A Maastrichtian insect assemblage from Patagonia sheds light on arthropod diversity previous to the K/Pg event
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Download PDF Article Open access Published: 11 December 2023 A Maastrichtian insect assemblage from Patagonia sheds light on arthropod diversity previous to the K/Pg event Ezequiel I. Vera1,
Mateo D. Monferran2, Julieta Massaferro ORCID: orcid.org/0000-0001-9370-00623, Lara M. Sabater2, Oscar F. Gallego2, Valeria S. Perez Loinaze1, Damián Moyano-Paz4, Federico L. Agnolín5,6,
Makoto Manabe ORCID: orcid.org/0000-0003-2490-61257, Takanobu Tsuhiji8 & …Fernando E. Novas ORCID: orcid.org/0000-0002-6901-86775 Show authors Communications Biology volume 6, Article
number: 1249 (2023) Cite this article
3224 Accesses
5 Citations
46 Altmetric
Metrics details
Subjects EntomologyPalaeontology AbstractInsect faunas from the latest Cretaceous are poorly known worldwide. Particularly, in the Southern Hemisphere, there is a gap regarding insect assemblages in the Campanian-Maastrichtian
interval. Here we present an insect assemblage from the Maastrichtian Chorrillo Formation, southern Argentina, represented by well-preserved and non-deformed, chitinous microscopic remains
including head capsules, wings and scales. Identified clades include Chironomidae dipterans, Coelolepida lepidopterans, and Ephemeroptera. The assemblage taxonomically resembles those of
Cenozoic age, rather than other Mesozoic assemblages, in being composed by diverse chironomids and lepidopterans. To the best of our knowledge, present discovery constitutes the first insect
body fossils for the Maastrichtian in the Southern Hemisphere, thus filling the gap between well-known Early Cretaceous entomofaunas and those of Paleogene age. The presented evidence shows
that modern clades of chironomids were already dominant and diversified by the end of the Cretaceous, in concert with the parallel radiation of aquatic angiosperms which became dominant in
freshwater habitats. This exceptional finding encourages the active search of microscopic remains of fossil arthropods in other geological units, which could provide a unique way of
enhancing our knowledge on the past diversity of the clade.
Similar content being viewed by others The contribution of the Middle Triassic fossil assemblage of Monte San Giorgio toinsect evolution Article Open access 20 August 2024 A twig-like insect stuck in the Permian mud indicates early origin of an ecological strategy in Hexapoda evolution Article Open access 21
October 2021 Unlocking the mystery of the mid-Cretaceous Mysteriomorphidae (Coleoptera: Elateroidea) and modalities in transiting from gymnosperms to angiosperms Article Open access 08
October 2020 Introduction
The fossil record of insects known for the Late Cretaceous is scarcely known across the globe and, in particular, it is almost inexistent in the Campanian-Maastrichtian interval1,2,3.
Information on Campanian-Maastrichtian insect diversity in South America consists on nests, pupal chambers, cocoons, and feeding activities on leaves and wood, recovered in central and
northern Patagonia4,5,6,7,8,9. These occurrences suggest a complex community of terrestrial insects represented by different types of herbivores, pollen feeders, and predators/parasites of
scavengers (wasps). However, body insect remains are still wanting. Further, there are no fossils belonging to aquatic insects from this time interval in South America (except for a mention,
without descriptions or illustrations, of nepomorph waterbugs in the La Colonia Formation10), which are otherwise of very rare occurrence in the global record11.
Here we present an insect assemblage from the lower Maastrichtian Chorrillo Formation, cropping out in Santa Cruz province, southern Argentina. Previous uppermost Cretaceous report from
insects in the region consisted of coleopteran elytra imprints presumably from the lowermost levels of the Dorotea Formation in southern Chile12,13. The Chorrillo Formation has yielded a
rich and variegated fossil content14 including titanosaurids, hadrosaurids, ankylosaurids15, non-avian theropods16, enantiornithine birds17,18, gondwanatherian and monotreme mammals19,20,21,
turtle, frogs, and fishes14,17, terrestrial mollusks14, and conifer woods, pollen and spores of different plant groups14,17,22,23. The entomofauna currently recognized is composed by
members of Chironomidae, Ephemeroptera, and Lepidoptera. Insect diversity may be greater, as suggested by the presence of abundant chitinous remains belonging to taxonomically unidentified
arthropods. To the best of our knowledge, these constitute the first insect assemblage from the Maastrichtian of southern South America, offering information about the diversity of water
dwelling insects previous to the Cretaceous–Paleocene massive extinction event.
Recovered arthropod remains consist of well-preserved cephalic capsules of aquatic larvae of chironomids, isolated scales and fragments of larval exuviae of lepidopterans, and an
ephemeropteran larval head, as well as diverse fragmentary remains of unknown arthropod affinities. They are within the size range of palynomorphs (c. 10–250 µm) and were identified in
palynological slides. The discovery of these chitinous remains was fortuitous, after preparing fragments of rocks with the usual techniques employed for palynology (see Methods). It is worth
noting that, for historical reasons, palynologists working on pre-Quaternary material infrequently focus on the study of arthropod remains associated with palynomorphs24, and they are
rarely included in the interpretation of the data25,26,27,28. The not-deformed, chitinous remains from both aquatic and terrestrial insects documented in palynological samples from the
Chorrillo beds, constitute an exceptional preservation for Upper Cretaceous fossils, resembling those frequently found in Quaternary samples29.
ResultsArthropod materials are represented by approximately 30 specimens, and include representatives of the Chironomidae, Ephemeroptera, and Lepidoptera, as well as remains of unknown
affinities.
Systematic PaleontologyPhylum Arthropoda Gravenhorst, 1843
Subphylum Hexapoda Latreille, 1825
Class Insecta Linnaeus, 1758
Order Diptera Linnaeus, 1758
Suborder Nematocera Latreille, 1825
Family Chironomidae Macquart, 1838
Subfamily Orthocladiinae Lenz, 1921 Morphotype 1Referred specimens. MPM-Pal 21835-24: M31/0, MPM-Pal 21835-3: P46/4.
Description. The specimen 1 (MPM-Pal 21835-24: M31/0, Fig. 1a) corresponds to a head capsule (135 µm long and 77 µm high) showing a (partial) mentum (at least 43 µm long and 9 µm high) with
5 lateral teeth and one mandible (41 µm long and 25 µm wide at its base) showing an apical short tooth and 3–4 inner teeth similar in shape.
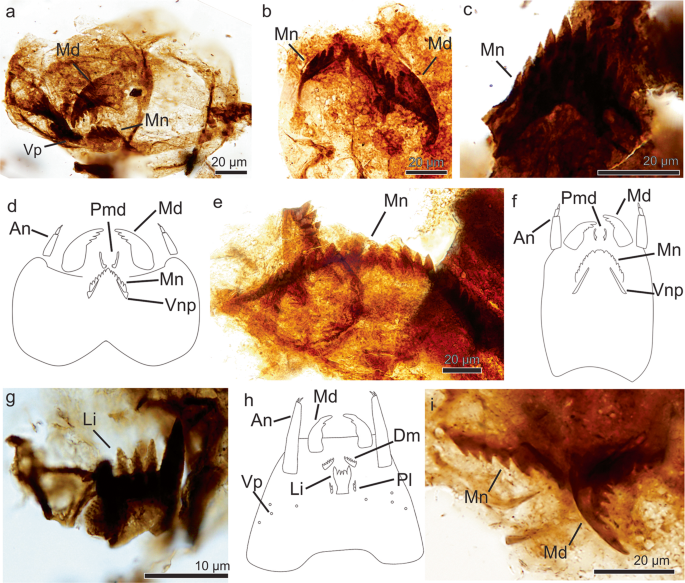
Fig. 1: Arthropod fossil remains from theChorrillo Formation referred to Chironomidae.
a–d Orthocladiinae. a, b, Morphotype 1; a MPM-Pal 21835-24: M31/0; b MPM-Pal 21835-3: P46/4; c Morphotype 2, MPM-Pal 21835-25: V26/3; d Schematic representation of an Orthocladiinae head
capsule; e, f Diamesinae, e, Morphotype 3, MPM-Pal 21835-19: V34/4; f, Schematic representation of a Diamesinae head capsule; g, h Tanypodinae, g Morphotype 4, MPM-Pal 21835-2:Y40/3; h
Schematic representation of a Tanypodinae head capsule; Morphotype 5, MPM-Pal 21835-9: Q58/3. An antennae, Li ligulae, Md mandible, Mnt mentum, Plg paraligulae, Prm premandible, Vnp
Ventromental plate, Vp ventromental pores. d, f, and h based on personal photo library of J. Massaferro.
Full size imageSpecimen 2 (MPM-Pal 21835-3: P46/4, Fig. 1b) preserves a complete mentum (47 µm long and 10 µm high) with double median teeth and 5 pairs of lateral teeth, and a single mandible with an
apical short tooth and 4 inner teeth. Both morphotypes exhibit sclerotized narrow ventromental plates, extending through the outermost lateral tooth.
The shape and position of the ventromental plates support these morphotypes unequivocally belong to the Subfamily Orthocladiinae (Fig. 1d). The double median teeth and the mandible resemble
the living genera Parapsectrocladius Cranston and a Botryocladius Cranston of the Orthocladiinae30,31, but preservation of the available remains are not enough for their correct
identification.
Morphotype 2Referred specimens. MPM-Pal 21835-25: V26/3.
Description. The specimen (Fig. 1c) consists of a mentum (38 µm wide and 22 µm high) detached from the head capsule. Double median teeth and 5 pairs of lateral teeth, median teeth slightly
taller and wider than 1st lateral; 2nd lateral tooth only slightly smaller than 1st lateral tooth. All teeth are pointed, taller than wide. The shape of the mentum in joint with the
inconspicuous ventromental plate, are features typical of an orthoclad (Fig. 1d); however, the mentum lacks similarities with any known living chironomid, thus it is interpreted as
Orthocladiinae incertae sedis.
Subfamily Diamesinae Pagast, 1947Tribe Heptagyini Brundin, 1966
Morphotype 3Referred specimens. MPM-Pal 21835-9:E34/4, MPM-Pal 21835-19: V34/4.
Description. This morphotype (Fig. 1e) consists of isolated menti (136 µm wide and 14 µm high) showing double median tooth and 7–11 lateral teeth. The arrangement of mental teeth, however,
gives an appearance of 3–6 median teeth. The presence of at least nine lateral teeth allows its placement in the Diamesinae, a clade of chironomids often bearing more than seven lateral
teeth on the mentum (Fig. 1f). The morphotype is similar to the living genus Paraheptagyia Brundin, although in the later one there is not differentiation of central teeth, suggesting it may
represent an extinct taxon.
Subfamily Tanypodinae sensu Cranston et al., 1983 Morphotype 4Referred specimens. MPM-Pal 21835-2:Y40/3
Description. The specimen (Fig. 1g) consists of a ligula (12 µm wide and 15 µm high) showing five teeth, the two outer teeth dark brown and the three central pale brown teeth. The ligula is
a common character of all the genera within the Subfamily Tanypodinae (Fig. 1h).
Subfamily indeterminate Morphotype 5Referred specimens. MPM-Pal 21835-9: Q58/3.
Description. The specimen (Fig. 1i) shows two overlapped menti and a mandible in the same slide. The left side of the mentum is approximately 31 µm long and 7 µm high, and is similar to
morphotypes 1 and 2 of Subfam. Orthocladiinae, whereas the right mentum (18 µm long and 7 µm high) looks similar to morphotype 4 (Diamesinae). In addition, the mandible (26 µm long and 11 µm
wide at its base) does not seem to correspond to any of the aforementioned morphotypes, but it resembles the mandible of Cricotopus spp32.
Order Ephemeroptera Hyatt and Arms, 1890Ephemeroptera indet
Referred specimens. MPM-Pal 21835-9:W37/1
Description. The specimen (Fig. 2a) corresponds to a small (340 µm long and 260 µm high) but heavily sclerotized head. A pair of small subcircular compound eyes is located near the
posterolateral corners of the head, bearing seven rows of lenses (ommatidia). The eyes are anteriorly delimited by prominent ocelar tubercles. The head surface is moderately densely covered
with hairlike setae. Patches of short, hairlike setae are present below and laterally to the compound eyes. The mouthparts are incomplete and not well preserved, the labrum and pharynx are
obscured by other structures. The head is prognathous and shows the presence of a region with strong sclerotization and acuminate ends that suggest that a pair of mandibles were
well-differentiated. There is no evidence of mandibular tusk.
Fig. 2: Arthropod fossil remains from the Chorrillo Formation referred to Ephemeroptera.a Ephemeroptera head MPM-Pal 21835-9:W37/1; b lineal drawing of a living caenid head for comparisons (based on Caenidae nymph.jpg by Dmccabe, 2017 CC BY SCA 4.0 - REF 85). Md mandible, Mx
maxilla, Lb labrum, Lp labial palpus.
Full size imageRemarks. The presence of sclerotized mandibles which are not expanded and lack prominent teeth, and transversely expanded head with eyes located at the posterolateral corners, indicate that
it corresponds to a Ephemeroptera naiad33,34,35 (Fig. 2b). Further, differs from the closely related odonates in that the latter lack sclerotized mandibles36. Presence of ocelar tubercle
delimiting the anterior margin of the eye and located at the corner of the head occurs in the extant families Caenidae and Ephemerellidae35.
The fossil record shows that mayflies were abundant and extremely numerous regionally during the Cretaceous, being reported from Asia, North America, and the early Cretaceous of Africa and
Australia36,37,38,39,40,41,42. In South America they are restricted to the Aptian–Albian Crato Formation of Brazil, where more than 17 species have been described42, and a single finding in
the Lower-Upper Cretaceous of Colombia43.
Order Lepidoptera Linnaeus, 1758 Lepidoptera indetReferred specimens. MPM-Pal 21835-10:X30/2, MPM-Pal 21835-7:S27/2
Description. Two chitinous remains are tentatively identified as belonging to lepidopteran larval exuvial remains. One of these is at least 227 µm.long and 74 µm wide (Fig. 3a), and shows at
least four pairs of 23–30 µm long thoracic legs. The other one (Fig. 3c) probably corresponds to the last sommite of a larval remain, at least 253 µm long bearing the anal proleg with
semicircular shaped uniordinal crochets, 31.5 µm.in diameter.
Fig. 3: Arthropod fossil remains from the Chorrillo Formation referred to Lepidoptera.a Larval exuvial fragment showing thoracic legs, MPM-Pal 21835-7:S27/2; b Probable last sommite larval exuvial fragment showing crochet-bearing prolegs, MPM-Pal 21835-10:X30/2; c Lineal
drawing of an extant lepidopteran larva for comparison (based on Lukas3, public domain. Gasienica_motyla_budowa.svg; REF 86), and on Moreira et al., 2019 (Fig 33 CC by-SA 4.0; REF 87). AbP
abdominal prolegs, Thl thoracic legs, Cr crochets.
Full size imageRemarks. Presence of thoracic legs and prolegs, the later ones exhibiting uniordinal crochets, could indicate that the studied elements are probably remains of a lepidopteran larval cuticle.
The presence of a single series of more or less uniform (uniordinal) crochets observed in the specimens, is the plesiomorphic condition for lepidopterans44 (Fig. 3b). However, there are
several insect groups that have abdominal appendices known as prolegs45,46, and there is a considerable diversity/variation in their number, position, and shape47, and thus, the specimens
here reported are identified as Lepidoptera indet.
Suborder Glossata Fabricius, 1775Clade Coelolepida Nielsen and Kristensen, 1996
Coelolepida indetReferred specimens. MPM-Pal 21835-3:W34/1, MPM-Pal 21835-23:W55/0, MPM-Pal 21835-22:R52/2,MPM-Pal 21835-20:Z47/3. MPM-Pal 21835-22: G55/0; MPM-Pal 2183-21: E42/3
Description. At least four morphological types of scales and scale fragments were recovered. They mainly differ in size, general shape, apical margin shape, and presence of perforations
(Fig. 4). All the specimens bear longitudinal ridges, cross ridges, and small inter-ridge perforations. In addition, they exhibit a clear pedicel below their basal margin, and the
longitudinal ridges seemingly do not extend beyond the apical margin. Broken scales indicate that they are hollow.
Fig. 4: Arthropod fossil remains from the Chorrillo Formation referredto Lepidoptera.
a Type 1 scale, MPM-Pal 21835-3:W34/1; b Detail of Type 1 scale showing small inter-ridge perforations; c Type 2 scale, MPM-Pal 21835-23:W55/0; d Type 3 scale, MPM-Pal 21835-22:R52/2; e Type
4 scale, MPM-Pal 21835-20:Z47/3. Ma serrated apical margin, LR longitudinal ridges, P pedicle.
Full size imageType 1 scale (specimen MPM-Pal 21835-3:W34/1; Fig. 4a, b) is 125 µm long and 42 µm wide, and shows longitudinal ridges (Fig. 4b), serrated apical margin, and rounded basal margin.
Type 2 (specimen MPM-Pal 21835-23:W55/0; Fig. 4c) is a fragmentary scale 147 µm long and 26 µm wide, similar to Type 1, but with a serrated apical margin having finger-like projections.
Type 3 scale (specimen MPM-Pal 21835-22:R52/2; Fig. 4d) is 178 µm long and approximately 48 µm wide, with a rounded apical margin, and a large pedicle.
Type 4 (specimens MPM-Pal 21835-20:Z47/3; MPM-Pal 21835-22: G55/0; MPM-Pal 2183-21: E42/3; Fig. 4e) is long and dark in a central zone, reaching 174 µm long and 30 µm wide, and bears a
slightly serrated apical margin.
Remarks. The scales described above are similar to the ones present in the lepidopteran clade Coelolepida48 due to the presence of serrated apical margins, dense cross-ridges, hollow
structure, and perforations in the inter-ridge areas of the upper lamina, characteristic of the vast majority of extant Lepidoptera27,48. Rhaetian–Hettangian lepidopterans and non-glossatan
moths differ in having rounded scales, with a solid inner structure47. Trichopterans were recently described for Cretaceous deposits49, but they exhibit scales lacking serrated apical
margins, and cross-ridges47, thus differing from lepidopteran scales recorded in the Chorrillo Formation.
In spite that the earliest lepidopteran body fossil dates back to the Early Jurassic of England50, lepidopteran wing scales were recorded from Triassic–Jurassic boundary sediments from
northern Germany47,51.
Lepidoptera are scarce in fossil records from South America, with representatives of the microlepidopteran grade occurring in the Crato Formation52,53,54.
Indeterminate arthropod remainsDescription. The Chorrillo insect assemblage also includes small arthropod remains of uncertain systematic position (Figs. 5 and 6). They hold irregularly distributed pores, setae, compound
eyes, and spines. The recorded arthropod cuticles have different shapes and sizes and, in most cases, they range from 100 to 150 μm wide. Among them, there are fragmentary remains of
compound eyes (Fig. 5d). However, none of these cuticles seem to be associated with any other diagnostic parts of other arthropods identified in the Chorrillo assemblage.
Fig. 5: Fossilremains of indeterminate arthropods from the Chorrillo Formation.
a Terminal process with six spine-like projections, MPM-Pal 21835-7:G42/4; b Cuticule fragment with small process, MPM-Pal 21835-23:H55/0; c Cuticle fragment with well-conspicuous setae at
the margins, MPM-Pal 21835-20:U27/0; d Fragment of compound eye, MPM-Pal 21835-4.
Full size imageFig. 6: Fossil remains of indeterminate arthropods from the Chorrillo Formation.a Folded cuticle fragment, MPM-Pal 21835-22:W55/2; b Cuticle fragment of arthropod affinity, MPM-Pal 21835-7:K21/2; c Cuticle fragment of possible arthropod exuvial origin, MPM-Pal
21835-23:L36/0; d Stiff conical structure, MPM-Pal 21835-4:R46/0; e Oval shape cuticle with wrinkles, MPM-Pal 21835-23:W46/0.
Full size image DiscussionCurrently known insect record of the Chorrillo Formation includes taxa representative of the Chironomidae, Ephemeroptera, and Lepidoptera, as well as arthropod remains of unknown affinities.
The presence of larval (Chironomidae) and naiad (Ephemeroptera) remains unambiguously indicates a freshwater environment for the Megafloral level 1, in agreement with the paleobotanical
content and sedimentological features17,22. The larval instars of living chironomids inhabit an enormous variety of aquatic and semi-terrestrial environments, from moist soils to pools in
tree holes, and from low-oxygen lake sediments to fast-flowing mountain streams55. The presence of Orthocladiinae and Diamesinae in the Chorrillo Formation suggest that Megafloral level 1
accumulated under low productive and vegetated, shallow waters. Even though, Diamesinae is usually a typical inhabitant of running waters, its presence in a lacustrine environment is not
surprising as it could have been transported and later deposited in the lacustrine environment by erosion and/or precipitation runoff. Indeed, these insects were found together with aquatic
plant remains in the Megafloral level 1 which was described as a stratum of swampy, low-energy environment located in the distal portion of low gradient meandering river floodplain (see
Geological and paleoenvironmental contexts).
Morphotypes of both subfamilies are characterized by having mixed functional feeding strategies, including detritivores, herbivores, and predators. Chironomid larvae represent one of the
main proteinaceous bases of freshwater food chains of post-Mesozoic ecosystems, mainly for fish and other aquatic predators56. The Chorrillo Formation assemblage is taxonomically congruent
with those of Cenozoic ecosystems because chironomids are numerically dominant57. In contrast, less than 10% of the specimens collected in Cretaceous amber inclusions correspond to
Chironomidae36,58. While chironomids (in particular larval stages) are typically rare or absent in amber deposits, as chances of being caught in the resinous secretions of the trees are
scarcer in the aquatic environment, its absence in Cretaceous lithic sediments is also noteworthy and, as far as we are aware, only a partial wing of a possible chironomid has been so far
reported from the Early Cretaceous of Brazil59.
Although available chironomid specimens collected in the Chorrillo Formation consist of fragmentary mouthparts, their morphological features show that they can be comfortably referred to
extant tribes. This supports previous interpretations60,61,62 that Chironomidae were morphologically stable since the Early Cretaceous, and that diversification of most of their tribes
occurred during the Cretaceous, mainly between 118 through 66 Mya63. Moreover, the evidence afforded here dismisses the hypothesis57,64,65,66 interpreting that chironomid subclades did not
survive the K/Pg boundary.
Some authors62,64 concluded that primitive chironomids (e.g., Buchonomyiinae, Aenneinae) were replaced during the Cenozoic by assemblages dominated by modern chironomid clades such as
Orthocladiinae and Chironominae. This ecological shift has been correlated with the emergence of angiosperm-dominated deciduous forests, which led to the enhanced transport of allochthonous
organic matter into the water bodies. Evidence yielded by the Chorrillo Formation shows that modern clades of chironomids were already abundant and diversified by the end of the Cretaceous,
in concert with the radiation, for the same period, of aquatic angiosperms which became dominant in freshwater habitats10.
The presence of Lepidoptera in the assemblage is congruent with the evolutionary renewal described above for chironomids, although in this case the four different morphotypes of wing scales
may not be a direct indicator of taxonomic richness, as variable morphology is recognized in the vestiture of lepidopterans67. In spite of this variation, the distinctions among
morphological types observed in scales described above, are higher than that known in extant lepidopterans67. As for example, Type 2 scale lacks pedicle, bears a low number of longitudinal
ridges, and its margin is decorated with digitiform serrations, being sharply different from Type 4, which has pedicle, the number of ridges is notably higher, a dark central area is
present, and the distal margin is weakly serrated. These strong morphological differences are not found in the vestiture of a single extant lepidopteran species, making the referral of all
scale types to a single biological species unlikely. In any case, the record of lepidopteran scales in this freshwater environment, along with exuvial remains also allied with the
Lepidoptera, may indicate the presence of aquatic or semi-aquatic lepidopterans, conforming a small, taxonomically diverse, and scarcely known group68. This may also be indicative that
lepidopterans may have acquired an outstanding role as pollinators and probably nectarivores by the latest Cretaceous. Such condition is reminiscent to Cenozoic insect associations, in which
lepidopterans constitute one of the most frequent and diversified clades of phytophagous insects36,69,70, contrasting with the Early Cretaceous times, when lepidopterans were numerically
scarce52,53,54,59.
The chironomid assemblage of the Chorrillo Formation resembles those of the Andean-Patagonian biogeographic region by the absence of Chironominae and the abundance and diversity of
Orthocladiinae71, and differs from those reported for Tropical lowlands or Neotropical Region58. These austral taxa generally inhabit cool, pristine environments, often upland to montane
streams61. Noteworthy, some floristic elements recovered from the Chorrillo Formation, as well as paleosoil analysis, contrarily indicate temperate to warm conditions23,72.
Chironomids recorded in the Chorrillo beds are anatomically close to some Australasian taxa, such as the Heptagyini diamesine Paraheptagya and the Orthocladiinae Parapsectrocladius,
Botryocladius, and Limnophyes, currently present in the Andean and Australasian regions30,31,61,73,74. The strong tie between chironomids from Chorrillo beds and those of the Australasian
Region is congruent with the evidence afforded by different fossil vertebrates: in the Chorrillo beds megaraptorid theropods are frequent, alongside with abundant elasmarian
ornithischians14,15,75, an assemblage resembling Cretaceous faunas of Australia. Recently, the monotreme Patagorhynchus pascuali was reported from the Chorrillo Formation, which exhibits
close affinities with the Cenozoic ornithorhynchid Obdurodon from Australia21, thus lending support to Transantarctic relationships with Australasia during Late Cretaceous times.
Summing up, the discovery of the Chorrillo Formation insect assemblage partially fills the gap between well-known Early Cretaceous entomofauna and those of Paleogene age. To our knowledge,
only remains of insects of terrestrial and herbivorous habits were known from the Maastrichtian of Patagonia, lacking evidences of aquatic forms. The Chorrillo Formation insect assemblage
resembles Cenozoic, rather than Mesozoic, insect assemblages in the abundance and diversity of chironomids, particularly of the Orthocladiinae/Diamesinae lineage, and in diversity of
lepidopterans. This suggests that modern insect assemblages were already established in southern Patagonia for end of the Cretaceous Period.
MethodsThe fossil specimens reported in this publication were obtained from the upper Maastrichtian Chorrillo Formation after a field trip carried out near El Calafate city, Santa Cruz Province,
Argentina, during March 2020. Exploration and fossil collecting was authorized by Secretaría de Estado de Cultura de la Provincia de Santa Cruz, Argentina. Studied specimens are deposited in
the Museo Regional Padre Jesús Molina (Río Gallegos, Santa Cruz Province), under MPM-Pal 21835 (Palynological slides) acronyms.
Geological and paleoenvironmental contextsThe Chorrillo Formation is a syn-orogenic stratigraphic unit that accumulated during the early Maastrichtian in the Austral-Magallanes Basin during a continental phase of the basin17,76. The
unit crops out on the south of the Lago Argentino, southwest of the Santa Cruz province, Argentina (Fig. 7A) and it overlies the coarse-grained braided fluvial deposits of the La Irene
Formation77,78 and is covered by the shallow marine deposits of the Calafate Formation79 (Fig. 7B). The Chorrillo Formation is a~500 m thick sequence (Figs. 7C and 8) interpreted as
accumulated in a low gradient fluvial environment dominated by thick fine-grained floodplain deposits which alternate with channel-shaped and lobe-shaped sandstone bodies interpreted and
fluvial channels and crevasse splay facies17. The fine-grained deposits constitute more than 60% of the unit and show evidence of both soil development and waterlogging17,22,72. The
Chorrillo Formation was initially named as ‘dinosaur-bearing strata’ because of the abundance of fossil vertebrate remains that were found on these deposits14,15,16,19,20,21,80,81. However,
studies carried out in the last years indicate that the Chorrillo Formation was not only rich in vertebrates but also in invertebrates, plants, and palynomorphs14,17,22,23.
Fig. 7:Location map indicating provenance of arthropod fossil remains.
A Location map of the Austral-Magallanes basin. B Stratigraphy of the sedimentary infill of the basin related with the three main tectonic stages. C Outcrop photopanel of the Chorrillo
Formation at the study area showing the path of the measured sedimentary section and location of the arthropod-bearing bed.
Full size imageFig. 8: Estratigraphic column indicatingprovenance of arthropod fossil remains.
Detail of the sedimentary measured section of the Chorrillo Formation showing the main lithological and sedimentological aspects of the unit, the fossil content, and the detail of the
arthropod-bearing bed (Megafloral level 1).
Full size imageThe arthropod specimens were obtained from a stratum located 215 m from the base of the Chorrillo Formation, which was named as Megafloral level 122, cropping out in uppermost part of the
gullies of the La Anita farm (50°30'49.3“S 72°33’ 35.9“W) (Fig. 7A). The insect-bearing strata is ~2.5 m thick and consists of a dark gray to black colored, organic-rich, mudstone bed with
diffuse parallel lamination17 (Fig. 8). This bed accumulated in a waterlogged, low energy, environment as a swamp/pond, located in the distal areas of the low-gradient floodplain of the
fluvial system, as the product of fluvial overbank processes17,22. This swamp/pond deposits are vertically related to Histosol-like paleosols72. The high content of organic matter in this
bed suggests that vegetation grew in proximity to the swamp/pond favoring the development of the Histosol-like paleosol. The poorly decomposed state of the organic matter indicates anoxic
and reducing conditions in the bottom water of the swamp/pond72,82,83, which may also favor the exceptional preservation of non-mineralized organisms such as the arthropods here studied.
Detailed paleobotanical studies22 from the same levels that contain the paleoentomofauna here presented have been recently published, allowing to characterize the floristic content
accompanying the arthropods. Terrestrial elements of the assemblage include an array of spore producing taxa (mosses, lycopsids, and ferns), pollen grains referable to conifers of the
families Araucariaceae, Podocarpaceae and Hirmeriellaceae ( = Cheirolepidiaceae), and dicot and monocot angiosperms22. While these floristic elements can have some degree of allochtony, the
assemblage is also composed of aquatic plants. Recognized aquatic elements (e.g., Nymphaeaceae, Marsileaceae, Salviniaceae, freshwater algae) indicate the presence of a freshwater community
that inhabited a low energy, paludal or marginal, mesotrophic environment. These taxa can be segregated in three functional groups, with the recognition of free floating macrophytes (Azolla
spp.), floating leaved macrophytes (Nuphar?: Nymphaeaceae), and emergent species (Gabonisporis vigorouxii: Marsileaceae), distributed along a depth gradient22. This floral community probably
acted as a food source and refugia for some of the aquatic insects here described.
Sample processingThe discovery of chitinous remains was fortuitous, after preparing fragments of rocks with the usual techniques employed for palynology. Rock samples were obtained from a stratigraphic level
named Megafloral Level 122. Superficial rock and debris was removed prior to collecting the samples, to avoid incorporating meteorized rock and/or foraneous organic content. Rock samples
were treated using standard palynological techniques for extraction and concentration of palynomorphs84 with a sequential combination of HCl (40%) and HF (70%). To increase the possibility
of recovering large specimens, rocks were not crushed before the acid treatment. The remaining organic residue was sieved using 80 μm mesh to obtain the larger fractions, to recover organic
macrofossils. The fractions of the residue with particles smaller than 80 μm were also revised. The organic residue was mounted and fixed in glass slides using glycerine jelly, as the
original intention was to use them for a palynological study. Thus, the arthropod remains were serendipitously discovered while analyzing the slides searching for plant-derived remains. The
fixed nature of the resulting microscope slides precludes the recovery or removal of any particular specimen (e.g., each chironomid remain), for example to rotate it, without destroying the
microscope slide sample. This fact also forbids the use of a scanning electronic microscope in the study, as the samples are encased between two fixed glasses. As a result, the illustration
and description of the arthropod remains were carried on using a transmitted light microscope exclusively.
Coincidence in color (i.e., yellow to pale brown) and preservation quality among arthropod remains and other preserved elements (spores, pollen, plant cuticular remains, and other non-pollen
palynomorphs22), fragmentation state, and diversity suggest the insect content cannot be interpreted as contamination of samples.
PhotographySpecimens were studied using a Nikon SMZ800 binocular microscope, photographed using an attached camera (Nikon Coolpix 995) or a Canon Powershot SX540 HS digital camera from Museo Argentino
de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires. For clarity’s sake, the CombineZP software (v.1.0) by Alan Hadley was used to produce photographic stackings of the identified
elements, as its size and volume resulted in many focal planes.
Reporting summaryFurther information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availabilityArthropod materials are represented by approximately 30 specimens, deposited in the Museo Regional Padre Jesús Molina (Río Gallegos, Santa Cruz Province), under MPM-Pal 21835 (Palynological
slides) acronyms. Respective collection numbers are as follows: MPM-Pal 21835-24: M31/0 (Orthocladiinae); MPM-Pal 21835-3: P46/4 (Orthocladiinae); MPM-Pal 21835-25: V26/3 (Orthocladiinae);
MPM-Pal 21835-9: E34/4 (Diamesinae); MPM-Pal 21835-19: V34/4 (Diamesinae); MPM-Pal 21835-2:Y40/3 (Tanypodinae); MPM-Pal 21835-9: Q58/3 (Chironomidae indet.); MPM-Pal 21835-9:W37/1
(Ephemeroptera indet.); MPM-Pal 21835-10: X30/2 (Lepidoptera indet.); MPM-Pal 21835-7:S27/2 (Lepidoptera indet.); MPM-Pal 21835-3: W34/1 (Coelolepida); MPM-Pal 21835-23: W55/0 (Coelolepida);
MPM-Pal 21835-22:R52/2 (Coelolepida); MPM-Pal 21835-20:Z47/3 (Coelolepida); MPM-Pal 21835-22: G55/0 (Coelolepida); MPM-Pal 2183-21: E42/3 (Coelolepida); MPM-Pal 21835-7:G42/4 (Arthropoda
indet.); MPM-Pal 21835-23:H55/0 (Arthropoda indet.); MPM-Pal 21835-20:U27/0 (Arthropoda indet.); MPM-Pal 21835-4 (Arthropoda indet.); MPM-Pal 21835-22:W55/2 (Arthropoda indet.); MPM-Pal
21835-7:K21/2 (Arthropoda indet.); MPM-Pal 21835-23:L36/0 (Arthropoda indet.); MPM-Pal 21835-4:R46/0 (Arthropoda indet.); MPM-Pal 21835-23:W46/0 (Arthropoda indet.).
References Szwedo, J. & Nel, A. The Cretaceous insects: a promising state of the art. Cretac. Res. 52, 628–630 (2015).
Article Google Scholar
Wang, B. & Szwedo, J. Introduction to thematic issue, “Cretaceous insects: diversity, palaeoecology and taphonomy”. Cretac. Res. 52, 313–315 (2015).
Article Google Scholar
Nel, A. Maastrichtian representatives of the dragonfly family Aeschnidiidae question the entomofaunal turnover of the early Late Cretaceous. Paleoentomology 4, 209–212 (2021).
Google Scholar
Fossa-Mancini, E. Hallazgos de insectos fósiles en la América del Sur. Notas Mus. La Plata, Paleontol. 6, 101140 (1941).
Google Scholar
Genise, J. F. Upper Cretaceous trace fossils in permineralized plant remains from Patagonia, Argentina. Ichnos 3, 283–299 (1995).
Article Google Scholar
Genise, J. F., Sciutto, J. C., Laza, J. H., González, M. G. & Bellosi, E. S. Fossil bee nests, coleopteran pupal chambers and tuffaceous paleosols from the Late Cretaceous Laguna Palacios
Formation, Central Patagonia (Argentina). Palaeogeogr. Palaeoclimatol. 177, 215235 (2002).
Article Google Scholar
Genise, J. F. & Sarzetti, L. C. Fossil cocoons associated with a dinosaur egg from Patagonia, Argentina. Palaeontology 54, 815823 (2011).
Article Google Scholar
Donovan, M. P., Iglesias, A., Wilf, P., Labandeira, C. C. & Cúneo, N. R. Rapid recovery of Patagonian plant–insect associations after the end-Cretaceous extinction. Nat. Ecol. Evol. 1, 0012
(2016).
Article Google Scholar
Vera, E. I., Perez Loinaze, V. S., Llorens, M. & Passalia, M. G. The fossil genus Aextoxicoxylon (Magnoliopsida) in the Upper Cretaceous Puntudo Chico Formation, Chubut Province, Argentina.
Cretac. Res. 107, 104315 (2020).
Article Google Scholar
Cúneo, N. R., Gandolfo, M. A., Zamaloa, M. C. & Hermsen, E. Late Cretaceous Aquatic Plant World in Patagonia, Argentina. PLoS ONE 9, e104749 (2014).
Article PubMed PubMed Central Google Scholar
Rasnitsyn, A. P. & Quickle, D. L. J. History of Insects 517 (Springer, Dordrecht, 2002).
Kuschel, G. Un curculiónido del Cretácico Superior. Primer insecto fósil de Chile. Invest. Zool. Chil. 5, 49–54 (1959).
Google Scholar
Menéndez, C. A. Paleophytología Kurtziana III-8. La flora del Cretácico Superior del Cerro Guido, Chile (1-2). Ameghiniana 9, 209–212 (1972).
Google Scholar
Novas, F. E. et al. Paleontological discoveries in the Chorrillo Formation (upper Campanian-lower Maastrichtian, Upper Cretaceous), Santa Cruz Province, Patagonia, Argentina. Rev. Mus.
Argent. Cienc. Nat. Nuevo Ser. 21, 217293 (2019).
Google Scholar
Rozadilla, S., Agnolín, F., Manabe, M., Tsuihiji, T. & Novas, F. E. Ornithischian remains from the Chorrillo Formation (Upper Cretaceous), southern Patagonia, Argentina, and their
implications on ornithischian paleobiogeography in the Southern Hemisphere. Cretac. Res. 125, 104881 (2021).
Article Google Scholar
Aranciaga Rolando, A. M. et al. A large Megaraptoridae (Theropoda: Coelurosauria) from Upper Cretaceous (Maastrichtian) of Patagonia, Argentina. Sci. Rep. 12, 6318 (2022).
Article PubMed PubMed Central CAS Google Scholar
Moyano-Paz, D. et al. The uppermost Cretaceous continental deposits at the southern end of Patagonia, the Chorrillo Formation case study (Austral-Magallanes Basin): Sedimentology, fossil
content and regional implications. Cretac. Res. 130, 105059 (2022).
Article Google Scholar
Álvarez-Herrera, G. Á. et al. New enantiornithine bird from the uppermost Cretaceous (Maastrichtian) of southern Patagonia, Argentina. Cretac. Res. 144, 105452 (2023).
Article Google Scholar
Chimento, N. R., Agnolín, F. L., Tsuihiji, T., Manabe, M. & Novas, F. E. New record of a Mesozoic gondwanatherian mammaliaform from Southern Patagonia. Sci. Nat. 107, 1–7 (2020).
Article Google Scholar
Chimento, N. R., Agnolín, F. L., Tsuihiji, T., Manabe, M. & Novas, F. E. New gondwanatherian (Mammaliaformes) remains from the Chorrillo Formation (Upper Cretaceous) of southern Patagonia,
Argentina. Cretac. Res. 127, 104947 (2021).
Article Google Scholar
Chimento, N. R. et al. First monotreme from the Late Cretaceous of South America. Commun. Biol. 16, 146 (2023).
Article Google Scholar
Vera, E. I. et al. Paleobotany of the uppermost Cretaceous Chorrillo Formation, Santa Cruz Province, Argentina: insights in a freshwater floral community. Cretac. Res. 138, 105296 (2022).
Article Google Scholar
Perez Loinaze, V. S. et al. Maastrichtian palynological assemblages from the Chorrillo Formation, Patagonia, Argentina. Rev. Palaeobot. Palynol. 314, 104893 (2023).
Article Google Scholar
Shumilovskikh, L., O’Keefe, J. M. K. & Marret, F. An overview of the taxonomic groups of non-pollen palynomorphs. Geol. Soc. Spec. Publ. 511, 13–61 (2021).
Article Google Scholar
Rudolph, K. Untersuchungen über den Aufbau Böhmischer Moore. I. Aufbau und Entwicklungsgeschichte Südböhmischer Hochmoore. Abh. K. K. Zool. -Bot. Ges. Wien. 9, 1–116 (1917).
Google Scholar
Hesmer, H. Mikrofossilien in Torfen. PalZ 11, 245–257 (1929).
Article Google Scholar
van Eldijk, T. J. B. et al. A Triassic-Jurassic window into the evolution of Lepidoptera. Sci. Adv. 4, e1701568 (2018).
Article PubMed PubMed Central Google Scholar
Fernández, D. A., Martínez, P. A., Palazzesi, L. & Barreda, V. D. Mites (Acari, Oribatida, Nanhermannidae) from the Eocene of Patagonia: first Southern Hemisphere fossil record in marine
sediments. Ameghiniana 58, 61–65 (2021).
Article Google Scholar
Massaferro, J. Paleoecología: el uso de los quironómidos (Diptera, Chironomidae) fósiles en reconstrucciones paleoambientales durante el Cuaternario. Rev. Soc. Entomol. Argent. 68, 209–217
(2009).
Google Scholar
Cranston, P. S. & Edward, D. H. Botryocladius gen. n.: a new transantarctic genus of orthocladiine midge (Diptera: Chironomidae). Syst. Entomol. 24, 305–333 (1999).
Article Google Scholar
Cranston, P. S. Parapsectrocladius: a new genus of orthocladiine Chironomidae (Diptera) from Patagonia, the southern Andes. Insect Syst. Evol. 31, 103–120 (2000).
Google Scholar
Drayson, N., Krosch, M. N. & Cranston, P. S. Taxonomic review of the chironomid genus Cricotopusv d.Wulp (Diptera: Chironomidae) from Australia: keys to males, females, pupae and larvae,
description of ten new species and comments on Paratrichocladius Santos Abreu. Zootaxa 3919, 1–40 (2015).
Article PubMed Google Scholar
Kukalova, J. Permian mayfly nymphs. J. Entomol. 75, 310–327 (1968).
Google Scholar
Sartori, M. & Brittain, J. E. Thorp and Covich’s Freshwater Invertebrates (ed. Thorp, J. H.) 873–891 (Academic 2015).
Salles, F. F. et al. Thorp and Covich’s Freshwater Invertebrates (ed. Thorp, J. H.) 61–117 (Academic, 2018).
Grimaldi, D. & Engel, M. S. Evolution of the Insects (Cambridge Univ. Press, 2005).
Kluge, N. J. Ephemeroptera & Plecoptera Biology-Ecology-Systematics (eds Landolt, P. & Sartori, M.) 527–535 (Fribourg, 1997).
Peters, W. L., & Peters, J. G. Studies on Fossils in Amber, with Particular Reference to the Cretaceous of New Jersey (ed. Grimaldi, D.) 127–131 (Backhuys Publ., 2000).
Sinitshenkova, N. D. History of Insects (ed. Rasnitsyn, A. P. & Quicke, D. L. J.) 388–426 (Kluwer Acad. Publ., 2002).
Schluter, T. Fossil insect in Gondwana-localities and palaeodiversity trends. Acta Zool. Cracov. 46, 345–371 (2003).
Google Scholar
Grimaldi, D. A., Engel, M. S. & Nascimbene, P. C. Fossiliferous Cretaceous amber from Myanmar (Burma): its rediscovery, biotic diversity, and paleontological significance. Am. Mus. Novit.
3361, 1–71 (2002).
Article Google Scholar
Storari, A. P. et al. An overview of the Hexagenitidae (Ephemeroptera) from the Crato Formation (Aptian, Lower Cretaceous) of Brazil, with the description of a new species. Hist. Biol. 34,
875–884 (2021).
Article Google Scholar
Alfonso-Rojas, A. & Cadena, E. A. The first benthic insects (Ephemeroptera and Coleoptera) from the Upper Cretaceous of Colombia. Cretac. Res. 132, 105116 (2002).
Article Google Scholar
Common, I. F. B. Evolution and Classification of the Lepidoptera. Ann. Rev. Entomol. 20, 183–203 (1975).
Article Google Scholar
Snodgrass, R. E. Principles of Insect Morphology (Cornell Univ. Press, 1935).
Nagy, L. M. & Grbic, M. in The Origin and Evolution of Larval Forms (eds Hall, B. K. & Wake, M. H.) 275–300 (Academic, 1999).
Suzuki, Y. & Palopoli, M. F. Evolution of insect abdominal appendages: are prolegs homologous or convergent traits? Dev. Genes Evol. 211, 456–492 (2001).
Article Google Scholar
Simonsen, T. J. & Kristensen, N. P. Agathiphaga wing vestiture revisited: evidence for complex early evolution of lepidopteran scales (Lepidoptera: Agathiphagidae). Insect Syst. Evol. 32,
169–175 (2001).
Article Google Scholar
Wang, J. et al. Early evolution of wing scales prior to the rise of moths and butterflies. Curr. Biol. 32, 3808–3814.e2 (2022).
Article PubMed CAS Google Scholar
Whalley, P. The systematics and palaeogeography of the Lower Jurassic insects of Dorset, England. Bull. Br. Mus. 39, 107–189 (1985).
Google Scholar
Zhang, Q. et al. Fossil scales illuminate the early evolution of lepidopterans and structural colors. Sci. Adv. 4, e1700988 (2018).
Article PubMed PubMed Central Google Scholar
Martins-Neto, R. G. & Vulcano, M. A. Amphiesmenoptera (Trichoptera+ Lepidoptera) na Formação Santana (Cretáceo Inferior) Bacia do Araripe, Nordeste do Brasil. I: Lepidoptera (Insecta). Acad.
Bras. Ciênc. 61, 459–466 (1989).
Google Scholar
Martins-Neto, R. G. New genus and new species of Lepidoptera (Insecta, Eolepidopterigidae) from Santana Formation (Lower Cretaceous, Northeast Brazil). Bol. 5th Symp. Cret. Bras. 5, 531–535
(1999).
Google Scholar
Martins-Neto, R. G. Review of some Insecta from Mesozoic and Cenozoic Brazilian deposits, with Descriptions of new taxa. Acta Geol. Leopold. 24, 115–124 (2001).
Google Scholar
da Silva, F. L. et al. Thorp and Covich’s Freshwater Invertebrates 4th edn, Vol. 3 (eds Hamada, N., Thorp, J. H. & Rogers, D. C.) 661–700 (Academic, 2018).
Rasnitsyn, A. P. & Quicke, D. L. J. History of Insects. (Kluwer Acad. Publ., 2002).
Doitteau, G. & Nel, A. Chironomid midges from early Eocene amber of France (Diptera: Chironomidae). Zootaxa 1404, 1–66 (2007).
Google Scholar
Grund, M. Chironomids (Diptera: Chironomidae) of Dominican amber. Ablabesmyia electrohispaniolana, sp. n. and paleoecological indications due to subfamily proportions. Insect Sys. Evol. 36,
29–34 (2005).
Article Google Scholar
Bechly, G. et al. The Crato Fossil Beds of Brazil: Window into an Ancient World (eds Martill, D. M., Bechly, G.& Loveridge, R. F.) 142–426 (Cambridge Univ. Press, 2007).
Veltz, I., Azar, D. & Nel, A. New chironomid flies in Early Cretaceous Lebanese amber (Diptera: Chironomidae). Afr. Invertebr. 48, 169–191 (2007).
Google Scholar
Brundin, L. Transantarctic relationships and their significance, as evidenced by chironomid midges, with a monograph of the subfamilies Podonominae and Aphroteniinae and the austral
Heptagyiae. K. Sven. Vetensk. Akad. Handl. 11, 1–472 (1966).
Google Scholar
Giłka, W. et al. Wanted, tracked down and identified: Mesozoic non-biting midges of the subfamily Chironominae (Chironomidae, Diptera). Zool. J. Linn. Soc. 194, 874–892 (2022).
Article Google Scholar
Krosch, M. N., Cranston, P. S., Bryant, L. M., Strutt, F. & McCluen, S. R. Towards a dated molecular phylogeny of the Tanypodinae (Chironomidae, Diptera). Invertebr. Syst. 31, 302–316
(2017).
Article CAS Google Scholar
Kalugina, N. S. [Changes in the subfamily composition of chironomids (Diptera, Chironomidae) as indicator of a possible eutrophication of water bodies during the Late Mesozoic)]. Byulleten
Mosk. Obschestva Ispyt. Prir. Otd. Biol. 79, 45–56 (1974). [in Russian].
Google Scholar
Kalugina, N. S. & Kovalev, V. G. [Jurassic Diptera of Siberia] 198. (Nauka, Moscow, 1985). [In Russian].
Baranov, V. A., Hoffeins, C., Hoffeins, H. W. & Haug, J. T. More than dead males: reconstructing the ontogenetic series of terrestrial non-biting midges from the Eocene amber forest. Bull.
Geosci. 94, 187–199 (2019).
Article Google Scholar
Simonsen, T. J. The wing vestiture of the non-ditrysian Lepidoptera (Insecta). Comparative morphology and phylogenetic implications. Acta Zool. 82, 275–298 (2001).
Article Google Scholar
Pabis, K. What is a moth doing under water? Ecology of aquatic and semi-aquatic Lepidoptera. Knowl. Manag. Aquat. Ecosyst. 219, 42 (2018).
Article Google Scholar
Moran, V. C. & Southwood, T. R. E. The guild composition of arthropod communities in trees. J. Anim. Ecol. 51, 289–306 (1982).
Article Google Scholar
Labandeira, C. Plant-Animal Interactions: An Evolutionary Approach (ed. Herrera, C. M. & Pellmyr, O. M.) 26–74 (Blackwell Sci., 2002).
Ashe, P., Murray, D. A. & Reiss, F. The zoogeographical distribution of Chironomidae (Insecta: Diptera). Ann. Limnol. 23, 27–60 (1987).
Article Google Scholar
Raigemborn, M. S. et al. Paleosols of the Maastrichtian dinosaur-bearing Chorrillo Formation (southern Patagonia, Argentina): paleoenvironmental and paleoclimateimplications. Cretac. Res.
150, 105587 (2023).
Article Google Scholar
Brundin, L. On the real nature of transantarctic relationships. Evolution 19, 496–505 (1965).
Google Scholar
Giribet, G. & Boyer, S. L. ‘Moa’s Ark’ or ‘Goodbye Gondwana’: is the origin of New Zealand’s terrestrial invertebrate fauna ancient, recent, or both? Invertebr. Syst. 24, 1–8 (2010).
Article Google Scholar
Rozadilla, S. et al. A new ornithopod (Dinosauria, Ornithischia) from the Upper Cretaceous of Antarctica and its palaeobiogeographical implications. Cretac. Res. 57, 311–324 (2016).
Article Google Scholar
Ghiglione, M. C. et al. Santonian-Campanian continentalization in the Austral-Magallanes basin: regional correlation, sediment sourcing and geodynamic setting. Cretac. Res. 128, 104968
(2021).
Article Google Scholar
Macellari, C. E., Barrio, C. A. & Manassero, M. J. Upper Cretaceous to Paleocene depositional sequences and sandstone petrography of southwestern Patagonia (Argentina and Chile). J. S. Am.
Earth Sci. 2, 223–239 (1989).
Article Google Scholar
Tettamanti, C. et al. Sedimentology and fluvial styles of the Uppermost Cretaceous Continental Deposits of the Austral-Magallanes Basin, Patagonia, Argentina. Lat. Am. J. Sedimentol. Basin
Anal. 25, 149–168 (2018).
Google Scholar
Odino-Barreto, A. L. et al. Sedimentology of the shallow marine deposits of the Calafate formation during the Maastrichtian transgression at Lago Argentino, austral Magallanes Basin,
Argentina. Lat. Am. J. Sedimentol. Basin Anal. 25, 169–191 (2018).
Google Scholar
Feruglio, E. Estudios geológicos y glaciológicos en la región del Lago Argentino (Patagonia). Bol. Acad. Nac. Cienc. Córdoba 37, 3–255 (1945).
Google Scholar
Bonaparte, J. F. Dinosaurios de América del Sur (Mus. Arg. Cienc. Nat., 1996).
Wright, V. P., Taylor, K. G. & Beck, V. H. The paleohydrology of Lower Cretaceous seasonal wetlands, Isle of Wight, southern England. J. Sediment. Res. 70, 619–632 (2000).
Article Google Scholar
Kraus, M. J. & Hasiotis, S. T. Significance of different modes of rhizolith preservation to interpreting paleoenvironmental and paleohydrologic settings: examples from Paleogene paleosols,
Bighorn Basin, Wyoming, U.S.A. J. Sediment. Res. 76, 633–646 (2006).
Article CAS Google Scholar
Phipps, G. & Playford, G. Techniques for extraction of palynomorphs from sediments. Pap. Dept. Geol. Univ. Qld. 11, 1–33 (1984).
Google Scholar
Dmccabe. Caenidae nymph.jpg; in Wikimedia Commons (2017).
Lukas3. Gasienica_motyla_budowa.svg; in Wikimedia Commons (2007).
Moreira, G. R. P., Pereira, C. M., Becker, V. O., Specht, A. & Gonçalves, G. L. A new cecidogenous species of many-plumed moth (Alucitidae) associated with Cordiera A. Rich. ex DC.
(Rubiaceae) in the Brazilian Cerrado. Zoologia 36, 1–15 (2019).
Article Google Scholar
Download references
AcknowledgementsThe present paper is the result of the first Argentine-Japanese exploration, carried out in March 2020. We thank Coleman Burke (New York), for his encouragement and financial assistance to
carry on first field explorations to La Anita farm. Special thanks to Dr. Y. Harashi, former General Director of the National Museum of Nature and Science, Japan. Thanks are extended to
editor George Inglis and three anonymous reviewers for their critical comments, which greatly improved the final version of the manuscript. Special thanks to Federico Braun for allowing
access to his property. Facundo Echeverría and his wife Daphne Fraser (La Anita farm) offered their valuable geographic knowledge of these territories, allowing us an easy access to fossil
sites with our 4x4 vehicles. Oscar Canto and Carla Almazán (Secretaría de Cultura) for supporting our projects and explorations in Santa Cruz Province. We thank Jorge Genise for reviewing an
early version of this manuscript.
Author informationAuthors and Affiliations División Paleobotánica, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” (MACN-CONICET), Av. Ángel Gallardo 470, C1405DJR, Ciudad
Autónoma de Buenos Aires, Argentina
Ezequiel I. Vera & Valeria S. Perez Loinaze
Centro de Ecología Aplicada del Litoral CONICET y Departamento de Biología, FaCENA-UNNE, Ruta Provincial N° 5, s/n, Km 2,5, 3400, Corrientes, Argentina
Mateo D. Monferran, Lara M. Sabater & Oscar F. Gallego
Programa de Estudios Aplicados a la Conservación de la Biodiversidad CENAC/APN, Fagnano 244, 8400, Bariloche, Argentina
Julieta Massaferro
Centro de Investigaciones Geológicas (CIG, CONICET-UNLP), Diagonal 113 #275, B1904DPK, La Plata, Buenos Aires, Argentina
Damián Moyano-Paz
Laboratorio de Anatomía Comparada y Evolución de los Vertebrados (LACEV), Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” (MACN-CONICET), Av. Ángel Gallardo 470, C1405DJR,
Ciudad Autónoma de Buenos Aires, Argentina
Federico L. Agnolín & Fernando E. Novas
Fundación de Historia Natural “Félix de Azara”, Departamento de Ciencias Naturales y Antropología, Universidad Maimónides, Hidalgo 775, C1405BDB, Ciudad Autónoma de Buenos Aires, Argentina
Federico L. Agnolín
Center for Collections, National Museum of Nature and Science, Tsukuba 305-0005, Tokio, Japan
Makoto Manabe
Department of Geology, National Museum of Nature and Science, 3-23-1 Hyakanin-cho, Shinjuku-lu, 1069-0073, Tokyo, Japan
Takanobu Tsuhiji
AuthorsEzequiel I. VeraView author publications You can also search for this author inPubMed Google Scholar
Mateo D. MonferranView author publications You can also search for this author inPubMed Google Scholar
Julieta MassaferroView author publications You can also search for this author inPubMed Google Scholar
Lara M. SabaterView author publications You can also search for this author inPubMed Google Scholar
Oscar F. GallegoView author publications You can also search for this author inPubMed Google Scholar
Valeria S. Perez LoinazeView author publications You can also search for this author inPubMed Google Scholar
Damián Moyano-PazView author publications You can also search for this author inPubMed Google Scholar
Federico L. AgnolínView author publications You can also search for this author inPubMed Google Scholar
Makoto ManabeView author publications You can also search for this author inPubMed Google Scholar
Takanobu TsuhijiView author publications You can also search for this author inPubMed Google Scholar
Fernando E. NovasView author publications You can also search for this author inPubMed Google Scholar
ContributionsE.I.V. and V.P.L. collected and processed the samples, and discovered the insect specimens. D.M.P. carried out the stratigraphic work. M.D.M., J.M., L.M.S., O.F.G., E.I.V., and F.L.A.
carried out systematics and descriptions of the specimens. E.I.V., F.L.A., and F.E.N. conceptualized the study. F.E.N., M.M., and T.T. acquired fundings. VPL, EIV, D.M.P., and F.L.A. made
the figures. All authors contributed to the writing and general revision of the manuscript.
Corresponding author Correspondence to Fernando E. Novas.
Ethics declarations Competing interestsThe authors declare no competing interests.
Peer review Peer review informationCommunications Biology thanks Bo Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: George Inglis. A peer review file
is available.
Additional informationPublisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary informationPeer Review FileReportingsummaryRights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or
other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in
the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and permissions
About this articleCite this article Vera, E.I., Monferran, M.D., Massaferro, J. et al. A Maastrichtian insect assemblage from Patagonia sheds light on arthropod diversity previous to the
K/Pg event. Commun Biol 6, 1249 (2023). https://doi.org/10.1038/s42003-023-05596-2
Download citation
Received: 31 May 2023
Accepted: 15 November 2023
Published: 11 December 2023
DOI: https://doi.org/10.1038/s42003-023-05596-2
Share this article Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article.
Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
