealing and probably under-appreciated potential cause of significantly increased diversity in evolving ecosystems. METHODS BASIC MODEL PROPERTIES It is well known that that
the basic quantity underlying the dynamic behavior of model (2) is the derivative _d__F_/_d__N_ evaluated at the equilibrium _K_: $${\left.\frac{dF}{dN}\right|}_{N = K}=1-\frac{\lambda
-1}{\lambda }\beta .$$ (5) For _λ_ > 1, the population dynamics converges to the steady state _K_ if and only if \(| 1-\frac{\lambda -1}{\lambda }\beta | <1\). Thus, for a given _λ_,
for small values of the exponent _β_ the system exhibits stable equilibrium dynamics, and increasing _β_ gives way to a period-doubling route to chaos (Fig. 1). Biologically, increasing _β_
can be viewed as reflecting a gradual change from contest to scramble competition23. This is reflected by the shape of the per capita number of offspring as a function of population size,
given by the right hand side of (2) divided by _N_(_t_), and viewed as a function of _N_(_t_): for any _λ_, and for high _β_, the per capita number of offspring is approximately constant
until the population size reaches the vicinity of _K_, but as _N_ increases above _K_ the number of offspring falls rapidly to very low values, essentially generating a population crash as
soon as the population size is above _K_. PROCEDURES FOR EVOLUTIONARY SIMULATIONS To simulate the evolutionary process, we set the scaling parameters _K_0 and _σ__K_ to _K_0 = 1 and _σ__K_ =
1, and we start with a number _S_ of phenotypes (typically, _S_ = 1, and the phenotype is randomly chosen in the vicinity of the maximum of the carrying capacity). We then simulate the
ecological dynamics in discrete time, using (3) for each of the phenotypes. In each generation, a new phenotype is generated with a probability _μ_ (typically _μ_ = 0.1). The new phenotype
is a mutant of one of the existing phenotypes. Of those, a parental phenotype is chosen with a probability proportional to its population size, and the offspring phenotype is chosen randomly
from a Gaussian distribution with the average centered at the parental phenotype and a small standard deviation Δ_x_ (typically Δ_x_ = 10−2). After addition of the new phenotype, the
community now comprises _S_ + 1 phenotypes, and the process is repeated for many generations. What one wants to know from this process is how the distribution of phenotypes changes over
time. To keep the number of phenotypes from increasing to very large numbers that would render the simulation computationally impossible, we periodically merge phenotypes that are very close
together. Specifically, once every _t__m__e__r__g__e_ generations (typically _t__m__e__r__g__e_ = 1000), phenotypes that are within a distance Δ_x_ of each other are merged (preserving
their phenotypic center of mass) and their population sizes added. In addition, every generation all clusters with populations densities below a threshold (typically = 10−12) are declared
extinct and removed from the system. Together, these procedures preserve the phenotypic variance necessary for evolution, but prevent undesirable computational complexity. To define and
count the number of phenotypically distinct species in the community at any given point in time, with each species possibly consisting of a number of similar phenotypes, the phenotypes in
the community are clustered with a larger distance Δ_x__s__p__e__c__i__e__s_ (typically Δ_x__s__p__e__c__i__e__s_ = 10−1). This phenotypic distance is still significantly smaller than the
typical scales of ecological interactions as long as _σ__α_ and _σ__K_ in (4) are of order 1. This implies that the phenotypes within a designated species experience very similar competitive
interactions and generally follow the same population dynamics. Note that species designation is only used to gather statistical data from simulations, but not in the actual computational
steps of the simulations. REPORTING SUMMARY Further information on research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY All data
used in this study was generated by computer simulations. The data that support the findings of this study are available from the authors upon reasonable request. The movie files referred to
in the context of Fig. 2 can be found at videos of various diversification scenarios corresponding to Fig. 2 can be found here: figshare.com/s/f2d8ecf480fa372319e1. CODE AVAILABILITY The
code for the computer simulations performed for this study was written in Fortran and can be found here:
https://github.com/jaros007/Fortran_code_Boom_bust_population_dynamics_increase_diversity_in_evolving_competitive_communities, and as described in ([Code depository] The code depository is
https://doi.org/10.5072/zenodo.747095.). REFERENCES * Rosindell, J., Hubbell, S. P., He, F., Harmon, L. J. & Etienne, R. S. The case for ecological neutral theory. _Trends Ecol. Evol._
27, 203–208 (2012). Article Google Scholar * Armstrong, R. A. & McGehee, R. Competitive exclusion. _Am. Nat._ 115, 151–170 (1980). Article Google Scholar * Hutchinson, G. E. The
paradox of the plankton. _Am. Nat._ 95, 137–145 (1961). Article Google Scholar * Koch, A. L. Coexistence resulting from an alternation of density dependent and density independent growth.
_J. Theor. Biol._ 44, 373–386 (1974). Article CAS Google Scholar * Koch, A. L. Competitive coexistence of two predators utilizing the same prey under constant environmental conditions.
_J. Theor. Biol._ 44, 387–395 (1974). Article CAS Google Scholar * Benincà, E. et al. Chaos in a long-term experiment with a plankton community. _Nature_ 451, 822–825 (2008). Article
Google Scholar * Chesson, P. Multispecies competition in variable environments. _Theor. Popul. Biol._ 45, 227–276 (1994). Article Google Scholar * Chesson, P. Mechanisms of maintenance of
species diversity. _Annu. Rev. Ecol. Syst._ 31, 343–366 (2000). Article Google Scholar * Huisman, J. & Weissing, F. J. Biodiversity of plankton by species oscillations and chaos.
_Nature_ 402, 407–410 (1999). Article Google Scholar * Barabás, G., Meszéna, G. & Ostling, A. Community robustness and limiting similarity in periodic environments. _Theor. Ecol._ 5,
265–282 (2012). Article Google Scholar * Kremer, C. T. & Klausmeier, C. A. Species packing in eco-evolutionary models of seasonally fluctuating environments. _Ecol. Lett._ 20,
1158–1168 (2017). Article Google Scholar * Roughgarden, J. _Theory of Population Genetics and Evolutionary Ecology: An Introduction_. (MacMillan, 1979). Google Scholar * Doebeli, M.
_Adaptive diversification (MPB-48)_. vol. 48 (Princeton University Press, 2011). * Doebeli, M. & Ispolatov, I. Complexity and diversity. _Science_ 328, 494–497 (2010). Article CAS
Google Scholar * Doebeli, M. & Ispolatov, I. Chaos and unpredictability in evolution. _Evolution_ 68, 1365–1373 (2014). Article Google Scholar * Ispolatov, I., Madhok, V. &
Doebeli, M. Individual-based models for adaptive diversification in high-dimensional phenotype spaces. _J. Theor. Biol._ 390, 97–105 (2016). Article Google Scholar * Doebeli, M. &
Ispolatov, I. Diversity and coevolutionary dynamics in high-dimensional phenotype spaces. _Am. Nat._ 189, 105–120 (2017). Article Google Scholar * Ispolatov, I., Alekseeva, E. &
Doebeli, M. Competition-driven evolution of organismal complexity. _PLoS Comput. Biol._ 15, e1007388 (2019). Article CAS Google Scholar * MaynardSmith, J. & Slatkin, M. The stability
of predator-prey systems. _Ecology_ 54, 384–391 (1973). Article Google Scholar * Bellows, T. The descriptive properties of some models for density dependence. _J. Animal Ecol._ 50, 139–156
(1981). Article Google Scholar * Doebeli, M. Dispersal and dynamics. _Theor. Popul. Biol._ 47, 82–106 (1995). Article Google Scholar * Yodzis, P. _Introduction to Theoretical Ecology_.
(Harper and Row, 1989). Google Scholar * Brännström, A. & Sumpter, D. J. The role of competition and clustering in population dynamics. _Proceed. Royal Soc. London B_ 272, 2065–2072
(2005). Google Scholar * Ricker, W. Stock and recruitment. _J. Fisheries Board oCanada_ 11, 559–623 (1954). Article Google Scholar * Gyllenberg, M. & Meszéna, G. On the impossibility
of coexistence of infinitely many strategies. _J. Math. Biol._ 50, 133–160 (2005). Article Google Scholar * Dieckmann, U. & Doebeli, M. On the origin of species by sympatric
speciation. _Nature_ 400, 354–357 (1999). Article CAS Google Scholar * Hernández-García, E., López, C., Pigolotti, S. & Andersen, K. H. Species competition: coexistence, exclusion and
clustering. _Phil. Trans. Roy. Soc. A_ 367, 3183–3195 (2009). Article Google Scholar * Scheffer, M. & van Nes, E. H. Self-organized similarity, the evolutionary emergence of groups of
similar species. _Proc. Nat. Acad. Sci. USA_ 103, 6230–6235 (2006). Article CAS Google Scholar * Vandermeer, J. Oscillating populations and biodiversity maintenance. _Bioscience_ 56,
967–975 (2006). Article Google Scholar * Shoresh, N., Hegreness, M. & Kishony, R. Evolution exacerbates the paradox of the plankton. _Proceed. Natl Acad. Sci. USA_ 105, 12365–12369
(2008). Article CAS Google Scholar * Parvinen, K., Metz, J. A. & Dieckmann, U. Environmental dimensionality determines species coexistence. _J. Theor. Biol._
https://doi.org/10.1016/j.jtbi.2020.110280 (2020). * Gavina, M. K. et al. Multi-species coexistence in Lotka-Volterra competitive systems with crowding effects. _Scientific Rep._ 8, 1–8
(2018). CAS Google Scholar * Xue, C. & Goldenfeld, N. Coevolution maintains diversity in the Stochastic “Kill the Winner” Model. _Phys. Rev. Lett._ 119, 268101 (2017). Article Google
Scholar * Benincà, E., Jöhnk, K. D., Heerkloss, R. & Huisman, J. Coupled predator-prey oscillations in a chaotic food web. _Ecol. Lett._ 12, 1367–1378 (2009). Article Google Scholar *
Lehahn, Y. et al. Decoupling physical from biological processes to assess the impact of viruses on a mesoscale algal bloom. _Curr. Biol._ 24, 2041–2046 (2014). Article CAS Google Scholar
* Uthicke, S., Schaffelke, B. & Byrne, M. A boom-bust phylum? Ecological and evolutionary consequences of density variations in echinoderms. _Ecol. Monogr._ 79, 3–24 (2009). Article
Google Scholar * Behrenfeld, M. J. et al. Annual boom-bust cycles of polar phytoplankton biomass revealed by space-based lidar. _Nat. Geosci._ 10, 118–122 (2017). Article CAS Google
Scholar * Mitchell, E. G. et al. The importance of neutral over niche processes in structuring Ediacaran early animal communities. _Ecol. Lett._ 22, 2028–2038 (2019). Article Google
Scholar * Louca, S., Parfrey, L. W. & Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. _Science_ 353, 1272–1277 (2016). Article CAS Google Scholar
Download references ACKNOWLEDGEMENTS M.D. was supported by NSERC Discovery Grant 219930. E.C.J. was supported funded by the National Agency for Research and Development (ANID) Scholarship
21190785. IY.I. acknowledges support from FONDECYT project 1200708. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Zoology and Department of Mathematics, University of British
Columbia, 6270 University Boulevard, Vancouver, BC, Canada Michael Doebeli * Physics Department, University of Santiago of Chile (USACH), Santiago, Chile Eduardo Cancino Jaque & Yaroslav
Ispolatov Authors * Michael Doebeli View author publications You can also search for this author inPubMed Google Scholar * Eduardo Cancino Jaque View author publications You can also search
for this author inPubMed Google Scholar * Yaroslav Ispolatov View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.D. provided the basic idea,
performed simulations, and wrote the paper. E.C.J. performed simulations and data analysis. IY.I. made conceptual contributions, performed simulations and analysis, and wrote the paper.
CORRESPONDING AUTHOR Correspondence to Michael Doebeli. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION PEER REVIEW FILE SUPPLEMENTARY INFORMATION REPORTING
SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes
were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Doebeli, M., Jaque, E.C. & Ispolatov, Y. Boom-bust population dynamics increase diversity in evolving competitive communities. _Commun Biol_ 4, 502 (2021).
https://doi.org/10.1038/s42003-021-02021-4 Download citation * Received: 24 August 2020 * Accepted: 24 March 2021 * Published: 23 April 2021 * DOI: https://doi.org/10.1038/s42003-021-02021-4
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy
to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
