Zyxin is important for the stability and function of podocytes, especially during mechanical stretch
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Podocyte detachment due to mechanical stress is a common issue in hypertension-induced kidney disease. This study highlights the role of zyxin for podocyte stability and function.
We have found that zyxin is significantly up-regulated in podocytes after mechanical stretch and relocalizes from focal adhesions to actin filaments. In zyxin knockout podocytes, we found
that the loss of zyxin reduced the expression of vinculin and VASP as well as the expression of matrix proteins, such as fibronectin. This suggests that zyxin is a central player in the
translation of mechanical forces in podocytes. In vivo, zyxin is highly up-regulated in patients suffering from diabetic nephropathy and in hypertensive DOCA-salt treated mice. Furthermore,
zyxin loss in mice resulted in proteinuria and effacement of podocyte foot processes that was measured by super resolution microscopy. This highlights the essential role of zyxin for
podocyte maintenance in vitro and in vivo, especially under mechanical stretch. SIMILAR CONTENT BEING VIEWED BY OTHERS ROLE OF BIOPHYSICS AND MECHANOBIOLOGY IN PODOCYTE PHYSIOLOGY Article 05
March 2024 ROLES OF THE MECHANOSENSITIVE ION CHANNEL PIEZO1 IN THE RENAL PODOCYTE INJURY OF EXPERIMENTAL HYPERTENSIVE NEPHROPATHY Article 25 December 2023 PIEZO ACTIVITY LEVELS NEED TO BE
TIGHTLY REGULATED TO MAINTAIN NORMAL MORPHOLOGY AND FUNCTION IN PERICARDIAL NEPHROCYTES Article Open access 16 November 2024 INTRODUCTION More than 10% of the population worldwide suffer
from chronic kidney disease (CKD)1. Diabetes and hypertension, which are the major causes for the development of end-stage kidney disease (ESKD)2,3, are often associated with glomerular
hypertension. Glomerular hypertension is assumed to damage specifically podocytes, a terminally differentiated epithelial cell type in the glomerulus with extended major processes and thin,
interdigitating foot processes (FP) attached to the glomerular basement membrane (GBM). This complex 3D morphology of podocytes is highly dependent on an intact actin cytoskeleton that is
mainly located subcortical and in podocyte FP. Alterations of the actin cytoskeleton and actin-associated proteins often lead to a broadening of FP and a detachment of podocytes, often
resulting in the loss of the size-selectivity of the glomerular filtration barrier4,5,6,7. Since podocyte detachment cannot be compensated, the loss of podocytes is terminal. To prevent
podocyte detachment and damage induced by glomerular hypertension, knowledge about the responsible mechanosensors and mechanotransducers is essential. In the past, our group has revealed
that cultured podocytes are mechanosensitive and change their actin cytoskeleton as well as the gene expression upon mechanical stress and flow-induced shear stress8,9. We found that
mechanically stressed podocytes reorganize their actin cytoskeleton from transversal stress fibers into radially oriented actin filaments converging into a so called _actin rich center_
(ARC)9. However, the mechanosensor or mechanotransducer, which is responsible for such an actin reorganization, is still unknown. There are different ways in which cells sense physical
forces10,11. It is postulated that ion channels become activated by mechanical stretch allowing ion influx that triggers signaling cascades11,12,13. Another mechanism is that focal adhesions
(FAs) and the actin cytoskeleton itself activate specific pathways in response to physical forces14,15,16. Based on this, FA proteins are predisposed to be the linker between integrins and
the actin cytoskeleton for such a mechanotransduction17,18,19,20,21,22,23,24. Zyxin is an important FA and actin-associated protein, which has already been described to play an important
role in the cellular response to mechanical forces25,26,27. In this regard, zyxin has been implicated in facilitating actin filament assembly and stabilization of actin stress fibers,
thereby propagating force transmission from the extracellular matrix to the nucleus14,28,29,30,31,32,33. Zyxin is a zinc-binding phosphoprotein with a N-terminal proline-rich domain and
three LIM domains proximal to the C-terminus34,35. The N-terminus of zyxin has been reported to bind the actin filament crosslinker α-actinin, the actin assembly modulator Ena/VASP, the
cytoskeletal protein LIM and SH3 domain protein 1 (LASP-1)36,37,38,39 as well as the stretch-sensitive protein p130Cas40. In particular, the LIM domains of zyxin are essential for actin
binding and stress fiber localization in cells exposed to uniaxial stretch. This might be sufficient for force-induced accumulation of zyxin during cell migration41,42,43 . In vivo, zyxin
was described to protect from hypertension-induced cardiac dysfunction44 as well as being able to function a mechanosensor26. The present study investigated the role of zyxin in mechanically
stretched podocytes, a model for glomerular hypertension. Here we describe that zyxin is essential for the adhesion of the postmitotic cell type, the podocytes, in vitro during mechanical
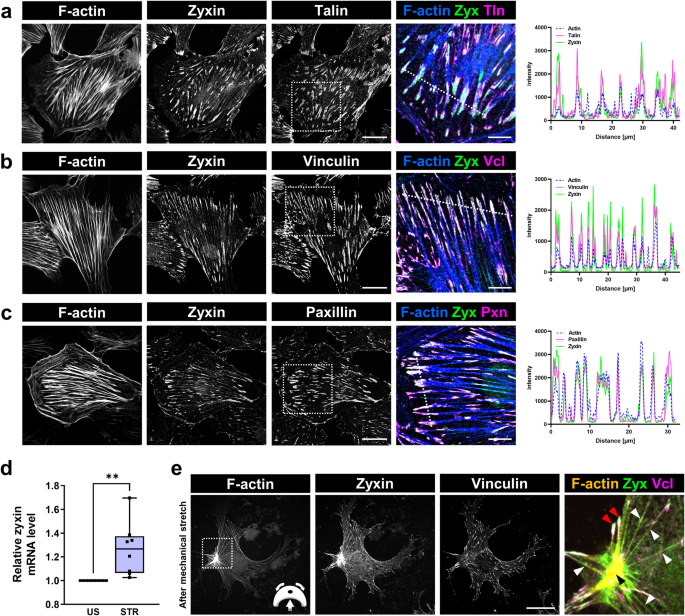
stretch as well as in vivo for the maintenance of the kidney filtration. RESULTS ZYXIN IS LOCALIZED AT FOCAL ADHESIONS AND ACTIN FIBERS IN CULTURED MURINE PODOCYTES To study the expression
of zyxin in cultured mouse podocytes, cells were stained with antibodies against zyxin and the focal adhesion proteins talin-1, vinculin and paxillin (Fig. 1a–c). By using confocal laser
scanning microscopy, we found that zyxin is colocalized with focal adhesion proteins as well as at actin fibers, as indicated by the plot profile intensity of multiple fluorescence images
(Fig. 1a–c, last panels). MECHANICAL STRETCH REGULATES ZYXIN EXPRESSION AND LOCALIZATION To find out whether mechanical stretch regulates the expression of zyxin, podocytes were cultured on
flexible silicone membranes and stretched for 3 days (0.5 Hz and 5% elongation) as described previously9. The expression of zyxin was analyzed in stretched (STR) and unstretched (US)
podocytes by immunofluorescence staining and qRT-PCR (Fig. 1d, e). We found a slight, but significant increase of the _Zyx_ mRNA level due to mechanical stretch (Fig. 1d). Furthermore, we
observed a reorganization of the podocyte actin cytoskeleton from transversal stress fibers into radial stress fibers due to mechanical stretch (Fig. 1e), as we have already described9. We
have found that mechanical stretch additionally induced increased zyxin localization along actin filaments as well as in the actin rich center (ARC) (Fig. 1e). This re-localization was also
observed in mechanically stretched primary podocytes. (Fig. S1a). ZYXIN KNOCKOUT PODOCYTES SHOW REDUCED CELL ADHESION AND HIGHER CELL LOSS AFTER MECHANICAL STRETCH To study the role of zyxin
in cultured podocytes that were exposed to mechanical stress for 3 days, we generated a zyxin knockout podocyte cell line (Zyx-KO) via the CRISPR/Cas9 technique and stretched Zyx-KO and
control podocytes (Ctrl) under the same conditions. The knockout was confirmed by immunofluorescence staining and Western blot analysis (Fig. 2a, b). After mechanical stretch, Zyx-KO
podocytes showed a higher cell loss compared to the Ctrl (-40 ± 10%) (Fig. 2d). However, we observed that mechanical stretch had no effect on the stretch-induced reorganization of the
F-actin cytoskeleton and the formation of ARCs of the remaining Zyx-KO podocytes (Fig. 2c). ZYXIN REGULATES THE EXPRESSION OF DISTINCT FOCAL ADHESIONS To investigate whether the reduced cell
adhesion under mechanical stress is due to a loss of focal adhesion proteins, we determined the expression levels of the typical focal adhesion (FA) proteins talin, vinculin and paxillin in
Zyx-KO podocytes by proteomics, qRT-PCR as well as immunocytochemistry analysis. Zyx-KO podocytes showed a significant decrease of vinculin protein (-23%) as well as mRNA level (-25%)
compared to Ctrl (Fig. 2e, f). Interestingly, the expression of talin and paxillin was not significantly affected by the zyxin knockout (Fig. 2e, f). Furthermore, immunocytochemistry
analysis showed that the loss of zyxin significantly influenced the size of FAs (Fig. 2g). The quantification revealed that the single FA area size was significantly reduced for vinculin
(mean FA size in µm2: Ctrl: 1.32; Zyx-KO: 1.24). The FA size for talin was unchanged (Ctrl: 1.78 µm2; Zyx-KO: 1.73 µm2) and paxillin-positive focal adhesions were even enlarged (Ctrl: 1.17
µm2; Zyx-KO: 1.26 µm2). Interestingly, the decrease in the mean FA size of vinculin is more pronounced under mechanical stretch in comparison to the unstretched conditions (Fig. 2h and Fig.
S1b). To find out which other proteins are regulated by zyxin, we performed proteomic profiling of three independent Ctrls (control podocytes) and three Zyx-KO podocyte clones by LC-MS/MS
(Supplementary Data 1). We detected 71 significant up- and 105 down-regulated proteins in the Zyx-KO podocytes compared to the control group (_p_ < 0.05 and fold change >1.5) (Fig.
2i). Figure 2k shows the top 25 up- and down-regulated proteins. It was remarkable that in the Zyx-KO podocytes the Gene Ontology clusters were strongly down-regulated, which are related to
cell adhesion or actin filament organization (Fig. 2j). ZYXIN REGULATES THE EXPRESSION OF VASP AND ACTIN FILAMENT ORGANIZATION PROTEINS IN PODOCYTES To study the influence of zyxin on the
expression of the zyxin-binding protein VASP, we performed immunostainings as well as Western blot analyses (Fig. 3a–c). Zyx-KO podocytes showed a significant decrease of the protein level
of VASP to 51 ± 22% compared to the Ctrl (Fig. 3a, b). The expression of other actin-associated proteins such as filamin A, α-actinin-1 or α-actinin-4 was not affected by the loss of zyxin
(Fig. 3a, b). Furthermore, immunostainings showed that the recruitment of VASP to the actin filaments seemed to be disrupted in Zyx-KO podocytes (Fig. 3c). This suggests that VASP is
recruited to actin stress fibers in a zyxin-dependent manner. In addition to VASP, other proteins of the zyxin interaction network were also significantly affected in Zyx-KO podocytes (Fig.
S2a, b). Proteomic data further showed a significant regulation of the Gene Ontology cluster “actin filament organization” in Zyx-KO podocytes (Fig. 3d). Especially, the protein levels of
SIRPA (tyrosine-protein phosphatase non-receptor type substrate 1) and IQGAP2 (Ras GTPase-activating-like protein) were significantly increased (SIRPA: 7.03-fold up-regulated; IQGAP2:
8.26-fold up-regulated). We confirmed this by qRT-PCR and found that mRNA levels of _Sirpa_ and _Iqgap2_ were significantly increased in Zyx-KO podocytes compared to Ctrl (_Sirpa_: 21.5-fold
up-regulated; _Iqgap2_: 5.8-fold up-regulated) (Fig. 3e). Transcriptional expression levels based on single‐cell RNA sequencing data45 revealed that _Sirpa_ and _Iqgap2_ are both highly
expressed in podocytes (Fig. 3f and Fig. S3). Looking at the expression levels of these proteins in patients suffering from diabetic nephropathy (DN), we found a down-regulation of the
expression (log2 fold change: _SIRPA_: -0.902; _IQGAP2_: -0.578) (Fig. 3g). Moreover, we found that the loss of zyxin did not only leads to an up-regulation of Rho GTPase activating protein
IQGAP2, but also affected the expression of other Rho GTPase-associated proteins like guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). A high number of
Arhgap and Arhgef proteins were also regulated by zyxin in cultured podocytes (Fig. 3h, i). ZYXIN KNOCKOUT PODOCYTES SHOW REDUCED EXPRESSION OF EXTRACELLULAR MATRIX PROTEINS AND DIMINISHED
CELL SPREADING Proteomic data impressively showed that extracellular matrix (ECM) proteins are significantly down-regulated in Zyx-KO podocytes (Fig. 4a). Therefore, we analyzed the mRNA
levels of specific ECM proteins by qRT-PCR. We have found that the mRNA levels of nidogen-2 (_Nid2_), thrombospondin-1 (_Thbs1_) and periostin (_Postn_) were significantly reduced in Zyx-KO
podocytes (Fig. 4b). To explore whether the loss of zyxin also influences the expression of fibronectin, we performed immunostainings and Western blot analyses of Zyx-KO and Ctrl podocytes
(Fig. 4c, d). The protein level of fibronectin was significantly reduced by 45% in Zyx-KO podocytes compared with control podocytes (Fig. 4d). This was also confirmed by immunofluorescence
analysis, which revealed a marked decrease of fibronectin fibrils in the Zyx-KO podocytes (Fig. 4c). Additionally, we found that the expression of the specific fibronectin receptor integrin
α5 was significantly reduced (-48%) in Zyx-KO podocytes. In contrast, the protein level of integrin β1 did not change (Fig. 4d). Since zyxin deficiency strongly reduced the expression of ECM
proteins, we wanted to investigate the role of zyxin in cell spreading. For this, both Ctrl and Zyx-KO podocytes were trypsinized, seeded on glass cover slips and incubated for 1–8 h. After
fixation at different time points, the cell size of the podocytes was quantified (Fig. 4e). We found that Zyx-KO podocytes showed a reduced spreading and cell size in the initial phase of
adhesion compared to Ctrl (Fig. 4e). Furthermore, the migration speed and distance increased significantly in Zyx-KO podocytes in contrast to the Ctrl (Fig. 4f and Fig. S1c). THE LOSS OF
ZYXIN REDUCES THE NUMBER AND LENGTH OF FILOPODIA IN CULTURED PODOCYTES DUE TO A DOWN-REGULATION OF FASCIN-1 By immunostaining and Western blot analyses, we observed a significant decrease of
the protein fascin-1 in Zyx-KO podocytes (Fig. 5a–c). Since fascin-1 is essential for the formation of filopodia in podocytes46, we speculated that the development of filopodia is altered
due to the loss of zyxin. Analysis of the proteome data of Zyx-KO and Ctrl podocytes by using the Ingenuity Pathway Analysis47 tool predicted a reduction of the “filopodia formation”
pathway. With the exception of Cdc42, many essential proteins involved in filopodia formation were significantly down-regulated in the Zyx-KO podocytes (Fig. 5d). Therefore, we quantified
the number and length of the filopodia and found that the loss of zyxin leads to a reduction of the number (-35%) and mean length of the filopodia (-47%) in Zyx-KO podocytes compared to Ctrl
(Fig. 5e, f). ZYXIN IS UP-REGULATED IN DIABETIC NEPHROPATHY (DN) To find out, whether zyxin is also regulated in human patients suffering from DN, we analyzed the mRNA expression of _ZYX_
in micro-dissected glomeruli originated from renal biopsies of patients suffering from DN and compared them to mRNA levels of patients with Minimal Change Disease (MCD) and healthy living
donors. We observed that patients with DN showed a significant increase of _ZYX_ mRNA compared to the control tissue (log2 fold change: 0.434; Fig. 6b). In contrast, the zyxin expression in
MCD patients did not change significantly (Fig. 6b). Analysis of human disease data confirmed the significant increase in the ZYX expression in DN, but not in MCD patients (Fig. 6c).
Additionally, we observed a strong co-localization of zyxin with synaptopodin in the foot processes of the podocytes in glomeruli of patients suffering from DN associated with hypertension
(Fig. 6a). Whereas zyxin in healthy living donors and in patient with MCD was only expressed slightly in podocytes, in DN patients, zyxin seemed to be expressed highly by podocytes (Fig.
6a). Furthermore, quantification of the mean ZYX fluorescence intensity in the foot processes of the podocytes also showed that zyxin expression increases podocyte-specifically in patients
with DN (Fig. 6d). In agreement with these data, we found an up-regulation of zyxin in the glomeruli of mice suffering from glomerular hypertension which was induced by uninephrectomy (UNX)
and desoxycorticosterone acetate (DOCA)-salt treatment (Fig. 6e). We were able to confirm this by quantification of the mean Zyx intensity (Fig. 6f). Furthermore, data analysis has shown
that zyxin expression is significantly correlated with proteinuria in FSGS, but not in MCD patients (Fig. 6g and Fig. S4) and blood pressure (Fig. 6h). _ZYX_ KO MICE SHOW EXPANSION OF THE
MESANGIAL COMPARTMENT, A REDUCED SLIT MEMBRANE DENSITY AND PROTEINURIA After validation of the knockout of zyxin by immunostaining (Fig. 7a) we analyzed the glomeruli by PAS staining (Fig.
7b, c). At the age of 6 months, an expansion of the mesangial compartment was detectable (Fig. 7b). Assessment of glomerulosclerosis (glomerular PAS+ area) confirmed glomerular damage in
_Zyx_ KO mice (Fig. 7c). To determine more precisely the changes of the foot process morphology using super-resolution microscopy 3D-SIM, we applied the podocyte effacement measurement
procedure on podocin-stained kidney sections (Fig. 7d), which was recently established by Siegerist et al. 48. In _Zyx_ KO mice, we found a significant reduction of the slit diaphragm
density compared to the control mice (4.25 µm−1 versus 4.57 µm−1) (Fig. 7e). By electron microscopy we were able to confirm the morphological glomerular changes. We observed a partial
podocyte foot process effacement, pseudocysts, expansion of the mesangial cells and a thickening of the glomerular basement membrane in _Zyx_ KO glomeruli (Fig. 7f, g and Fig. S5). Moreover,
evaluation of albumin-creatinine ratios (ACR) showed a significant increase of proteinuria in male _Zyx_ KO mice compared to the wildtype mice (ACR in µg/mg: WT: 39; KO: 161) (Fig. 7h). The
protein-creatinine ratio was not changed significantly (Fig. 7i). DISCUSSION Podocyte damage and detachment caused by mechanical stress is a common issue in hypertension-induced glomerular
disease49,50. However, it is unknown whether mechanical stress induces a dynamic change in the podocyte integrin adhesion complex or how such modulations affect the mechano-sensitive link
with the GBM and the integrity of the glomerular filtration barrier. In this study, a comprehensive analysis of zyxin for signaling and cell adhesion highlighted the essential role of zyxin
for podocyte stability and function. We have found that zyxin is significantly up-regulated in podocytes after three days of mechanical stretch and relocalized from focal adhesions to actin
filaments as well as to the actin-rich center (ARC), a characteristic structure in stretched podocytes9. Yoshigi and coworkers were the first to describe, that cyclic stretch or shear stress
in vitro induces such a relocation of zyxin from focal adhesions to actin filaments in cultured fibroblasts26, whereas other focal adhesion proteins remained unchanged. In contrast, our
zyxin knockout podocyte cell line showed a significant reduced size of vinculin-positive focal adhesions compared to control, whereas talin, a protein proposed to be a mechanosensor51,
remained unchanged. A down-regulation of vinculin was also found in zyxin knockdown chondrocytes52. A translocation of zyxin into the nucleus, as it was observed by Gosh et al. in stretched
vascular smooth muscle cells, was not found in podocytes53. Since zyxin and vinculin can recruit Ena/VASP proteins by their EVH1 domain32,54,55, we studied the influence of zyxin on the
localization and expression of VASP, a protein that plays a central role in cell adhesion, motility as well as in the regulation of integrin-extracellular matrix signaling pathways56,57,58.
Especially the observation of different researcher that force can induce an accumulation of actin filaments at adhesion sites through a zyxin–VASP-dependent actin polymerization59,60,61 was
highly interesting. Here, we show that zyxin is essential for the recruitment of VASP to actin filaments in podocytes as it was also described by Cheah et al., who found that VASP
accumulated along force-bearing actin fibers in a zyxin-dependent manner62. We believe that the decrease of VASP and vinculin that were observed in Zyx-KO podocytes in contrast to other
focal adhesion proteins such as α-actinin-4 and talin, led to an impaired cellular adhesion that we have seen during mechanical stretch. We found that 40% of the zyxin knockout podocytes
detached during the three-day mechanical stress in contrast to the control cells. Since zyxin knockout podocytes also showed reduced extracellular matrix protein expression, such as
fibronectin, periostin and nidogen, as well as a regulation of Rho GTPase-associated proteins (GEFs and GAPs), we hypothesized that zyxin might be a mechanosensor in podocytes. However, the
number of ARC formations, a readout for mechanical stretch sensing of podocytes, was not changed indicating that zyxin is not a mechanosensor, but a mechanotransducer. Small Rho GTPases also
regulate the motility and spreading of cells63,64,65. Therefore, we looked whether the dynamics of zyxin knockout podocytes were affected by the knockout. We observed that the cell size as
well as the motility were altered after the loss of zyxin, which is in accordance with data reported for vascular smooth muscle cells, fibroblasts and MDCK cells56,61,66,67. In the past, we
have observed that the actin-associated protein fascin-1, which is responsible for actin bundling, focal adhesion stability and filopodia formation by tightly crosslinking actin filaments
into non contractile stress fiber68,69,70, is up-regulated by mechanical stretch46. Therefore, we studied the influence of zyxin expression on fascin-1. We found that the number and length
of filopodia is significantly diminished after the loss of zyxin, suggesting that zyxin acts upstream of fascin-1. The down-regulation of fascin-1 might also reduce the stability of focal
adhesion leading to the detachment of podocytes under mechanical stress. The important role of zyxin in podocytes was underlined by in vivo results. We have seen that not only in diabetic
nephropathy, a disease which is often associated with glomerular hypertension, but also in a classical glomerular hypertension model, the DOCA-salt treated mice, we have found a massive
up-regulation of zyxin expression. We assume that zyxin is increasingly required in hypertensive glomerular injuries as a part of the mechano-adaptive response for podocytes to withstand
mechanical stress. Furthermore, Z_yx_ KO mice showed proteinuria and severe changes of podocyte foot process morphology, which were identified by super resolution microscopy and confirmed by
electron microscopy. Taken together, zyxin is an important protein that is critical for podocyte stability and function in vitro and in vivo, especially under mechanical stretch. METHODS
CELL CULTURE Conditionally immortalized podocytes (SVI; CLS Cell Line Service GmbH, Eppelheim, Germany) were handled as described previously9. Briefly, podocytes were maintained in RPMI-1640
medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS; Boehringer Mannheim, Mannheim, Germany), 100 U/ml penicillin, and 0.1 mg/ml streptomycin (Thermo
Fisher Scientific, Waltham, MA, USA). To propagate podocytes, we cultivated cells at 33 °C. To induce podocyte differentiation, we maintained podocytes at 38 °C for at least two weeks before
applying mechanical stress. MECHANICAL STRETCH EXPERIMENTS Mechanical stretch experiments were performed according to our previous study9. Differentiated mouse podocytes were seeded on
flexible silicone membranes of a six well plate (Bioflex, Flexcell® International Corporation, Burlington, NC, USA). The flexible silicone membranes were pre-coated with collagen IV to
facilitate cell attachment. After three days, the six well plate was mounted on a manifold connected to a custom-built stretch apparatus (NIPOKA GmbH, Greifswald, Germany), which induced
cyclic pressure variations resulting in upward and downward motion of the silicone membranes. Pressure amplitude was chosen to give a maximum upward deflection of the membrane center of 6
mm, being equal to an increase in membrane area by 11%, or 5% mean linear cell strain. Cycle frequency was adjusted to 0.5 Hz. This frequency was chosen because autoregulation of renal blood
flow is capable of damping pressure fluctuations below 0.1 Hz, whereas faster pressure fluctuations ( > 0.1 Hz) are fully transmitted to glomerular capillaries10,71. Number of adherent
cells was determined by semiautomatic-counting of stained podocytes with the StarDist ImageJ/Fiji Plugin72 from at least three biological replicates. ZYXIN KNOCKOUT PODOCYTES Cultured
podocytes were transfected with CRISPR-Cas9 RNP complexes from Integrated DNA Technologies (Coralville, Iowa, USA) and Lipofectamine™ RNAiMAX as transfection reagent (Thermo Fisher
Scientific) according to the manufacturer’s specifications. The following crRNAs were used: Mm.Cas9.ZYX.1.AD (ACTGGAGCAAACTTAGGTGC), Mm.Cas9.ZYX.1.AH (CGAGATCCCACCACCACCCC) or negative
Control crRNA. After single cell clonal expansion zyxin knockout podocytes (Zyx-KO) were selected and verified by immunostaining, (q)RT-PCR, Western blot and DNA sequencing. ZYXIN KNOCKOUT
MICE (_ZYX_ KO) The _Zyx_ KO mice were kindly provided by the group of M. Hecker44. The strain was originally designed by M. Beckerle73, but backcrossed more than 10-times onto the C57BL/6 J
background. As wildtype control age-matched C57BL/6 J mice were used. Experiments were done with 6-month-old and 12-month-old male and female mice. All studies were carried out in strict
accordance with regulations in Germany regarding the use of laboratory animals and were approved by the regional council in Karlsruhe. DOCA-SALT INDUCED HYPERTENSION Induction of glomerular
hypertension by uninephrectomy (UNX) and desoxycorticosterone acetate (DOCA) salt treatment has been described previously74. RNA EXTRACTION, CDNA SYNTHESIS AND QRT-PCR Samples were processed
in Tri-Reagent (Sigma-Aldrich) according to manufacturer´s instructions. For cDNA synthesis, 1 µg of the isolated total RNA was transcribed using the QuantiTect Reverse Transcription Kit
(Qiagen, Hilden, Germany). The quantitative real-time PCR (qRT-PCR) analysis was performed on a QuantStudio™ 5 Real-Time PCR System (Thermo Fisher Scientific) using the iTaq Universal SYBR
Green Supermix (Bio-Rad) with _Gapdh_ as reference gene. Relative quantifications of the mRNA levels were done by the efficiency corrected calculation model by Pfaffl75 and are shown with
standard deviations (SD) or standard error of the mean (SEM) from at least three biological replicates. Used primers can be viewed in Supplementary Table 1. WESTERN BLOT ANALYSIS Protein
lysates were separated using a 4-20% Mini-PROTEAN® TGX™ Gel, (Bio-Rad Laboratories) and transferred to a nitrocellulose membrane using a Trans-Blot® Turbo™ Transfer System (Bio-Rad
Laboratories) for 10 min at 2 A. Membranes were immersed for 1 h in blocking buffer (10 mM Tris, 100 mM NaCl, 5% non-fat dry milk, 0.2% Tween-20, pH 7.5) and incubated overnight at 4°C in
TBS-Tween (0.5%) with the following antibodies: anti-zyxin (Z4751, Sigma-Aldrich, St. Louis, MO, USA; immunoblot dilution 1:4000), anti-paxillin (610051, BD Biosciences; immunoblot dilution
1:25000), anti-talin (T3287, Sigma Aldrich; immunoblot dilution 1:10000), anti-vinculin (V9131, Sigma-Aldrich; immunoblot dilution 1:20000), anti-fibronectin (ab2413, Abcam; immunoblot
dilution 1:2000), anti-fascin-1 (HPA005723, Sigma-Aldrich; immunoblot dilution 1:1000), anti-VASP (HPA005724, Sigma-Aldrich; immunoblot dilution 1:2000), anti-filamin A (SAB4500951,
Sigma-Aldrich; immunoblot dilution 1:4000), anti-β-actin (sc-47778, Santa Cruz; immunoblot dilution 1:1000), anti-α-actinin-1 (A5044, Sigma-Aldrich; immunoblot dilution 1:2500), anti-
α-actinin-4 (0042-05, immunoGlobe; immunoblot dilution 1:2000) and anti-Gapdh (10494-1-AP, Proteintech Group; immunoblot dilution 1:40000). Blots were incubated for 1 h at RT with
HRP–conjugated secondary antibody anti-mouse (SA00001-1, Proteintech Group; immunoblot dilution 1:15000) or anti-rabbit (SA00001-2, Proteintech Group; immunoblot dilution 1:15000) and
developed using Clarity™ Western ECL Blotting Substrate (Bio-Rad Laboratories) and finally exposed to X-ray films (Fujifilm Super RX, FUJIFILM, Tokyo, Japan). For the relative
quantification, developed x-ray films were scanned and analyzed using the Fiji distribution of Image J (ImageJ J 1.51)76,77. Mean gray values of specific signals were determined and
normalized to mean gray values of Gapdh signals. LIQUID CHROMATOGRAPHY-MASS SPECTROMETRY (LC-MS/MS) Zyx-KO and control podocytes of three independent bioreplicates were harvested in 8 M
urea/2 M thiourea and proteins extracted by five freeze-thaw cycles. Protein containing supernatant was collected by centrifugation (16,000 × _g_, 60 min, 20 oC) and nucleic acid degraded
enzymatically with universal nuclease 0.125 U/µg protein, (Pierce/Thermo, Rockford, IL, USA). Protein concentration was determined using a Bradford assay (Biorad). Four µg protein were
reduced by 2.5 mM dithiothreitol for 1 h at 60°C and alkylated with 10 mM iodoacetic acid for 30 min at 37 °C in the dark. Samples were diluted with 20 mM ammoniumbicarbonate to 1 M
urea/thiourea before digestion by trypsin (Promega, Walldorf, Germany) in a protein to enzyme ratio 25:1 overnight at 37°C. The reaction was stopped with acetic acid (1%) and the peptide
mixtures were desalted on C-18 reverse phase material (ZipTip μ-C18 Millipore Corporation, Burlington, MA, USA). Peptide eluates were lyophilised and resuspended in 2% ACN in 0.1% acetic
acid. Peptides were separated by LC (Ultimate 3000, Thermo Electron, Bremen, Germany) before data-independent acquisition of MS data on an Exploris 480 mass spectrometer (Thermo Electron).
MS data were analysed via the DirectDIA algorithm implemented in Spectronaut (v15, Biognosys, Zurich, Switzerland) using an Uniprot database (version 2021_2) limited to _Mus musculus_ (n =
17,063). Carbamidomethylation at cysteine was set as static modification, oxidation at methionine and protein N-terminal acetylation were defined as variable modifications, and up to two
missed cleavages were allowed. Proteins were only considered for further analyses, if two or more unique+razor peptides were identified and quantified per protein. Data were median
normalized on ion level before statistical analysis was carried out on peptide level after exclusion of peptides with oxidized methionine using the algorithm ROPECA78. Binary differences
were identified by application of a reproducibility-optimized test statistic (using the ROTS package). Only proteins that showed different abundance (_p_ value < 0.05) were used for
further considerations. Detailed description of data acquisition and search parameters are provided in Supplementary tables 2 and 3. Furthermore, data were analysed by Gene Set Enrichment
Analysis (GSEA) and the use of QIAGEN IPA (QIAGEN Inc.). For the analysis of the differentially abundant proteins R scripts including the libraries EnhancedVolcano and ComplexHeatmap were
used for the visualization. The heatmaps include on the Y-axis a dendrogram of the hierarchical clustering after Z-score normalization of the protein abundance values in the different
samples. The heatmaps are used to represent the abundance of proteins as ratio from treatment versus control of specific biological pathways from the GeneOntology (GO). To detect the
enriched and overrepresented pathways and their protein protein interactions the cnetplots and dotplots from the GO biological processes were created using the R library clusterProfiler79.
IMMUNOCYTOCHEMISTRY Cells were fixed using 2% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 10 min at room temperature (RT), washed and subjected to blocking solution (PBS,
2% fetal bovine serum, 2% bovine serum fraction V, 0.2% fish gelatin) for 1 h at RT. Primary antibodies were incubated for 60 min at RT. The following antibodies were used: anti-zyxin
(Z4751, Sigma-Aldrich; IF dilution 1:200), anti-paxillin (610051, BD Biosciences; IF dilution 1:200), anti-talin (T3287, Sigma-Aldrich; IF dilution 1:100), anti-vinculin (V9131,
Sigma-Aldrich; IF dilution 1:400), anti-fibronectin (ab2413, Abcam; IF dilution 1:250), anti-fascin-1 (HPA005723, Sigma-Aldrich; IF dilution 1:100) and anti-VASP (HPA005724, Sigma-Aldrich;
IF dilution 1:200). Actin was stained using Alexa Fluor® 488 phalloidin (Thermo Fisher Scientific; dilution 1:100). After a washing step with PBS (3 × 3 min) cells were incubated with
secondary antibodies for 45 min at RT. Bound antibodies were visualized with Cy2- or Cy3-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, USA; IF dilution
1:300). For staining of nuclei DAPI (Sigma-Aldrich, 1 µg/ml) was used for 5 min. All samples were mounted in Mowiol (Carl Roth, Karlsruhe, Germany) and used for laser scanning microscopy
(LSM). Pictures for focal adhesion analysis were taken randomly on the microscope slides. HISTOLOGY For paraffin sections, samples were dehydrated and embedded in paraffin by standard
procedures. Paraffin sections (2 µm) were cut on a Leica SM 2000R (Leica Microsystems, Wetzlar, Germany). After rehydration, sections were unmasked in citrate buffer (0.1 M, pH 6.0) by
heating for 5 min in a pressure cooker. The nuclei were stained with 1 mg/100 ml Hoechst 33342 (Sigma-Aldrich) for 30 min. For immunofluorescence double-staining, samples were incubated with
an antibody against zyxin (HPA004835 for human and HPA073497 for mice FFPE material; both from Sigma-Aldrich; IF dilution 1:100) and synaptopodin (61094, Progen Biotechnik GmbH, Heidelberg,
Germany; IF dilution 1:100) overnight. Samples were washed with 1x PBS for 3 × 5 min and incubated with Cy2- and Cy3-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories; IF
dilution 1:300) for 1 h. After additional washing, the samples were mounted in Mowiol (Carl Roth) for fluorescence microscopy. PAS stainings were performed by standard procedures. For
transmission electron microscopy, kidneys were embedded in EPON 812 (SERVA, Heidelberg, Germany). Ultrathin sections were cut and contrasted with 5% uranyl acetate and lead citrate. All
grids were examined with a LIBRA® 120 transmission electron microscope (Carl Zeiss Microscopy, Jena, Germany). Scanning electron microscopy was performed according to Artelt et al. 80
HISTOLOGY ON HUMAN KIDNEY BIOPSIES Kidney biopsies were received from the Department of Nephropathology, Institute of Pathology, University Hospital Erlangen, Germany. The use of remnant
kidney biopsy material was approved by the Ethics Committee of the Friedrich-Alexander-University of Erlangen-Nürnberg, waiving the need for retrospective consent for the use of archived
rest material (No. 22-150-D). Sample fixation (4% PBS-buffered formalin pH 7.6) and preparation of the control, minimal change disease (MCD) and diabetic nephropathy (DN) group were
identical. All MCD patients (n = 3) had proteinuria (1,7–3,2 g/d) and effacement of the podocyte foot processes (examined by electron microscopy). MICROARRAYS ON HUMAN KIDNEY BIOPSIES Human
renal biopsy specimens and Affymetrix microarray expression data were procured within the framework of the European Renal cDNA Bank - Kröner-Fresenius Biopsy Bank81,82. Biopsies were
obtained from patients after informed consent and with approval of the local ethics committees. Following renal biopsy, the tissue was transferred to RNase inhibitor and microdissected into
glomeruli and tubulointerstitium. Total RNA was isolated from microdissected glomeruli, reverse transcribed, and linearly amplified according to a protocol previously reported83. Previously
generated microarray data from microdissected human glomeruli sourced from individuals with kidney disease and healthy donors were used (GEO accession numbers: GSE32591, GSE37463, GSE47185,
GSE99340). Pre-transplantation kidney biopsies from living donors were used as control. CEL file normalization was performed with the Robust Multichip Average method using RMAExpress
(version 1.20) and the human Entrez‐Gene custom CDF annotation from Brain Array (version 25). The log-transformed dataset was corrected for batch effect using ComBat from the GenePattern
pipeline. To identify differentially expressed genes, the SAM (Significance Analysis of Microarrays) method was applied using SAM function in Multiple Experiment Viewer (TiGR MeV, Version
4.9)84. A q-value below 5% was considered to be statistically significant. Analysis included gene expression profiles from patients with diabetic nephropathy (n = 14), minimal change disease
(n = 14) as well as controls (living donors, n = 41). IMAGE ANALYSIS For quantification of focal adhesions size, we developed the custom software “Focal Contact Segmentation and Analysis
Tool”. The focal contacts (FC) are segmented by a gradient-based local-threshold method. The specificity of this segmentation is further increased by relating the peripheral region of each
FC to its center intensity. Slightly connected FCs are separated. Finally, specific shape parameters and the fluorescence activities of each FCs have been computed. Additionally, the cells
are segmented by a semi-automatic technique and therefore the exact position of each FC within the cell is known46,85. PODOCYTE FOOT PROCESS EFFACEMENT MEASUREMENT PROCEDURE The evaluation
of the filtration slit density was performed using a recently established super-resolution microscopy-based methodology (structured illumination microscopy, SIM; N-SIM [Nikon] with a 100×
silicone objective) termed as podocyte exact morphology measurement procedure (PEMP)48. The three-dimensional (3D) SIM (z-stack) images of slit diaphragms were colorized according to their
position on the z axis. Filtration slit density values of six glomeruli in five mice per group were quantified. The slit diaphragm density was stated as length of the slit diaphragm per
glomerular capillary area in _µ_m−1. CONFOCAL LASER SCANNING MICROSCOPY Images were captured with an Olympus FV3000 confocal microscope (Olympus, Tokyo, Japan) with 20x/40x/60x oil immersion
objectives and Olympus FV3000 CellSense software. DEEP-LEARNING ENHANCED CELL MIGRATION ASSAY Cells were cultured in flat-bottom 24 well plates. Medium was exchanged to phenol-free medium
containing 100 ng/ml HOECHST 33342 and image sequences (a single frame every 20–30 min were acquired in the Acquifer Imaging Machine (Acquifer GmbH, Heidelberg, Germany)). Image sequences
were imported to FIJI76 and cell nuclei detected using the StarDist extension72 and an elsewhere published custom-trained StarDist network86. Cell tracks were merged and random cell velocity
was quantified with the TrackMate plugin87,88. STATISTICS AND REPRODUCIBILITY The GraphPad Prism 9 software was used for statistical analysis of experimental data and preparation of graphs.
Scatter plots indicate individual units used for statistical testing (samples, cells or replicates), as specified in the respective figure legends. Data are given as means ± SD or ±SEM,
analyzed by unpaired _t_ test with repeated measurements (n). For multiple groups statistical analyses were done by ANOVA followed by a Benjamini-Hochberg post-hoc test. Statistical
significance was defined as p < 0.05 and significance levels are indicated as *_p_ < 0.05, **_p_ < 0.01, ***_p_ < 0.001, ****_p_ < 0.0001 or non-significant (ns). To verify
the results, we conducted the experiments with n ≥ 3 to confirm their reproducibility. The exact number of independent experiments and analyzed units are stated in the figure legends.
REPORTING SUMMARY Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY All data supporting the findings of
this study are available within the paper and its Supplementary Information. The mass spectrometry proteomics data are available in supplementary data files (Supplementary Data 1) and have
been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD050861. All uncropped blots and raw data are available within the article and
its supplementary data files (Fig. S6 and Supplementary Data 2). All other relevant data and materials are available from the corresponding author on reasonable request. REFERENCES *
Global, regional, and national burden of chronic kidney disease, 1990-2017. a systematic analysis for the Global Burden of Disease Study 2017. _Lancet (London, England)_ 395, 709–733 (2020).
Article Google Scholar * Saran, R. et al. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. _Am. J. Kidney Dis._ 66, S1–305 (2015).
Article Google Scholar * Hill, N. R. et al. Global prevalence of chronic kidney disease – A systematic review and meta-analysis. _PLoS ONE_ 11, e0158765 (2016). Article PubMed PubMed
Central Google Scholar * Kretzler, M., Koeppen-Hagemann, I. & Kriz, W. Podocyte damage is a critical step in the development of glomerulosclerosis in the
uninephrectomised-desoxycorticosterone hypertensive rat. _Vichows Archiv A Pathol. Anat_. 425; https://doi.org/10.1007/bf00230355 (1994). * Kriz, W., Hosser, H., Hähnel, B., Gretz, N. &
Provoost, A. P. From segmental glomerulosclerosis to total nephron degeneration and interstitial fibrosis: A histopathological study in rat models and human glomerulopathies. _Nephrol.
Dialysis Transp._ 13, 2781–2798 (1998). Article CAS Google Scholar * Simons, J. L. et al. Pathogenesis of glomerular injury in the fawn-hooded rat: Early glomerular capillary hypertension
predicts glomerular sclerosis. _J. Am. Soc. Nephrol._ 3, 1775–1782 (1993). Article CAS PubMed Google Scholar * van Dokkum, R. P., Sun, C. W., Provoost, A. P., Jacob, H. J. & Roman,
R. J. Altered renal hemodynamics and impaired myogenic responses in the fawn-hooded rat. _Ame. J. Physiol._ 276, R855–R863 (1999). Google Scholar * Friedrich, C., Endlich, N., Kriz, W.
& Endlich, K. Podocytes are sensitive to fluid shear stress in vitro. _Am. J. Physiol. Renal Physiol._ 291, F856–F865 (2006). Article CAS PubMed Google Scholar * Endlich, N. et al.
Podocytes respond to mechanical stress in vitro. _J. Am. Soc. Nephrol._ 12, 413–422 (2001). Article PubMed Google Scholar * Endlich, N. & Endlich, K. The challenge and response of
podocytes to glomerular hypertension. _Seminars Nephrol._ 32, 327–341 (2012). Article CAS Google Scholar * Endlich, K., Kliewe, F. & Endlich, N. Stressed podocytes-mechanical forces,
sensors, signaling and response. _Pflugers Archiv: Eur. J. Physiol._ 469, 937–949 (2017). Article CAS Google Scholar * Martinac, B. Mechanosensitive ion channels: molecules of
mechanotransduction. _J. Cell Sci._ 117, 2449–2460 (2004). Article CAS PubMed Google Scholar * Nourse, J. L. & Pathak, M. M. How cells channel their stress: Interplay between Piezo1
and the cytoskeleton. _Seminars Cell Dev. Biol._ 71, 3–12 (2017). Article CAS Google Scholar * Bershadsky, A. D., Balaban, N. Q. & Geiger, B. Adhesion-dependent cell
mechanosensitivity. _Ann. Rev. Cell Dev. Biol._ 19, 677–695 (2003). Article CAS Google Scholar * Ingber, D. E. Tensegrity II. How structural networks influence cellular information
processing networks. _J. Cell Sci._ 116, 1397–1408 (2003). Article CAS PubMed Google Scholar * Endlich, N. & Endlich, K. Stretch, tension and adhesion - adaptive mechanisms of the
actin cytoskeleton in podocytes. _Eur. J. Cell Biol._ 85, 229–234 (2006). Article CAS PubMed Google Scholar * Naruse, K., Yamada, T., Sai, X. R., Hamaguchi, M. & Sokabe, M. Pp125FAK
is required for stretch dependent morphological response of endothelial cells. _Oncogene_ 17, 455–463 (1998). Article CAS PubMed Google Scholar * Chen, K. D. et al. Mechanotransduction
in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. _J. Biol. Chem._ 274, 18393–18400 (1999). Article CAS PubMed Google Scholar * Helmke, B. P. &
Davies, P. F. The cytoskeleton under external fluid mechanical forces: hemodynamic forces acting on the endothelium. _Ann. Biomed. Eng._ 30, 284–296 (2002). Article PubMed Google Scholar
* Katsumi, A., Orr, A. W., Tzima, E. & Schwartz, M. A. Integrins in mechanotransduction. _J. Biol. Chem._ 279, 12001–12004 (2004). Article CAS PubMed Google Scholar * Kuo, J.-C.
Focal adhesions function as a mechanosensor. _Progr. Mol. Biol. Transl. Sci._ 126, 55–73 (2014). Article Google Scholar * Parsons, J. T., Horwitz, A. R. & Schwartz, M. A. Cell
adhesion: integrating cytoskeletal dynamics and cellular tension. _Nat. Rev. Mol. Cell Biol._ 11, 633–643 (2010). Article CAS PubMed PubMed Central Google Scholar * Sun, Z., Guo, S. S.
& Fässler, R. Integrin-mediated mechanotransduction. _J. Cell Biol._ 215, 445–456 (2016). Article CAS PubMed PubMed Central Google Scholar * Wozniak, M. A., Modzelewska, K., Kwong,
L. & Keely, P. J. Focal adhesion regulation of cell behavior. _Biochimica et Biophysica Acta_ 1692, 103–119 (2004). Article CAS PubMed Google Scholar * Hirata, H., Tatsumi, H. &
Sokabe, M. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. _J. Cell Sci._ 121, 2795–2804 (2008). Article CAS PubMed Google Scholar *
Yoshigi, M., Hoffman, L. M., Jensen, C. C., Yost, H. J. & Beckerle, M. C. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal
reinforcement. _J. Cell Biol._ 171, 209–215 (2005). Article CAS PubMed PubMed Central Google Scholar * Wang, Y.-X., Wang, D.-Y., Guo, Y.-C. & Guo, J. Zyxin: a mechanotransductor to
regulate gene expression. _Eur. Rev. Med. Pharmacol. Sci._ 23, 413–425 (2019). PubMed Google Scholar * Nix, D. A. & Beckerle, M. C. Nuclear-cytoplasmic shuttling of the focal contact
protein, zyxin: a potential mechanism for communication between sites of cell adhesion and the nucleus. _J. Cell Biol._ 138, 1139–1147 (1997). Article CAS PubMed PubMed Central Google
Scholar * Mori, M. et al. Zyxin mediates actin fiber reorganization in epithelial-mesenchymal transition and contributes to endocardial morphogenesis. _Mol. Biol. Cell_ 20, 3115–3124
(2009). Article CAS PubMed PubMed Central Google Scholar * Fradelizi, J. et al. ActA and human zyxin harbour Arp2/3-independent actin-polymerization activity. _Nat. Cell Biol._ 3,
699–707 (2001). Article CAS PubMed Google Scholar * Beckerle, M. C. Zyxin: Zinc fingers at sites of cell adhesion. _BioEssays_ 19, 949–957 (1997). Article CAS PubMed Google Scholar *
Smith, M. A. et al. A zyxin-mediated mechanism for actin stress fiber maintenance and repair. _Dev. Cell_ 19, 365–376 (2010). Article CAS PubMed PubMed Central Google Scholar *
Hoffman, L. M., Jensen, C. C., Chaturvedi, A., Yoshigi, M. & Beckerle, M. C. Stretch-induced actin remodeling requires targeting of zyxin to stress fibers and recruitment of actin
regulators. _Mol. Biol. Cell_ 23, 1846–1859 (2012). Article CAS PubMed PubMed Central Google Scholar * Drees, B. E., Andrews, K. M. & Beckerle, M. C. Molecular dissection of zyxin
function reveals its involvement in cell motility. _J. Cell Biol._ 147, 1549–1560 (1999). Article CAS PubMed PubMed Central Google Scholar * Zamir, E. & Geiger, B. Components of
cell-matrix adhesions. _J. Cell Sci._ 114, 3577–3579 (2001). Article CAS PubMed Google Scholar * Li, B. & Trueb, B. Analysis of the alpha-actinin/zyxin interaction. _J. Biol. Chem._
276, 33328–33335 (2001). Article CAS PubMed Google Scholar * Reinhard, M. et al. An alpha-actinin binding site of zyxin is essential for subcellular zyxin localization and alpha-actinin
recruitment. _J. Biol. Chem._ 274, 13410–13418 (1999). Article CAS PubMed Google Scholar * Steele, A. N., Sumida, G. M. & Yamada, S. Tandem zyxin LIM sequences do not enhance force
sensitive accumulation. _Biochem. Biophys. Res. Commun._ 422, 653–657 (2012). Article CAS PubMed PubMed Central Google Scholar * Li, B., Zhuang, L. & Trueb, B. Zyxin interacts with
the SH3 domains of the cytoskeletal proteins LIM-nebulette and Lasp-1. _J. Biol. Chem._ 279, 20401–20410 (2004). Article CAS PubMed Google Scholar * Yi, J. et al. Members of the Zyxin
family of LIM proteins interact with members of the p130Cas family of signal transducers. _J. Biol. Chem._ 277, 9580–9589 (2002). Article CAS PubMed Google Scholar * Smith, M. A.,
Hoffman, L. M. & Beckerle, M. C. LIM proteins in actin cytoskeleton mechanoresponse. _Trends Cell Biol._ 24, 575–583 (2014). Article CAS PubMed PubMed Central Google Scholar *
Uemura, A., Nguyen, T.-N., Steele, A. N. & Yamada, S. The LIM domain of zyxin is sufficient for force-induced accumulation of zyxin during cell migration. _Biophys. J._ 101, 1069–1075
(2011). Article CAS PubMed PubMed Central Google Scholar * Smith, M. A. et al. LIM domains target actin regulators paxillin and zyxin to sites of stress fiber strain. _PLoS ONE_ 8,
e69378 (2013). Article CAS PubMed PubMed Central Google Scholar * Al-Hasani, J. et al. Zyxin protects from hypertension-induced cardiac dysfunction. _Cell. Mol. Life Sci._ 79, 93
(2022). Article CAS PubMed PubMed Central Google Scholar * Ransick, A. et al. Single-cell profiling reveals sex, lineage, and regional diversity in the mouse kidney. _Dev. Cell_ 51,
399–413.e7 (2019). Article CAS PubMed PubMed Central Google Scholar * Kliewe, F. et al. Studying the role of fascin-1 in mechanically stressed podocytes. _Sci. Rep._ 7, 9916 (2017).
Article PubMed PubMed Central Google Scholar * Krämer, A., Green, J., Pollard, J. & Tugendreich, S. Causal analysis approaches in ingenuity pathway analysis. _Bioinformatics (Oxford,
England)_ 30, 523–530 (2014). PubMed Google Scholar * Siegerist, F. et al. Structured illumination microscopy and automatized image processing as a rapid diagnostic tool for podocyte
effacement. _Sci. Rep._ 7, 11473 (2017). Article PubMed PubMed Central Google Scholar * Feng, D., DuMontier, C. & Pollak, M. R. Mechanical challenges and cytoskeletal impairments in
focal segmental glomerulosclerosis. _Am. J. Physiol.-Renal Physiol._ 314, F921–F925 (2018). Article CAS PubMed PubMed Central Google Scholar * Naik, A. S. et al. Podocyte stress and
detachment measured in urine are related to mean arterial pressure in healthy humans. _Kidney Int._ 98, 699–707 (2020). Article CAS PubMed PubMed Central Google Scholar * Yao, M. et al.
The mechanical response of talin. _Nat. Commun._ 7, 11966 (2016). Article PubMed PubMed Central Google Scholar * Li, G. et al. Zyxin-involved actin regulation is essential in the
maintenance of vinculin focal adhesion and chondrocyte differentiation status. _Cell Proliferation_ 52, e12532 (2019). Article PubMed Google Scholar * Ghosh, S. et al. Loss of the
mechanotransducer zyxin promotes a synthetic phenotype of vascular smooth muscle cells. _J. Am. Heart Assoc._ 4, e001712 (2015). Article PubMed PubMed Central Google Scholar * Reinhard,
M., Jouvenal, K., Tripier, D. & Walter, U. Identification, purification, and characterization of a zyxin-related protein that binds the focal adhesion and microfilament protein VASP
(vasodilator-stimulated phosphoprotein). _Proc. Natl. Acad. Sci. USA._ 92, 7956–7960 (1995). Article CAS PubMed PubMed Central Google Scholar * Reinhard, M., Rüdiger, M., Jockusch, B.
M. & Walter, U. VASP interaction with vinculin: a recurring theme of interactions with proline-rich motifs. _FEBS Lett._ 399, 103–107 (1996). Article CAS PubMed Google Scholar *
Hoffman, L. M. et al. Genetic ablation of zyxin causes Mena/VASP mislocalization, increased motility, and deficits in actin remodeling. _J. Cell Biol._ 172, 771–782 (2006). Article CAS
PubMed PubMed Central Google Scholar * Holt, M. R., Critchley, D. R. & Brindle, N. P. The focal adhesion phosphoprotein, VASP. _Int. J. Biochem. Cell Biol._ 30, 307–311 (1998).
Article CAS PubMed Google Scholar * Samarin, S. et al. How VASP enhances actin-based motility. _J. Cell Biol._ 163, 131–142 (2003). Article CAS PubMed PubMed Central Google Scholar
* Mise, N. et al. Zyxin is a transforming growth factor-β (TGF-β)/Smad3 target gene that regulates lung cancer cell motility via integrin α5β1. _J. Biol. Chem._ 287, 31393–31405 (2012).
Article CAS PubMed PubMed Central Google Scholar * Rottner, K., Krause, M., Gimona, M., Small, J. V. & Wehland, J. Zyxin is not colocalized with vasodilator-stimulated
phosphoprotein (VASP) at lamellipodial tips and exhibits different dynamics to vinculin, paxillin, and VASP in focal adhesions. _Mol. Biol. Cell_ 12, 3103–3113 (2001). Article CAS PubMed
PubMed Central Google Scholar * Nguyen, T. N., Uemura, A., Shih, W. & Yamada, S. Zyxin-mediated actin assembly is required for efficient wound closure. _J. Biol. Chem._ 285,
35439–35445 (2010). Article CAS PubMed PubMed Central Google Scholar * Cheah, J. S. et al. Spatial proximity of proteins surrounding zyxin under force-bearing conditions. _Mol. Biol.
Cell_ 32, 1221–1228 (2021). Article CAS PubMed PubMed Central Google Scholar * Bos, J. L., Rehmann, H. & Wittinghofer, A. GEFs and GAPs: Critical elements in the control of small G
proteins. _Cell_ 129, 865–877 (2007). Article CAS PubMed Google Scholar * Guan, X., Guan, X., Dong, C. & Jiao, Z. Rho GTPases and related signaling complexes in cell migration and
invasion. _Exp. Cell Res._ 388, 111824 (2020). Article CAS PubMed Google Scholar * Ridley, A. J. Rho GTPase signalling in cell migration. _Curr. Opin. Cell Biol._ 36, 103–112 (2015).
Article CAS PubMed PubMed Central Google Scholar * Sun, Z., Huang, S., Li, Z. & Meininger, G. A. Zyxin is involved in regulation of mechanotransduction in arteriole smooth muscle
cells. _Front. Physiol._ 3, 472 (2012). Article CAS PubMed PubMed Central Google Scholar * Yip, A. K. et al. Zyxin is involved in fibroblast rigidity sensing and durotaxis. _Front. Cell
Dev. Biol._ 9, 735298 (2021). Article PubMed PubMed Central Google Scholar * Elkhatib, N. et al. Fascin plays a role in stress fiber organization and focal adhesion disassembly. _Curr.
Biol._ 24, 1492–1499 (2014). Article CAS PubMed Google Scholar * Jayo, A. & Parsons, M. Fascin: a key regulator of cytoskeletal dynamics. _Int. J. Biochem. Cell Biol._ 42, 1614–1617
(2010). Article CAS PubMed Google Scholar * Vignjevic, D. et al. Role of fascin in filopodial protrusion. _J. Cell Biol._ 174, 863–875 (2006). Article CAS PubMed PubMed Central
Google Scholar * Holstein-Rathlou, N. H., Wagner, A. J. & Marsh, D. J. Tubuloglomerular feedback dynamics and renal blood flow autoregulation in rats. _Am. J. Physiol.-Renal Physiol._
260, F53–F68 (1991). Article CAS Google Scholar * Schmidt, U., Weigert, M., Broaddus, C. & Myers, G. Cell Detection with Star-Convex Polygons. In _Medical Image Computing and Computer
Assisted Intervention – MICCAI 2018_, edited by A. F. Frangi, J. A. Schnabel, C. Davatzikos, C. Alberola-López & G. Fichtinger (Springer International Publishing, Cham), Vol. 11071, pp.
265–273. (2018). * Hoffman, L. M. et al. Targeted disruption of the murine zyxin gene. _Mol. Cellular Biol._ 23, 70–79 (2003). Article CAS Google Scholar * Schordan, S. et al. OPN
deficiency results in severe glomerulosclerosis in uninephrectomized mice. _Am. J. Physiol.-Renal Physiol._ 304, F1458–F1470 (2013). Article CAS PubMed Google Scholar * Pfaffl, M. W. A
new mathematical model for relative quantification in real-time RT-PCR. _Nucleic Acids Res._ 29, e45 (2001). Article CAS PubMed PubMed Central Google Scholar * Schindelin, J. et al.
Fiji: an open-source platform for biological-image analysis. _Nat. Methods_ 9, 676–682 (2012). Article CAS PubMed Google Scholar * Rueden, C. T. et al. ImageJ2: ImageJ for the next
generation of scientific image data. _BMC Bioinf._ 18, 529 (2017). Article Google Scholar * Suomi, T. & Elo, L. L. Enhanced differential expression statistics for data-independent
acquisition proteomics. _Sci. Rep._ 7, 5869 (2017). Article PubMed PubMed Central Google Scholar * Wu, T. et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics
data. _Innovation (Cambridge (Mass.))_ 2, 100141 (2021). CAS Google Scholar * Artelt, N. et al. The Role of Palladin in Podocytes. _J. Am. Soc. Nephrol._ 29, 1662–1678 (2018). Article CAS
PubMed PubMed Central Google Scholar * Cohen, C. D., Frach, K., Schlöndorff, D. & Kretzler, M. Quantitative gene expression analysis in renal biopsies: a novel protocol for a
high-throughput multicenter application. _Kidney Int._ 61, 133–140 (2002). Article CAS PubMed Google Scholar * Martini, S. et al. Integrative biology identifies shared transcriptional
networks in CKD. _J. Am. Soc. Nephrol._ 25, 2559–2572 (2014). Article CAS PubMed PubMed Central Google Scholar * Cohen, C. D. et al. Comparative promoter analysis allows de novo
identification of specialized cell junction-associated proteins. _Proc. Natl. Acad. Sci. USA._ 103, 5682–5687 (2006). Article CAS PubMed PubMed Central Google Scholar * Tusher, V. G.,
Tibshirani, R. & Chu, G. Significance analysis of microarrays applied to the ionizing radiation response. _Proc. Natl. Acad. Sci. USA._ 98, 5116–5121 (2001). Article CAS PubMed PubMed
Central Google Scholar * Kliewe, F. et al. Fibronectin is up-regulated in podocytes by mechanical stress. _FASEB J._ 33, 14450–14460 (2019). Article CAS PubMed Google Scholar *
Siegerist, F. et al. ScoMorphoFISH: A deep learning enabled toolbox for single-cell single-mRNA quantification and correlative (ultra-)morphometry. _J. Cellular Mol. Med._ 26, 3513–3526
(2022). Article CAS Google Scholar * Tinevez, J.-Y. et al. TrackMate: An open and extensible platform for single-particle tracking. _Methods (San Diego, Calif.)_ 115, 80–90 (2017).
Article CAS PubMed Google Scholar * Ershov, D. et al. TrackMate 7: integrating state-of-the-art segmentation algorithms into tracking pipelines. _Nat. Methods_ 19, 829–832 (2022).
Article CAS PubMed Google Scholar * Ju, W. et al. Defining cell-type specificity at the transcriptional level in human disease. _Genome Res._ 23, 1862–1873 (2013). Article CAS PubMed
PubMed Central Google Scholar * Hodgin, J. B. et al. A molecular profile of focal segmental glomerulosclerosis from formalin-fixed, paraffin-embedded tissue. _Am. J. Pathol._ 177,
1674–1686 (2010). Article CAS PubMed PubMed Central Google Scholar * Neusser, M. A. et al. Human nephrosclerosis triggers a hypoxia-related glomerulopathy. _Am. J. Pathol._ 176, 594–607
(2010). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank Claudia Weber and Mandy Weise for technical assistance. This work was supported
by the Research Network Molecular Medicine of the University Medicine Greifswald [FOVB-2021-05] and by grants of the Federal Ministry of Education and Research (BMBF, grant 01GM1518B;
STOP‐FSGS and grant 01ZX1908B; 01ZX2208B, Sys_CARE) to Nicole Endlich and Uwe Völker. Maja Lindenmeyer is supported by BMBF, grant 01GM1518A; STOP‐FSGS and BMBF grant 01EK2105D, UPTAKE. We
thank all participating centers of the European Renal cDNA Bank-Kroener-Fresenius Biopsy Bank (ERCB-KFB) and their patients for their cooperation. Active members at the time of the study are
listed in ref. (Martini et al. JASN 2014, Vol 25, No 11, 2559-2572). This work was generously supported by the Südmeyer Stiftung für Nieren- und Gefäßforschung and the Dr. Gerhard
Büchtemann fund, Hamburg, Germany. Christoph Daniel and Kerstin Amann are supported by the DFG Project-ID 509149993, TRR 374, SP C2. The funders had no role in study design, data collection
and analysis, decision to publish, or preparation of the manuscript. FUNDING Open Access funding enabled and organized by Projekt DEAL. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Anatomy and Cell Biology, University Medicine Greifswald, Greifswald, Germany Felix Kliewe, Florian Siegerist, Theodor Rolf Jakob Amling, Jonas Zeno Eddy Hollemann, Maximilian
Schindler & Nicole Endlich * Interfaculty Institute for Genetics and Functional Genomics, University Medicine Greifswald, Greifswald, Germany Elke Hammer & Uwe Völker * Department of
Cardiovascular Physiology, Heidelberg University, Heidelberg, Germany Jaafar Al-Hasani & Markus Hecker * NIPOKA GmbH, Center of High-End Imaging, Greifswald, Germany Vedran Drenic &
Nicole Endlich * Institute of Bioinformatics, University Medicine Greifswald, Greifswald, Germany Stefan Simm * Department of Nephropathology; Friedrich-Alexander University (FAU)
Erlangen-Nuremberg, Erlangen, Germany Kerstin Amann & Christoph Daniel * III. Department of Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany Maja Lindenmeyer *
Hamburg Center for Kidney Health (HCKH), University Medical Center Hamburg-Eppendorf, Hamburg, Germany Maja Lindenmeyer Authors * Felix Kliewe View author publications You can also search
for this author inPubMed Google Scholar * Florian Siegerist View author publications You can also search for this author inPubMed Google Scholar * Elke Hammer View author publications You
can also search for this author inPubMed Google Scholar * Jaafar Al-Hasani View author publications You can also search for this author inPubMed Google Scholar * Theodor Rolf Jakob Amling
View author publications You can also search for this author inPubMed Google Scholar * Jonas Zeno Eddy Hollemann View author publications You can also search for this author inPubMed Google
Scholar * Maximilian Schindler View author publications You can also search for this author inPubMed Google Scholar * Vedran Drenic View author publications You can also search for this
author inPubMed Google Scholar * Stefan Simm View author publications You can also search for this author inPubMed Google Scholar * Kerstin Amann View author publications You can also search
for this author inPubMed Google Scholar * Christoph Daniel View author publications You can also search for this author inPubMed Google Scholar * Maja Lindenmeyer View author publications
You can also search for this author inPubMed Google Scholar * Markus Hecker View author publications You can also search for this author inPubMed Google Scholar * Uwe Völker View author
publications You can also search for this author inPubMed Google Scholar * Nicole Endlich View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
The study was designed by F.K., N.E.; F.K., J.Z.E.H. contributed to the cell culture experiments; biopsies were handled and analyzed by F.K., F.S., M.T.L., C.D. and K.A.; LC-MS/MS was
performed by E.H. and U.V.; animal experiments were performed by F.S., J.A., M.H. and F.K.; western blots were performed and quantified by F.K., T.R.J.A. and J.Z.E.H.; V.D. performed PEMP;
all other experiments were performed by F.K.; experimental data were analyzed by F.K., F.S., E.H., U.V., S.S., M.S., T.R.J.A, J.Z.E.H.; F.K. and N.E. wrote the main manuscript text. F.K. and
F.S. prepared figures. All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Felix Kliewe. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. PEER REVIEW PEER REVIEW INFORMATION _Communications Biology_ thanks Heon Gee, John Crean, and Justin Chun for their contribution to the peer review of this work. Primary Handling
Editors: Ashwani Gupta, Anam Akhtar and Christina Karlsson Rosenthal. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2
REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Kliewe, F., Siegerist, F., Hammer, E. _et al._ Zyxin is important for the stability and function of podocytes, especially during mechanical stretch. _Commun Biol_ 7, 446
(2024). https://doi.org/10.1038/s42003-024-06125-5 Download citation * Received: 18 July 2023 * Accepted: 29 March 2024 * Published: 11 April 2024 * DOI:
https://doi.org/10.1038/s42003-024-06125-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
