A viral assembly inhibitor blocks sars-cov-2 replication in airway epithelial cells
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The ongoing evolution of SARS-CoV-2 to evade vaccines and therapeutics underlines the need for innovative therapies with high genetic barriers to resistance. Therefore, there is
pronounced interest in identifying new pharmacological targets in the SARS-CoV-2 viral life cycle. The small molecule PAV-104, identified through a cell-free protein synthesis and assembly
screen, was recently shown to target host protein assembly machinery in a manner specific to viral assembly. In this study, we investigate the capacity of PAV-104 to inhibit SARS-CoV-2
replication in human airway epithelial cells (AECs). We show that PAV-104 inhibits >99% of infection with diverse SARS-CoV-2 variants in immortalized AECs, and in primary human AECs
cultured at the air-liquid interface (ALI) to represent the lung microenvironment in vivo. Our data demonstrate that PAV-104 inhibits SARS-CoV-2 production without affecting viral entry,
mRNA transcription, or protein synthesis. PAV-104 interacts with SARS-CoV-2 nucleocapsid (N) and interferes with its oligomerization, blocking particle assembly. Transcriptomic analysis
reveals that PAV-104 reverses SARS-CoV-2 induction of the type-I interferon response and the maturation of nucleoprotein signaling pathway known to support coronavirus replication. Our
findings suggest that PAV-104 is a promising therapeutic candidate for COVID-19 with a mechanism of action that is distinct from existing clinical management approaches. SIMILAR CONTENT
BEING VIEWED BY OTHERS SARS-COV-2 PROMOTES RIPK1 ACTIVATION TO FACILITATE VIRAL PROPAGATION Article Open access 18 October 2021 STRUCTURAL BIOLOGY OF SARS-COV-2 AND IMPLICATIONS FOR
THERAPEUTIC DEVELOPMENT Article 17 September 2021 PROLYL ISOMERASE PIN1 PLAYS AN ESSENTIAL ROLE IN SARS-COV-2 PROLIFERATION, INDICATING ITS POSSIBILITY AS A NOVEL THERAPEUTIC TARGET Article
Open access 17 September 2021 INTRODUCTION Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the etiological agent of the ongoing COVID-19 pandemic, belongs to a highly
contagious betacoronavirus1. The high transmission and variation of SARS-CoV-2 poses an ongoing threat to global public health. Despite multiple vaccine options, only 65.4% of people are
currently fully vaccinated worldwide, due in part to lack of vaccine access as well as behavioral resistance to vaccination. Moreover, many people remain at high risk for severe COVID-19 due
to decreased vaccine efficacy and increased risk of respiratory failure associated with immune compromise. For the treatment of SARS-CoV-2, anti-SARS-CoV-2 monoclonal antibodies (mAbs) that
target the spike protein represent one class of therapeutic candidates approved by the FDA for COVID-19 patients2,3. However, the efficacy of anti-SARS-CoV-2 mAbs is negligible in the face
of currently circulating viral variants4. Beyond mAbs, antiviral small-molecule drugs have been developed that target specific parts of the viral life cycle to prevent infectivity, severe
illness, and death attributed to COVID-19. Three antiviral agents that have been shown to directly inhibit SARS-CoV-2 replication in vitro are currently authorized by the FDA or FDA-granted
emergency use authorization (EUA) for the treatment of COVID-19: viral RNA-dependent RNA polymerase (RdRp) inhibitors, remdesivir and molnupiravir4,5,6, and a viral 3C-like protease
inhibitor, paxlovid, which consists of nirmatrelvir and ritonavir7. Clinical studies have shown that remdesivir is not associated with statistically significant clinical benefits8. In vitro
studies have shown that molnupiravir exposure may increase the risk of mutagenesis in the host genome9,10. Paxlovid treatment is often associated with COVID-19 rebound following the
treatment cycle11. Taken together, these realities illustrate the need for effective and broad-spectrum antiviral drugs for COVID-19 with minimal off-target effects. Recent studies have
focused on the SARS-CoV-2 viral life cycle to find additional targets for drug therapy, providing candidate SARS-CoV-2 entry and attachment inhibitors12,13, viral protease inhibitors14,15,
and N assembly inhibitors that reduce viral nucleocapsid (N)-genome RNA interaction16,17. However, data are limited describing agents that selectively inhibit the late stage of the
SARS-CoV-2 viral life cycle of particle formation/budding18. The viral assembly/budding process is a dynamic program dependent on transient multi-protein assembly complexes19. One approach
to identifying these host-viral drug targets is by interrogation of the pathway of viral assembly/budding in cell-free protein synthesis and assembly systems20,21,22. Small molecules have
been identified in this manner that are active against various viral families, including those of rabies virus, HIV-1, and influenza virus23,24,25. The small-molecule PAV-104, identified
through a moderate-throughput screen involving cell-free protein synthesis and assembly (CFPSA), was recently shown to target host protein assembly machinery in a manner specific to viral
assembly24. This compound has minimal host toxicity, and once-daily oral dosing in rats achieves >200-fold of the 90% effective concentration (EC90) in blood. Pharmacokinetic studies of
PAV-104 in rats showed a lung-to-plasma ratio of 0.3 after oral administration24. The chemotype shows broad activity against respiratory viral pathogens, including _Orthomyxoviridae_,
_Paramyxoviridae_, _Adenoviridae_, _Herpesviridae_, and _Picornaviridae_, with low susceptibility to evolutionary escape. Here, we evaluated the antiviral effect of PAV-104 against
SARS-CoV-2 infection in immortalized and primary human airway epithelial cells (AECs), and elucidated the mechanism underlying this antiviral activity. Our results show that PAV-104
treatment potently inhibits the replication of diverse SARS-CoV-2 variants. We further demonstrate that PAV-104 specifically inhibits the late stages of the SARS-CoV-2 replication cycle,
perturbing the oligomerization of viral N, and thereby blocking viral capsid assembly and budding. PAV-104 treatment also reverses SARS-CoV-2 induction of the type-I interferon (IFN)
response and the maturation of nucleoprotein signaling pathway, which supports coronavirus replication within the target cell. Together, our data suggest that PAV-104 is a promising
therapeutic candidate for COVID-19, and its mechanism of action is distinct from existing clinical strategies. RESULTS PAV-104 SUPPRESSES SARS-COV-2 INFECTION IN CALU-3 CELLS The synthesis
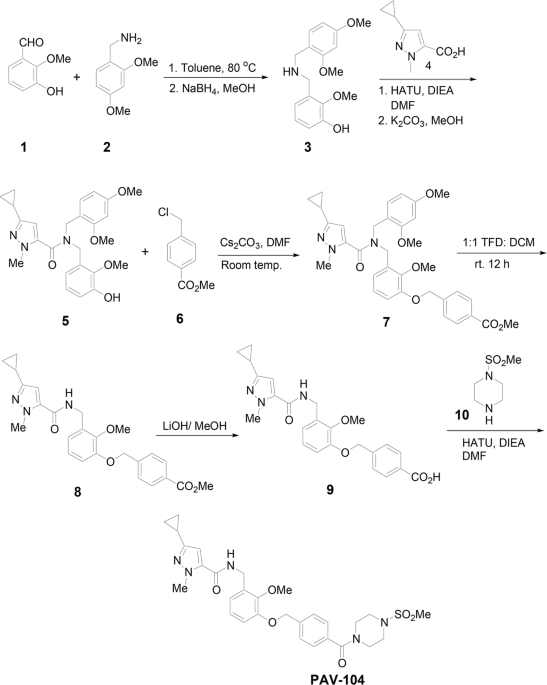
and molecular structure of PAV-104 (Fig. 1), a small-molecule drug recently associated with pan-respiratory virus antiviral activity24, is described in detail in the Methods section. To
investigate the impact of PAV-104 on SARS-CoV-2 infection, we first determined the cytotoxicity of PAV-104, to optimize dosing in Calu-3 cells. The 50% cytotoxic concentration (CC50) value
detected by MTT assay was 1306 nM for PAV-104 in Calu-3 cells (Fig. 2a). Next, we evaluated the effects of PAV-104 on SARS-CoV-2 (USA-WA1/2020) replication at 6.25 nM, 25 nM, 50 nM, and 100
nM concentrations. Calu-3 cells were pretreated for one hour with PAV-104 followed by infection with SARS-CoV-2 at an MOI of 0.01, and PAV-104 was maintained in the media until 24 h
following viral infection. SARS-CoV-2 replication, as measured by quantitation of viral nucleocapsid (_N_) gene expression, was decreased significantly by treatment with PAV-104 in a
dose-dependent manner (_p_ < 0.01) (Fig. 2b). Similarly, release of infectious virus in the supernatant was suppressed significantly by PAV-104 in a dose-dependent manner, as measured by
median tissue culture infectious dose (TCID50) (_p_ < 0.01) (Fig. 2c), with up to 75-fold reduction at the highest concentration of PAV-104. The inhibition of virus production by PAV-104
was confirmed by using an immunofluorescence assay (IFA) (Fig. 2d, e). Taken together, these data demonstrate that PAV-104 decreases SARS-CoV-2 viral production in susceptible Calu-3 cells.
PAV-104 INHIBITS SARS-COV-2 MORE POTENTLY THAN REMDESIVIR IN CALU-3 CELLS Remdesivir is the first approved small-molecule anti-SARS-CoV-2 drug for COVID-19 treatment and shows potent
anti-SARS-CoV-2 activity in Calu-3 cells26,27. To compare the anti-SARS-CoV-2 efficacies of PAV-104 with remdesivir in Calu-3 cells, Calu-3 cells were infected with SARS-CoV-2 (USA-WA1/2020)
for 48 h at the MOI of 0.001 and treated with varying doses of PAV-104 or remdesivir. Cells and supernatants were harvested for quantification of _N_ expression by RT-qPCR, and
quantification of infectious viral titers by TCID50, respectively. Both compounds displayed dose-dependent inhibition of viral replication (Fig. 3a–d). Remdesivir inhibited SARS-CoV-2 with a
mean 90% maximal EC90 value of 219.9 nM, as determined by RT-qPCR measurement of the SARS-CoV-2 _N_ gene (Fig. 3b). PAV-104 was more potent (mean EC90 = 24.5 nM) than remdesivir (_p_ <
0.0005) (Fig. 3b). EC50 value determined by quantification of infectious virus in the supernatants showed a similar trend (Fig. 3d). Thus, PAV-104 inhibits SARS-CoV-2 more potently than
remdesivir in Calu-3 cells. PAV-104 IS A HIGHLY POTENT ANTIVIRAL INHIBITOR OF SARS-COV-2 IN PRIMARY AIRWAY EPITHELIAL CELLS Upper and lower airways in humans are known to be the first
gateway for SARS-CoV-2 infection28. To investigate the antiviral activity of PAV-104 against SARS-CoV-2 in human primary AECs, we performed antiviral assays in air/liquid interface
(ALI)-cultured AECs, which is useful in modeling the in vivo effects of PAV-104 on SARS-CoV-2 infection ex vivo29. We pretreated primary AECs from three healthy donors with PAV-104 and then
infected them with the SARS-CoV-2 Gamma variant (Pango lineage designation P.1) for 36 h. In PAV-104-treated, SARS-CoV-2-infected AECs cultures, there was >99% inhibition of infection
with PAV-104 treatment at the highest tested concentration (_p_ < 0.01) (Fig. 4a). We also tested the antiviral effect of PAV-104 on the Delta and Omicron SARS-CoV-2 variants in AECs.
Administration of PAV-104 also significantly reduced Delta and Omicron replication in primary AECs (_p_ < 0.01) (Fig. 4b). Together, these data demonstrate that PAV-104 exerts potent
antiviral activity against a broad range of SARS-CoV-2 variants in primary AECs. PAV-104 INTERFERES WITH POST-ENTRY STEPS OF THE SARS-COV-2 LIFE CYCLE Since PAV-104 was identified based on
the inhibition of the viral particle assembly/budding process, we next sought to determine whether PAV-104 inhibits SARS-CoV-2 replication by acting on a post-entry step of the SARS-CoV-2
viral replication cycle as expected. We detected the effect of PAV-104 on SARS-CoV-2 entry by using VSV-SARS-CoV-2 spike-ΔG-luciferase reporter pseudovirus (hereafter referred to as
SARS-2-S). Positive serum (P serum), which was predetermined to possess SARS-CoV-2 neutralizing activity, potently reduced SARS-2-S infection (_p_ < 0.0001) but did not suppress the
infection of VSV-spike G glycoprotein-luciferase reporter pseudovirus (hereafter referred to as VSV-G). PAV-104 showed no effect on SARS-2-S infection or VSV-G infection, indicating that
PAV-104 has no effect on SARS-CoV-2 entry (Fig. 5a). To further clarify the stage of the virus replication cycle targeted by PAV-104, we infected Calu-3 cells with SARS-CoV-2 (USA-WA1/2020,
MOI = 0.01), and administered PAV-104 at different time points to enable specific assessment of drug effects on viral entry/attachment (early), and post-entry (late) stages of the viral life
cycle, using established experimental timelines30,31,32 (Fig. 5b). Our data showed that PAV-104 does not inhibit SARS-CoV-2 infection in the 1 h incubation assay, or the pre-infection
treatment condition (Fig. 5c), indicating that PAV-104 does not act on SARS-CoV-2 attachment or entry. Post-infection treatment with PAV-104 did strikingly reduce SARS-CoV-2 viral titer in
the supernatant as represented by TCID50 (_p_ < 0.01), as compared to post infection treatment with DMSO (negative control) or pre-infection treatment with PAV-104 (Fig. 5c). Consistent
with these data, SARS-CoV-2 replication in primary AECs was decreased significantly by treatment with PAV-104 in the post infection condition, but not in the pre-infection treatment scenario
(_p_ < 0.05) (Fig. 5d). These results suggest that PAV-104 activity can be entirely attributed to blocking the late stage of the SARS-CoV-2 viral life cycle after viral entry. PAV-104
BLOCKS SARS-COV-2 VIRAL PARTICLE FORMATION Transient coexpression of four SARS-CoV-2 structural proteins (N, M, E, and S) in cell culture has been shown to produce assembling virus-like
particles (VLPs), which can be used to study the viral life cycle such as assembly/budding, egress, and entry33,34. To explore whether PAV-104 results in the inhibition of SARS-CoV-2 viral
formation/budding, we quantified production of SARS-CoV-2 structural proteins in VLPs from cell culture supernatants of transfected HEK-293T cells treated with PAV-104 or DMSO. Viral
assembly was quantified by western blot and nanoparticle tracking analysis (NTA) of extracellular vesicles and viral particles. Western blots were performed on proteins from the pellet after
ultracentrifugation of transfected cell lysates and culture supernatants. PAV-104 significantly reduced structural protein production in the pellet collected from cell supernatants in a
dose-dependent manner (_p_ < 0.01), but did not inhibit structural protein synthesis and steady-state levels of actin in the cell lysates (Fig. 6a–c). Consistent with western blot data
(Fig. 6a), our NTA results showed that cells transfected with the four SARS-CoV-2 structural proteins displayed increased nanoparticle production as compared to cells transfected with empty
vectors (_p_ < 0.001) (Fig. 6d), reflecting production and release of SARS-CoV-2 VLPs. PAV-104 treatment inhibited the concentration of nanoparticles in the supernatants of cells
transfected with the four SARS-CoV-2 structural proteins in a dose-dependent manner (_p_ < 0.01) (Fig. 6d), while no effect on nanoparticle secretion in empty vector-transfected cell
supernatants was observed (suggesting that extracellular vesicle secretion is not affected by PAV-104). These data indicate that PAV-104 specifically inhibits VLP production in our model. To
further investigate the role of PAV-104 on viral particle formation, we infected Calu-3 cells with single-cycle infectious SARS-CoV-2 virus (hereafter referred to as ΔS-VRP) and treated
cells with PAV-104. Our data show that PAV-104 does not affect ΔS-VRP _N_ mRNA level (Fig. 7a) or protein synthesis (Fig. 7b, c). However, PAV-104 did significantly inhibit the concentration
of viral particles released into the supernatant (Fig. 7d). These data confirm that PAV-104 has no effect on protein synthesis, and blocks SARS-CoV-2 replication specifically through
targeting the viral assembly/budding process. PAV-104 INHIBITS THE OLIGOMERIZATION OF THE SARS-COV-2 N To investigate the main drug target of PAV-104 involved in the SARS-CoV-2 viral
particle assembly/budding process, drug resin affinity chromatography (DRAC) was performed24. PAV-104 was coupled to the 4% crosslinked agarose resin24. Cellular extracts from Calu-3 cells
with or without SARS-CoV-2 infection were incubated on the PAV-104 drug resin columns with or without PAV-104 covalently attached, allowing the target to bind. After washing, specifically
bound material was eluted with free drug (PAV-104), followed by stripping of the remaining bound material from the drug and control columns with 1% SDS. Based on western blotting with an
anti-SARS-CoV-2 N antibody, negligible SARS-CoV-2 N was bound to or eluted from control columns to which infected lysates were applied, while abundant SARS-CoV-2 N was bound to and eluted
from columns of PAV-104 attached to resin (Fig. 8a), indicating that SARS-CoV-2 N is a major component of the target multi-protein complex. No N reactivity was observed in columns loaded
with uninfected lysates as expected. The oligomerization of SARS-CoV-2 N has been demonstrated to be responsible for helping virus envelope formation and particle assembly35,36,37. To
determine if PAV-104 affects the oligomerization of SARS-CoV-2 N to inhibit SARS-CoV-2 viral particle assembly, cells were transfected with N in the presence or absence of PAV-104, followed
by analysis with glycerol gradient ultracentrifugation and a commercial ELISA kit to determine N concentrations in fractions. N-expressing cells treated with PAV-104 showed that there is a
reduction of the N intermediate complex (Fraction 20–22) as compared to DMSO treatment (Fig. 8b), indicating that the oligomerization of SARS-CoV-2 N is inhibited by PAV-104 treatment. These
data support a model in which PAV-104 directly or indirectly affects the oligomerization of SARS-CoV-2 N to inhibit viral particle formation/assembly. PAV-104 TREATMENT SUPPRESSES THE IFN
SIGNALING AND MATURATION OF NUCLEOPROTEIN GENE EXPRESSION PATHWAYS Finally, to understand the transcriptional impact of PAV-104 in the setting of SARS-CoV-2 infection and immunopathology, we
performed RNA-seq analysis on ALI-cultured primary AECs from five different healthy donors infected for 36 h with SARS-CoV-2 (MOI = 0.1) in the presence or absence of PAV-104. Uninfected,
untreated cells (Control) were characterized as a reference for both experimental conditions. The effects of PAV-104 administration alone (in the absence of SARS-CoV-2 infection) were
evaluated in three additional donors (Supplementary Fig. 1). Differentially expressed gene (DEG) analysis showed that 81 genes were significantly upregulated by SARS-CoV-2 infection alone
(Fig. 9a, Supplementary Data 1), with most of them being IFN-related genes, such as OAS3, HELZ2, IFIT3, OAS1, and ISG15. SARS-CoV-2 infection in the presence of PAV-104 exhibited a dramatic
impact on the host transcriptome when compared to uninfected control, with 10,255 DEGs identified (Fig. 9b, Supplementary Data 2), including 5843 downregulated and 4412 upregulated genes. In
addition, when compared with SARS-CoV-2 infection alone, SARS-CoV-2 infection in the presence of PAV-104 exhibited a distinct transcriptomic signature, with 9,319 DEGs identified (Fig. 9c,
Supplementary Data 3). We additionally examined the impact of PAV-104 treatment on SARS-CoV-2 mRNA levels in the spreading infection by aligning sequencing reads against the SARS-CoV-2
reference genome. The number of reads mapping to each region of the viral genome was calculated and interpreted to infer viral expression patterns (Fig. 9d)29. Consistent with the antiviral
effect of PAV-104, the mRNA levels of SARS-CoV-2 were profoundly reduced in the presence of PAV-104. These results confirm the highly potent antiviral activity of PAV-104 against SARS-CoV-2.
Fifteen genes that were significantly upregulated by SARS-CoV-2 infection were significantly down-regulated in the SARS-CoV-2 infection with PAV-104 treatment group: CXCL11, CXCL9, IFIT2,
IFIT3, DDX58, SAMD9L, OAS2, IFI44, USP18, SAMD9, DDX60, HERC6, XAF1, RSAD2, XAF1M, DDX60L. Most of these genes are IFN-stimulated genes (ISGs) (Fig. 9e). Gene set enrichment analysis (GSEA)
using Reactome database pathway definitions presents a counteracting pattern between the SARS-CoV-2 infection group and the PAV-104 treated group, suggesting that PAV-104 could reverse the
viral infection-regulated pathways (Fig. 9f). GSEA revealed that the IFN signaling pathway was the most upregulated pathway by SARS-CoV-2 infection (Fig. 9f, Supplementary Data 4).
Virus-induced IFN signaling was reversed by PAV-104 treatment. The regulation of genes involved in the IFN signaling pathway is shown in Supplementary Fig. 2a, and the modulation of select
ISGs was verified by RT-qPCR (Supplementary Fig. 2b). Of particular interest in our transcriptomic data is the set of genes related to the maturation of nucleoprotein pathway that are
selectively upregulated by SARS-CoV-2 infection but not by SARS-CoV-2 infection with PAV-104 treatment (Fig. 9f). SARS-CoV-2 nucleoprotein is found in the host cell cytosol, the nucleus and
plasma membrane38. The maturation of nucleoprotein signaling pathway, including oligomerization, ADP-ribosylation, phosphorylation, sumoylation, methylation, and other post-translational
modifications of nucleoprotein, is responsible for N movement, interaction with genomic RNAs, interaction with other proteins, and viral particle assembly17,39,40,41. The regulation of all
genes that are involved in the SARS-CoV-2 maturation of nucleoprotein pathway (as defined in the Reactome database) is shown in Supplementary Fig. 2c, and the modulation of select pathway
genes was verified by RT-qPCR (Supplementary Fig. 2d). SARS-CoV-2 infection increased the expression of the PARP9, PARP14, and PARP10 genes, but SARS-CoV-2 infection in the presence of
PAV-104 treatment down-regulated the expression of these genes (as compared to untreated/uninfected control). Beyond host factors defined by Reactome as members of the maturation of
nucleoprotein pathway, PIAS1, and SRPK3, which have been reported to play roles in SUMOylating42 and phosphorylating SARS-CoV-2 N43, respectively, were significantly down-regulated in the
SARS-CoV-2 infection with PAV-104 treatment group (Supplementary Fig. 2c). The protein arginine methyltransferase (PRMT) 5, 6, 7, and 9 genes were significantly down-regulated by PAV-104
treatment, and members of the PRMT family are known to methylate N17. Lastly, G3BP1, down-regulated by PAV-104 treatment, is known to be sequestered by SARS-CoV-2 N, leading to suppression
of the host immune response to favor virus replication44. Further unsupervised analysis of the gene expression data revealed four distinct clusters (Supplementary Fig. 3a). Gene Ontology
(GO) analysis demonstrated that SARS-CoV-2 induction of genes associated with the IFN signaling and response to chemokine pathways was reversed by PAV-104 treatment (cluster 2)
(Supplementary Fig. 3b). Genes associated with negative regulation of inclusion body assembly and protein refolding signaling pathways were induced by PAV-104 treatment (cluster 3)
(Supplementary Fig. 3b). Taken together, our findings demonstrate that PAV-104 modulates a diverse repertoire of host factors that are reported to be key players in the trafficking and
post-translation modifications of SARS-CoV-2 N that are critical to SARS-CoV-2 maturation and immune evasion. DISCUSSION The rapid emergence and spreading of the SARS-CoV-2 Omicron variant
that evades many monoclonal antibody therapies illustrates the need for antiviral treatments with low susceptibility to evolutionary escape. Capsid assembly is an essential step in the viral
life cycle mediated by the interaction of viral capsid proteins. Inhibition of this process can be used as a therapeutic approach; any proteins, any modifications, or any interactions that
participate in or stabilize viral particle assembly in the producer cell can be manipulated to inhibit assembly, prevent release, and protect as-yet-uninfected target cells from subsequent
infection. In our prior work, we identified three small molecules, PAV-431, PAV-471, and PAV-104, as inhibitors of influenza virus assembly using our cell-free protein synthesis and viral
assembly screening system24. We further demonstrated that PAV-104, in particular, exerted highly potent antiviral effects against Nipah virus with minimal toxicity24. Here, building on these
observations, we investigated the capacity of PAV-104 to inhibit SARS-CoV-2 infection. Our results show that PAV-104 inhibits SARS-CoV-2 replication in AECs, exhibiting potent antiviral
effects against multiple viral variants. We have established that PAV-104 interferes with a post-entry step of the SARS-CoV-2 life cycle and blocks SARS-CoV-2 viral particle assembly/budding
based on the following observations: (1) the chemotype of PAV-104 investigated here has no effects on early viral life cycle events (e.g., viral entry), (2) PAV-104 reduces virus release
into the cell culture supernatant, (3) PAV-104 treatment does not reduce steady-state levels of cellular proteins and does not impede the translation of viral structural proteins, and (4)
PAV-104 interacts with SARS-CoV-2 N and interferes with its oligomerization. Dimerization and oligomerization of SARS-CoV-2 N proteins are essential to enable associations with viral genomic
RNA and other viral structural proteins (M, E, and S), playing a critical role in virus particle assembly37,45. In addition to viral particle assembly, the coronavirus N is required for
viral mRNA and genome synthesis, viral core formation, and virus budding/envelope formation46. Given that some compounds that perturb capsid assembly can result in formation of noninfectious
virions47, PAV-104 effects could potentially extend beyond viral assembly/budding and attenuate the infectivity of SARS-CoV-2, further enhancing its antiviral activity. Previously, we
showed that PAV-104 bound a small subset of the known allosteric modulator 14-3-3, itself implicated in the interactome of SARS-CoV-224,48,49. Binding of phosphorylated SARS-CoV N to the
host 14-3-3 protein in the cytoplasm was reported to regulate nucleocytoplasmic N shutting and other functions of N50. In addition, human 14-3-3 proteins were reported to bind the mutational
hotspot region of SARS-CoV-2 N and modulate SARS-CoV-2 N phosphoregulation51. In accordance with these observations, our transcriptomic data showed that PAV-104 treatment negatively
regulates the maturation of nucleoprotein signaling pathway of SARS-CoV-1/2. For example, sumoylation of SARS-CoV-2 N protein can enhance its interaction affinity with itself and is critical
for its nuclear translocation, which is, in turn, critical for N-mediated viral RNA genome packaging and interaction with M protein41. Phosphorylation of SARS-CoV-2 N protein was reported
to be responsible for its localization, phase-phase separation, and interaction with host factors35. Moreover, our transcriptomic data showed that the assembly of inclusion bodies where
viral protein modification, aggregation, and assembly occurs52,53, was negatively regulated by PAV-104 treatment. The precise manner in which PAV-104 affects the post-translational
modification of SARS-CoV-2 N warrants additional investigation, which may reveal antiviral mechanisms and pharmacological targets. Our transcriptomic analysis also revealed that PAV-104
treatment of infected cells reversed SARS-CoV-2 induction of the IFN signaling pathway. IFN signaling is critical to antiviral responses54,55. To counteract host defense, multiple studies
have demonstrated that SARS-CoV-2 uses a multitude of mechanisms to avoid type-I IFN-mediated immune responses56. On the other hand, robust type-I IFN responses have been associated with
severe COVID-19 disease, and may exacerbate hyperinflammation during the development of severe COVID-1957,58. Therefore, beyond inhibition of viral replication, PAV-104 may exert adjunctive
anti-inflammatory effects via selective suppression of IFN pathway members that enhances its clinical potential as a therapeutic for COVID-19. In summary, our findings demonstrate that
PAV-104, a host-targeted pan-respiratory virus small-molecule inhibitor, is a promising therapeutic candidate for SARS-CoV-2. METHODS CELL LINES Human lung adenocarcinoma epithelial Calu-3
cells (ATCC, HTB-55) were cultured in Eagle’s Minimum Essential Medium (EMEM) (ATCC, 30-2003). African green monkey kidney Vero E6 cells (ATCC, CRL-1586) and human kidney HEK29T cells (ATCC,
CRL-3216) were cultured in Dulbecco’s Modified Eagle’s medium (DMEM) (Thermo Fisher Scientific, 11965092). All media were supplemented with 10% fetal bovine serum (FBS, Corning, 35010CV)
and 1% penicillin/streptomycin (Thermo Fisher Scientific, 10378016). Vero E6 cells stably expressing TMPRSS2 (Vero E6-TMPRSS2) were established and cultured in DMEM in the presence of
puromycin (1 µg/ml) (Thermo Fisher Scientific, A1113803). All cells had been previously tested for mycoplasma contamination and incubated at 37 °C in a humidified atmosphere with 5% CO2.
PRIMARY AECS Human unused donor tracheobronchial tissue was obtained at the time of lung transplant. The tissue was washed and placed in DMEM with 0.1% protease and antibiotics overnight at
4 °C. The next day, the solution was agitated, and the remaining tissue was removed. Cell pellets were treated with 0.05% trypsin-EDTA and then filtered through a cell strainer. Cells were
plated onto 6 mm/0.4 mm Transwell ALI insert (Corning, 3470) after treatment with FNC coating mixture (Athena Enzyme Systems, 0407). 10% FBS in DMEM and ALI media were added in equal volumes
to each basal compartment, and cultures were incubated at 37 °C with 5% CO2. The next day, the media was removed, and both compartments were washed with PBS and antibiotics. ALI media was
then added to each basal compartment and changed every three days for at least 28 days until differentiated airways were ready for use29. VIRUSES The severe acute respiratory syndrome
coronavirus-2 (SARS-CoV-2) strains USA-WA1/2022, lineage P.1 (Gamma), lineage B.1.617.2 (Delta), and lineage B.1.1.529 (Omicron) were obtained from BEI Resources of the National Institute of
Allergy and Infectious Diseases (NIAID) and propagated in Vero E6-TMPRSS2 cells. Virus titer was measured in Vero E6 cells by TCID50 assay. All the studies involving live viruses were
conducted in the Vitalant Research Institute BSL-3 under approved safety protocols. ETHICS STATEMENT The studies involving human participants were reviewed and approved by the Human Research
Protection Program, University of California, San Francisco. The patients/participants provided their written informed consent to participate in this study. All ethical regulations relevant
to human research participants were followed. DRUG CYTOTOXICITY ASSAY The cytotoxic effect of PAV-104 on Calu-3 cells was measured using an MTT assay kit (Abcam, ab211091) following the
manufacturer’s instructions. In brief, Calu-3 cells were seeded in 96-well cell culture plates. Appropriate concentrations of PAV-104 were added to the medium (0–5000 nM). After 48 h, the
media was removed, and 100 μl MTT reagent (1:1 dilution in DMEM medium (serum-free)) was added to each well and incubated for 3 h at 37 °C. Then the medium was removed, and 150 μl MTT
solvent was added to each well. Quantification was performed by reading absorbance at OD = 590 nm. The data from three independent experiments was used to calculate the CC50 by nonlinear
regression using GraphPad Prism 8.0 software. SARS-COV-2 INFECTION AND DRUG ADMINISTRATION Calu-3 cells were seeded at 0.5 × 106 cells per well in 0.5 ml volumes using a 24-well plate, or
were seeded at 1 × 105 cells per well in 0.1 ml volumes using a 96-well plate. The following day, cells were pretreated with or without PAV-104 or remdesivir (SIGMA, 1809249-37-3) for one
hour. Then viral inoculum (MOI of 0.01; 500 μl/well or 100 μl/well) was prepared using EMEM containing indicated concentrations of PAV-104 or remdesivir and added to the wells. The
inoculated plates were incubated at 37 °C with 5% CO2. At indicated infection time points, supernatants were collected and stored at −80 °C. Cells were lysed with TRizol (Thermo Fisher
Scientific, 15596026) for RNA extraction. For infection of primary AECs in ALI culture, cells were pretreated with PAV-104 in the basal compartment for one hour. SARS-CoV-2 (diluted in
ALI-culture medium, MOI = 0.1) was added to the apical chamber of inserts (250 μl) and the basal compartment (500 μl). Then the cultures were incubated for 2 h at 37 °C (5% CO2) to enable
virus entry. Subsequently, the cells were washed, and fresh ALI medium (500 μl) containing PAV-104 was added into the basal compartment. Cells were incubated at 37 °C (5% CO2) and harvested
for analysis at 36 h post infection. ΔS-VRP INFECTION AND DRUG ADMINISTRATION Stocks of ΔS-VRP were prepared by Dr. Manicassamy Lab59. Calu-3 cells were seeded at 1 × 106 cells per well in 1
mL volumes using a 12-well plate. The following day, cells were pretreated with or without PAV-104 for one hour. Then the viral inoculum (1 mL/12 wells) was prepared using EMEM containing
indicated concentrations of PAV-104 and added to the wells. The inoculated plates were incubated at 37 °C with 5% CO2 for 2 h. Cells were washed three times with PBS to remove extracellular
virus, and then supplied with EMEM containing indicated concentrations of PAV-104 for another 18 h. Supernatants were collected and stored at −80 °C for nanoparticle measurement. Cells were
lysed with TRizol (Thermo Fisher Scientific, 15596026) for RNA extraction or lysed with RIPA (Thermo Fisher Scientific, 89900) for immunoblotting. VIRAL TITER BY TCID50 ASSAY Virus
production in the supernatant was measured by quantifying TCID50. Vero E6 cells were plated in 96-well plates at 5 × 104 cells per well. The next day, supernatants collected from Calu-3
cells were subjected to 10-fold serial dilutions (101–1011) and inoculated onto Vero E6 cells. The cells were incubated at 37 °C with 5% CO2. Three to five days post infection, each
inoculated well was evaluated for the presence or absence of viral CPE. TCID50 was calculated based on the method of Reed and Muench60. RT-QPCR Total RNA was extracted using TRIzol reagent
according to the manufacturer’s instructions. Reverse transcription was performed using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, K1622) in accordance with the
manufacturer’s instructions. RT-qPCR was performed for each sample using Taqman Universal Master mix II, with UNG (Thermo Fisher Scientific, 4440038) on a ViiA7 Real-time PCR system. Primers
and probes for detection of the _RNaseP_ gene and SARS-CoV-2 _nucleocapsid_ (_N_) gene were obtained from IDT (2019-nCoV RUO Kit (Integrated DNA Technologies, 10006713)). The expression
level of the _N_ gene was determined relative to the endogenous control of the cellular _RNaseP_ gene. RNA-SEQUENCING ANALYSIS RNA concentration and quality were measured using High
Sensitivity RNA ScreenTape Analysis (Agilent, 5067-1500). cDNA libraries were constructed and sequencing was performed by Novogene using their mRNA sequencing protocol. The raw
RNA-sequencing data were aligned to the human genome (GRCh38) using STAR (version 2.7.3a). Donor effects were removed using the “removeBatchEffect” limma package, implemented in the R
computing environment (Supplementary Fig. 4)61. Analysis of differential expression was performed using DESeq2 according to a standard protocol. Genes with adjusted _P_ value < 0.05 were
considered as significantly differentially expressed. GSEA was performed using the fgsea package (version 1.22.0) in R. Enrichment of GO biological process terms in cluster genes from each
module was tested using a hypergeometric test in the clusterProfiler package (v4.10.0) with the Bonferroni procedure. Terms enriched with an adjusted _p_ value < 0.05 were considered
significant. The Reactome database (version 7.5.1) was downloaded from MSigDB (https://www.gsea-msigdb.org). IMMUNOFLUORESCENCE MICROSCOPY AND IMAGE ANALYSIS Cells were fixed and
permeabilized with cold methanol:acetone (1:1) for 10 min at 4 °C according to our previous method29. In brief, cells were washed with 1× PBS and incubated in a blocking buffer (5% goat
serum, Seracare Life Sciences Inc, 55600007) at room temperature for 30 min. Cells were then incubated with a primary antibody (monoclonal rabbit anti-SARS-CoV-2 N antibody, GeneTex,
GTX135357) in 1× PBS (1:1000) overnight at 4 °C. The following day, cells were washed three times with 1× PBS and incubated with a secondary antibody (Goat anti-Rabbit IgG (H + L) secondary
antibody, FITC (Thermo Fisher, 65-6111)) in 1× PBS (1:200) for 1 h at 37 °C. Then cells were washed three times with 1× PBS and incubated with DAPI (300 nM) (Thermo Fisher Scientific, D1306)
for 5 min at room temperature. Images were acquired using a fluorescence microscope (ECHO, Revolve). To measure the frequency of infected cells, randomly-selected areas were imaged. Each
treatment had three replicates. The FITC-positive cells and DAPI-positive cells were quantified using CellProfiler software (4.2.1)29. The same threshold value was applied to the images of
each area. Quantification of the western blots was carried out with Image J software (1.52q). β-actin/GAPDH were used as loading controls. PRODUCTION OF SARS-COV-2 VLPS HEK293T cells were
seeded in T75 cell culture flasks. The next day, cells were transfected with empty pcDNA3.1 plasmid or pcDNA3.1 plasmid encoding the SARS-CoV-2 M (Addgene, 158078), E (Addgene, 158080), N
(Addgene, 158079), and S proteins (Addgene, 158074), as indicated. 1 μg of each plasmid was used, with 5 μg of total plasmid in each transfection, normalized using empty vectors, in 400 μl
Opti-MEM and 18 μl of PEI. The transfection mixture was incubated at room temperature for 15 min and dropped into the HEK293T cells. Six hours post transfection, the media was removed and
supplemented with fresh medium containing PAV-104 at indicated concentrations. The supernatant and cell lysate were collected after 60 h. For the purification of SARS-CoV-2 VLPs, the
supernatant was passed through a 0.45 μm syringe filter (SIGMA, SLGVM33RS), then loaded on top of a 20% sucrose cushion in PBS, and ultracentrifuged at 30,000 rpm in an SW41 rotor for two
hours. VLP-containing pellets were washed with ice cold PBS and resuspended in SDS loading buffer, followed by sonication in an ice-water bath. Or VLP-containing pellets were resuspended in
PBS (passed through 0.22 μm syringe filter (SIGMA, SLGVM33RS)) for quantification by NTA. Cells were lysed in RIPA buffer (Thermo Fisher Scientific, 89900) and sonicated in an ice-water
bath. IMMUNOBLOTS OF SARS-COV-2 VLPS OR ΔS-VRP NUCLEOCAPSID PROTEIN Total protein in pellet and cell lysate samples were separated by SDS-PAGE, and subsequently electro-transferred onto a
supported PVDF membrane. Membranes were cut and probed for M with rabbit anti-SARS-CoV-2 M (Thermo Fisher Scientific, PA1-41160), N with rabbit anti-SARS-CoV-2 N (Rockland Immunochemicals,
200-401-A50), E with rabbit anti-SARS-CoV-2 E (Thermo Fisher Scientific, PA5-112047), and S with rabbit anti-SARS-CoV-2 S1/S2 (Thermo Fisher Scientific, PA5-112048) or with mouse
anti-SARS-CoV-2 spike (Genetex Inc, GTX632604). Goat anti-rabbit IgG HRP and goat anti-mouse IgG HRP secondary antibodies were used as appropriate. β-actin was used as a cell lysate and
pellet loading control by probing membranes with rabbit anti-human β-actin, conjugated with HRP (Cell Signaling Technology, 12620). All antibodies were diluted in 5% milk, and membranes were
washed with Tween 20 washing buffer (Thermo Fisher Scientific, J60304.K3). Chemiluminescent signal was visualized using SuperSignal West Femo Substrate (Thermo Fisher Scientific, PI34094)
or using ECL Blotting Reagents (SIGMA, GERPN2109), and imaged using ImageQuant LAS 4000. QUANTIFICATION OF VLPS BY NTA VLP-containing pellets were diluted in PBS (passed through 0.22 μm
syringe filter) to a concentration in the range of 107–109/ml and examined using a NanoSight NS300 (NanoSight Ltd) equipped with a 405 nm laser. Five 60 s-long videos were taken for each
sample with camera level 16 and the detection threshold set at 5. Raw data of particle movement and laser scattering were analyzed using NTA software (version 3.3, NanoSight Ltd). The output
data were presented as nanoparticle concentration and size. DRUG RESIN AFFINITY CHROMATOGRAPHY DRAC experiments were performed where 30 μl of extract prepared from Calu-3 cells under
different infection and treatment conditions were adjusted to a protein concentration of ~2.3 mg/ml in column buffer (50 mM HEPES, pH 7.6, 100 nM KAc, 6 mM MgAc, 1 mM EDTA, and 4 mM TGA) and
supplemented with an “energy cocktail” (to a final concentration of 1 mM rATP, 1 mM rGTP, 1 mM rCTP, 1 mM UTP, 4 mM creatine phosphate, pH 7.6) and 5 μg/mL creatine kinase and incubated on
a column containing 30 μl of Affi-gel resin (BIO-RAD, 1536099) coupled to either PAV-104 or a 4% agarose matrix (control) for one hour at room temperature. The PAV-104 resin conditions were
run side-by-side in triplicate, while the control resin conditions were done in single point. The flow-through material was collected, and the resin was washed with 1.5 mL column buffer then
eluted with 100 μl PAV-104 plus the energy cocktail at room temperature for 2 hours, then stripped with 100 μl 1% SDS. The eluate and SDS-stripped material run on agarose gels and are
analyzed by western blot for SARS-CoV-2 N protein (Rockland Immunochemicals, 200-401-A50). GLYCEROL GRADIENT SEDIMENTATION AND ELISA-BASED ASSESSMENT OF SARS-COV-2 N Cell extracts from
SARS-CoV-2 N-transfected HEK-293T cells in the presence or absence of PAV-104 were centrifuged at 15,000 × _g_ for 10 min at 4 °C to obtain the supernatant. 200 μl of supernatant were loaded
on the top of a 5 ml continuous 10–40% glycerol gradient in lysis buffer (v/v, Pierce IP Lysis Buffer (Thermo Fisher, 87787)) prepared using the Gradient Master machine (Biocomp, Gradient
Station). After concentration at 135,000 × _g_ for 20 h at 4 °C in a SW55 rotor (Beckman Coulter), 22 fractions of 250 μl were collected from the top to the bottom of the gradient. Proteins
were assessed by the commercial SARS-CoV-2 N protein sandwich ELISA kit (GeneTex, GTX535824) following the manufacturer’s instructions. In brief, each fraction was diluted to 1:1000 using an
assay dilute reagent. 50 μl of each standard and samples were added into the appropriate wells, then incubated at room temperature for 2 h. The solutions in the wells were aspirated, and
the wells were washed with a washing buffer six times. Then the conjugate solution was added and incubated at room temperature for 1 h. The solutions in the wells were aspirated, and the
wells were washed with a washing buffer six times once again. TMB solution was added to the wells and incubated in darkness for 15 min at room temperature. A stop solution was added to each
well. Finally, optical density at 450 nm was read within 15 min. STATISTICS AND REPRODUCIBILITY Statistical analysis was performed using GraphPad Prism version 8 software. Sample sizes are
indicated in the figure legends. Data were collected from a minimum of three independent experiments, with the exception of the data describing nucleocapsid concentrations detected by ELISA,
which were obtained from two independent experiments. Data were presented as means ± SEM or median. Data were analyzed for statistical significance using an unpaired or paired Student’s _t_
test to compare two groups, or using a paired _t_ test. Only _p_ values of 0.05 or lower were considered statistically significant (_p_ > 0.05 [ns], _p_ ≤ 0.05 [*], _p_ ≤ 0.01 [**], _p_
≤ 0.001 [***], _p_ ≤ 0.0001 [****]). REPORTING SUMMARY Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY
The DEG lists are shown in Supplementary Data 1–3. The GSEA pathway enrichment data are shown in Supplementary Data 4. Sequencing data are available in the NCBI Gene Expression Ominibus
under the GEO accession number GSE261002. All of the data generated or analyzed during this study are included in this published article or are available from the corresponding author upon
reasonable request. The source data underlying the graphs in the figure are shown in Supplementary Data 5. Supplementary Figs. 5–13 contain the original uncropped blot images associated with
the main figures. CODE AVAILABILITY Source codes and accompanying information can be found at https://github.com/ldu1/PAV104.git. REFERENCES * Hu, B., Guo, H., Zhou, P. & Shi, Z.-L.
Characteristics of SARS-CoV-2 and COVID-19. _Nat. Rev. Microbiol._ 19, 141–154 (2021). Article CAS PubMed Google Scholar * Razonable, R. R. et al. Casirivimab–Imdevimab treatment is
associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. _eClinicalMedicine_ 40, 101102 (2021). Article PubMed PubMed Central
Google Scholar * Thilagar, B. P. et al. Anti-spike monoclonal antibody therapy in pregnant women with mild-to-moderate coronavirus disease 2019 (COVID-19). _Obstet. Gynecol._ 139, 616–618
(2022). Article CAS PubMed PubMed Central Google Scholar * Chen, R. E. et al. In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains. _Nature_ 596, 103–108 (2021).
Article CAS PubMed PubMed Central Google Scholar * Beigel, J. H. et al. Remdesivir for the treatment of Covid-19 - final report. _N. Engl. J. Med._ 383, 1813–1826 (2020). Article CAS
PubMed Google Scholar * Jayk Bernal, A. et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. _N. Engl. J. Med._ 386, 509–520 (2022). Article PubMed Google
Scholar * Owen, D. R. et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. _Science_ 374, 1586–1593 (2021). Article CAS PubMed Google Scholar *
Wang, Y. et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. _Lancet_ 395, 1569–1578 (2020). Article CAS PubMed PubMed
Central Google Scholar * Miranda, J. A., McKinzie, P. B., Dobrovolsky, V. N. & Revollo, J. R. Evaluation of the mutagenic effects of Molnupiravir and N4-hydroxycytidine in bacterial
and mammalian cells by HiFi sequencing. _Environ. Mol. Mutagen_ 63, 320–328 (2022). Article CAS PubMed Google Scholar * Zhou, S. et al. β-d-N4-hydroxycytidine inhibits SARS-CoV-2 through
lethal mutagenesis but is also mutagenic to mammalian cells. _J. Infect. Dis._ 224, 415–419 (2021). Article CAS PubMed PubMed Central Google Scholar * Wang, L. et al. COVID-19 rebound
after paxlovid and molnupiravir during January-June 2022. _medRxiv_ https://doi.org/10.1101/2022.06.21.22276724 (2022). * Hashimoto, R. et al. Dual inhibition of TMPRSS2 and Cathepsin B
prevents SARS-CoV-2 infection in iPS cells. _Mol. Ther. Nucleic Acids_ 26, 1107–1114 (2021). Article CAS PubMed PubMed Central Google Scholar * Zhang, Q. et al. Molecular mechanism of
interaction between SARS-CoV-2 and host cells and interventional therapy. _Sig. Transduct. Target Ther._ 6, 1–19 (2021). Article Google Scholar * Mengist, H. M., Dilnessa, T. & Jin, T.
Structural basis of potential inhibitors targeting SARS-CoV-2 main protease. _Front. Chem._ 9, 622898 (2021). Article CAS PubMed PubMed Central Google Scholar * Narayanan, A. et al.
Identification of SARS-CoV-2 inhibitors targeting Mpro and PLpro using in-cell-protease assay. _Commun. Biol._ 5, 169 (2022). Article CAS PubMed PubMed Central Google Scholar * Ahamad,
S., Gupta, D. & Kumar, V. Targeting SARS-CoV-2 nucleocapsid oligomerization: insights from molecular docking and molecular dynamics simulations. _J. Biomol. Struct. Dyn._ 40, 2430–2443
(2022). Article CAS PubMed Google Scholar * Cai, T., Yu, Z., Wang, Z., Liang, C. & Richard, S. Arginine methylation of SARS-Cov-2 nucleocapsid protein regulates RNA binding, its
ability to suppress stress granule formation, and viral replication. _J. Biol. Chem._ 297, 100821 (2021). Article CAS PubMed PubMed Central Google Scholar * Bhowmik, D. et al.
Identification of potential inhibitors against SARS-CoV-2 by targeting proteins responsible for envelope formation and virion assembly using docking based virtual screening, and
pharmacokinetics approaches. _Infect. Genet. Evol._ 84, 104451 (2020). Article CAS PubMed PubMed Central Google Scholar * He, R. et al. Characterization of protein–protein interactions
between the nucleocapsid protein and membrane protein of the SARS coronavirus. _Virus Res._ 105, 121–125 (2004). Article CAS PubMed Google Scholar * Khambhati, K. et al. Exploring the
potential of cell-free protein synthesis for extending the abilities of biological systems. _Front. Bioeng. Biotechnol._ 7, 248 (2019). Article PubMed PubMed Central Google Scholar *
Rodríguez-Limas, W. A., Sekar, K. & Tyo, K. E. J. Virus-like particles: the future of microbial factories and cell-free systems as platforms for vaccine development. _Curr. Opin.
Biotechnol._ 24, 1089–1093 (2013). Article PubMed PubMed Central Google Scholar * Spice, A. J., Aw, R., Bracewell, D. G. & Polizzi, K. M. Synthesis and assembly of hepatitis B
virus-like particles in a pichia pastoris cell-free system. _Front. Bioeng. Biotechnol._ 8, 72 (2020). Article PubMed PubMed Central Google Scholar * Lingappa, U. F. et al. Host–rabies
virus protein–protein interactions as druggable antiviral targets. _Proc. Natl. Acad. Sci._ 110, E861–E868 (2013). Article CAS PubMed PubMed Central Google Scholar * Müller-Schiffmann,
A. et al. A pan-respiratory antiviral chemotype targeting a transient host multiprotein complex. Preprint at https://doi.org/10.1101/2021.01.17.426875 (2022). * Reed, J. C. et al.
Identification of an antiretroviral small molecule that appears to be a host-targeting inhibitor of HIV-1 assembly. _J. Virol._ 95, e00883-20 (2021). Article PubMed PubMed Central Google
Scholar * Grein, J. et al. Compassionate use of remdesivir for patients with severe Covid-19. _N. Engl. J. Med._ 382, 2327–2336 (2020). Article CAS PubMed Google Scholar * Pruijssers,
A. J. et al. Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. _Cell Rep._ 32, 107940 (2020). Article CAS PubMed
PubMed Central Google Scholar * Ryu, G. & Shin, H.-W. SARS-CoV-2 infection of airway epithelial cells. _Immune Netw._ 21, e3 (2021). Article PubMed PubMed Central Google Scholar *
Du, L. et al. Human galectin-9 potently enhances SARS-CoV-2 Replication and inflammation in airway epithelial cells. _J. Mol. Cell. Biol_. 15, mjad030 (2023). * Cheng, Y.-W. et al. Furin
inhibitors block SARS-CoV-2 spike protein cleavage to suppress virus production and cytopathic effects. _Cell Rep._ 33, 108254 (2020). Article CAS PubMed PubMed Central Google Scholar *
Sasaki, M. et al. S-217622, a SARS-CoV-2 main protease inhibitor, decreases viral load and ameliorates COVID-19 severity in hamsters. _Sci. Transl. Med._ 15, eabq4064 (2022). Article
Google Scholar * Marín-Palma, D. et al. Curcumin inhibits in vitro SARS-CoV-2 infection in vero E6 cells through multiple antiviral mechanisms. _Molecules_ 26, 6900 (2021). Article PubMed
PubMed Central Google Scholar * Plescia, C. B. et al. SARS-CoV-2 viral budding and entry can be modeled using BSL-2 level virus-like particles. _J. Biol. Chem._ 296, 100103 (2021).
Article CAS PubMed Google Scholar * Yurkovetskiy, L. et al. Structural and functional analysis of the D614G SARS-CoV-2 spike protein variant. _Cell_ 183, 739–751.e8 (2020). Article CAS
PubMed PubMed Central Google Scholar * Lu, S. et al. The SARS-CoV-2 nucleocapsid phosphoprotein forms mutually exclusive condensates with RNA and the membrane-associated M protein.
_Nat. Commun._ 12, 502 (2021). Article CAS PubMed PubMed Central Google Scholar * Cong, Y., Kriegenburg, F., de Haan, C. A. M. & Reggiori, F. Coronavirus nucleocapsid proteins
assemble constitutively in high molecular oligomers. _Sci. Rep._ 7, 5740 (2017). Article PubMed PubMed Central Google Scholar * Wu, C. et al. Characterization of SARS-CoV-2 N protein
reveals multiple functional consequences of the C-terminal domain. _bioRxiv_ https://doi.org/10.1101/2020.11.30.404905 (2020). * Scherer, K. M. et al. SARS-CoV-2 nucleocapsid protein adheres
to replication organelles before viral assembly at the Golgi/ERGIC and lysosome-mediated egress. _Sci. Adv._ 8, eabl4895 (2022). Article CAS PubMed PubMed Central Google Scholar *
Cubuk, J. et al. The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA. _Nat. Commun._ 12, 1936 (2021). Article CAS PubMed PubMed Central Google
Scholar * Grunewald, M. E., Fehr, A. R., Athmer, J. & Perlman, S. The coronavirus nucleocapsid protein is ADP-ribosylated. _Virology_ 517, 62–68 (2018). Article CAS PubMed Google
Scholar * Li, F. Q., Xiao, H., Tam, J. P. & Liu, D. X. Sumoylation of the nucleocapsid protein of severe acute respiratory syndrome coronavirus. _FEBS Lett._ 579, 2387–2396 (2005).
Article CAS PubMed PubMed Central Google Scholar * Madahar, V. et al. Human post-translational SUMOylation modification of SARS-CoV-2 nucleocapsid protein enhances its interaction
affinity with itself and plays a critical role in its nuclear translocation. _Viruses_ 15, 1600 (2023). Article CAS PubMed PubMed Central Google Scholar * Yaron, T. M. et al. Host
protein kinases required for SARS-CoV-2 nucleocapsid phosphorylation and viral replication. _Sci. Signal._ 15, eabm0808 (2022). * Yang, Z. et al. Interaction between host G3BP and viral
nucleocapsid protein regulates SARS-CoV-2 replication. _bioRxiv_ https://doi.org/10.1101/2023.06.29.546885 (2023). * Ye, Q., West, A. M. V., Silletti, S. & Corbett, K. D. Architecture
and self-assembly of the SARS-CoV-2 nucleocapsid protein. _Protein Sci._ 29, 1890–1901 (2020). Article CAS PubMed PubMed Central Google Scholar * McBride, R., van Zyl, M. &
Fielding, B. C. The coronavirus nucleocapsid is a multifunctional protein. _Viruses_ 6, 2991–3018 (2014). Article PubMed PubMed Central Google Scholar * Carnes, S. K., Sheehan, J. H.
& Aiken, C. Inhibitors of the HIV-1 capsid, a target of opportunity. _Curr. Opin. HIV AIDS_ 13, 359–365 (2018). Article CAS PubMed PubMed Central Google Scholar * Gordon, D. E. et
al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. _Nature_ 583, 459–468 (2020). Article CAS PubMed PubMed Central Google Scholar * Zhou, Y. et al. A
comprehensive SARS-CoV-2–human protein–protein interactome reveals COVID-19 pathobiology and potential host therapeutic targets. _Nat. Biotechnol._ 41, 128–139 (2023). Article PubMed
Google Scholar * Surjit, M. et al. The severe acute respiratory syndrome coronavirus nucleocapsid protein is phosphorylated and localizes in the cytoplasm by 14-3-3-mediated translocation.
_J. Virol._ 79, 11476–11486 (2005). Article CAS PubMed PubMed Central Google Scholar * Tugaeva, K. V. et al. Human 14-3-3 proteins site-selectively bind the mutational hotspot region of
SARS-CoV-2 nucleoprotein modulating its phosphoregulation. _J. Mol. Biol._ 435, 167891 (2023). Article CAS PubMed Google Scholar * Hoenen, T. et al. Inclusion bodies are a site of
ebolavirus replication. _J. Virol._ 86, 11779–11788 (2012). Article CAS PubMed PubMed Central Google Scholar * Zhu, N. et al. Morphogenesis and cytopathic effect of SARS-CoV-2 infection
in human airway epithelial cells. _Nat. Commun._ 11, 3910 (2020). Article CAS PubMed PubMed Central Google Scholar * Diamond, M. S. & Kanneganti, T.-D. Innate immunity: the first
line of defense against SARS-CoV-2. _Nat. Immunol._ 23, 165–176 (2022). Article CAS PubMed PubMed Central Google Scholar * Kim, Y.-M. & Shin, E.-C. Type I and III interferon
responses in SARS-CoV-2 infection. _Exp. Mol. Med._ 53, 750–760 (2021). Article CAS PubMed PubMed Central Google Scholar * Gu, W. et al. The molecular mechanism of SARS-CoV-2 evading
host antiviral innate immunity. _Virol. J._ 19, 49 (2022). Article CAS PubMed PubMed Central Google Scholar * Akamatsu, M. A., de Castro, J. T., Takano, C. Y. & Ho, P. L. Off
balance: interferons in COVID-19 lung infections. _EBioMedicine_ 73, 103642 (2021). Article CAS PubMed PubMed Central Google Scholar * Hadjadj, J. et al. Impaired type I interferon
activity and inflammatory responses in severe COVID-19 patients. _Science_ 369, 718–724 (2020). Article CAS PubMed PubMed Central Google Scholar * Malicoat, J. et al. Development of a
single-cycle infectious SARS-CoV-2 virus replicon particle system for use in biosafety level 2 laboratories. _J. Virol._ 96, e0183721 (2022). Article PubMed Google Scholar * Reed, L. J.
& Muench, H. A simple method of estimating fifty per cent endpoints12. _Am. J. Epidemiol._ 27, 493–497 (1938). Article Google Scholar * Smyth, G. K. Linear models and empirical bayes
methods for assessing differential expression in microarray experiments. _Stat. Appl. Genet. Mol. Biol._ 3, Article3 (2004). Article PubMed Google Scholar Download references
ACKNOWLEDGEMENTS This study was supported by The Sandler Program for Breakthrough Biomedical Research (UCSF PBBR) and NIH grant R01MH112457 (to S.K.P.); Veterans Affairs Office of Research
and Development CX002011 and NIH R01HL151552 (to J.R.G.). V.R.L., S.S., L.D., and S.K.P. received funding from NIH AViDD grant 1U19 AI171443; V.R.L. and S.S. received funding from Prosetta
Biosciences, Inc. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Vitalant Research Institute, 360 Spear St., San Francisco, CA, 94105, USA Li Du, Mohamed S. Bouzidi, Graham Simmons, Prerna
Dabral & Satish K. Pillai * University of California, San Francisco, CA, 94143, USA Li Du, Fred Deiter, Mohamed S. Bouzidi, Graham Simmons, Prerna Dabral, Homer Boushey, John R.
Greenland & Satish K. Pillai * Veterans Administration Health Care System, 4150 Clement St., San Francisco, CA, 94121, USA Fred Deiter & John R. Greenland * DNAnexus, 1975 W EI
Camino Real, Mountain View, CA, 94040, USA Jean-Noël Billaud * Prosetta Biosciences Inc, 670 5th St., San Francisco, CA, 94107, USA Suganya Selvarajah, Anuradha F. Lingappa, Maya Michon,
Shao Feng Yu, Kumar Paulvannan & Vishwanath R. Lingappa * University of Iowa, Iowa City, IA, 52242, USA Balaji Manicassamy Authors * Li Du View author publications You can also search
for this author inPubMed Google Scholar * Fred Deiter View author publications You can also search for this author inPubMed Google Scholar * Mohamed S. Bouzidi View author publications You
can also search for this author inPubMed Google Scholar * Jean-Noël Billaud View author publications You can also search for this author inPubMed Google Scholar * Graham Simmons View author
publications You can also search for this author inPubMed Google Scholar * Prerna Dabral View author publications You can also search for this author inPubMed Google Scholar * Suganya
Selvarajah View author publications You can also search for this author inPubMed Google Scholar * Anuradha F. Lingappa View author publications You can also search for this author inPubMed
Google Scholar * Maya Michon View author publications You can also search for this author inPubMed Google Scholar * Shao Feng Yu View author publications You can also search for this author
inPubMed Google Scholar * Kumar Paulvannan View author publications You can also search for this author inPubMed Google Scholar * Balaji Manicassamy View author publications You can also
search for this author inPubMed Google Scholar * Vishwanath R. Lingappa View author publications You can also search for this author inPubMed Google Scholar * Homer Boushey View author
publications You can also search for this author inPubMed Google Scholar * John R. Greenland View author publications You can also search for this author inPubMed Google Scholar * Satish K.
Pillai View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.D. and S.P. initiated the project and designed the experiments. L.D., F.D. and
J.G. performed and processed RNA-seq. F.D. and J.G. isolated and prepared ALI-cultured primary AECs. L.D., M.B. and G.S. performed MTT and glycerol gradient sedimentation assay. J.B.
analyzed RNA-seq data. L.D. and P.D. quantified VLPs by NTA. G.S. performed SARS-CoV-2 stock. B.M. generated ΔS-VRP stocks. S.S., A.L., M.M., S.Y., K.P., V.L. and H.B. prepared PAV-104 and
performed the DRAC assay. L.D., V.L. and S.P. prepared the manuscript. J.G. and S.P. jointly supervised the work. CORRESPONDING AUTHOR Correspondence to Satish K. Pillai. ETHICS DECLARATIONS
COMPETING INTERESTS S.S., A.F.L., M.M., S.F.Y. and K.P. are employees of Prosetta Biosciences. V.R.L. is the CEO of Prosetta Biosciences, which manufactures PAV-104 presented in the
manuscript. All other authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Communications Biology_ thanks Thomas Gramberg and the other, anonymous, reviewer(s) for
their contribution to the peer review of this work. Primary Handling Editors: Caroline Goujon and David Favero. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with
regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES
SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2 SUPPLEMENTARY DATA 3 SUPPLEMENTARY DATA 4 SUPPLEMENTARY DATA 5 REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are
included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Du, L., Deiter, F., Bouzidi, M.S. _et al._ A viral assembly
inhibitor blocks SARS-CoV-2 replication in airway epithelial cells. _Commun Biol_ 7, 486 (2024). https://doi.org/10.1038/s42003-024-06130-8 Download citation * Received: 05 May 2023 *
Accepted: 01 April 2024 * Published: 22 April 2024 * DOI: https://doi.org/10.1038/s42003-024-06130-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
