Intranasal oxytocin suppresses seizure-like behaviors in a mouse model of ngly1 deficiency
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT NGLY1 deficiency is a genetic disease caused by biallelic mutations of the _Ngly1_ gene. Although epileptic seizure is one of the most severe symptoms in patients with NGLY1
deficiency, preclinical studies have not been conducted due to the lack of animal models for epileptic seizures in NGLY1 deficiency. Here, we observed the behaviors of male and female
_Ngly1_−/− mice by video monitoring and found that these mice exhibit spontaneous seizure-like behaviors. Gene expression analyses and enzyme immunoassay revealed significant decreases in
oxytocin, a well-known neuropeptide, in the hypothalamus of _Ngly1_−/− mice. Seizure-like behaviors in _Ngly1_−/− mice were transiently suppressed by a single intranasal administration of
oxytocin. These findings suggest the therapeutic potential of oxytocin for epileptic seizure in patients with NGLY1 deficiency and contribute to the clarification of the disease mechanism.
SIMILAR CONTENT BEING VIEWED BY OTHERS TRANSIENT TARGETING OF HYPOTHALAMIC OREXIN NEURONS ALLEVIATES SEIZURES IN A MOUSE MODEL OF EPILEPSY Article Open access 10 February 2024 INTRANASAL
OXYTOCIN IN A GENETIC ANIMAL MODEL OF AUTISM Article Open access 15 December 2023 RESCUE OF OXYTOCIN RESPONSE AND SOCIAL BEHAVIOUR IN A MOUSE MODEL OF AUTISM Article 05 August 2020
INTRODUCTION Peptide:_N_-glycanase (NGLY1) is an enzyme that removes the _N_-linked glycans from misfolded _N_-glycoproteins1,2. NGLY1 is mainly localized in the cytoplasmic side of the
endoplasmic reticulum (ER) and involved in the ER-associated degradation of misfolded _N_-glycoproteins, which plays a crucial role in the quality control of newly synthesized
_N_-glycoproteins3,4. NGLY1 also activates the transcription factor NRF1 by deglycosylating its glycan chains and substituting their amino acid from asparagine to aspartic acid residues5,6.
NGLY1 deficiency is a genetic disease caused by biallelic mutations in the _Ngly1_ gene7. Patients with NGLY1 deficiency exhibit several symptoms, including developmental delay, intellectual
disability, movement disorders, alacrima, and epileptic seizures8. The first patient with NGLY1 deficiency was reported in 20129. The number of patients has increased to over 10010;
however, a satisfactory treatment method has not yet been established for NGLY1 deficiency, probably due to the lack of knowledge on the disease mechanism11,12,13. We previously established
_Ngly1_−/− mice and _Ngly1_−/− rats by deleting the exons 11 and 12 of the _Ngly1_ gene. These animals exhibited developmental delay and movement disorders14,15. Movement disorders in
_Ngly1_−/− rats were significantly improved by a single intracerebroventricular administration of AAV-NGLY116,17. This replacement therapy could be a fundamental treatment, but it requires
invasive surgery. Other treatment options are anticipated to reduce patient burden. For epileptic seizures in patients with NGLY1 deficiency, some approved drugs, including levetiracetam,
valproate, lamotrigine, and clobazam, have been tested; however, their efficacies were limited11. Researching the disease mechanism is necessary to discover more effective anti-epileptic
drugs. The _Ngly1_−/− mouse model is a candidate preclinical model of epileptic seizure in NGLY1 deficiency that is used to uncover the disease mechanism, although it is unknown whether
_Ngly1_−/− mice exhibit epileptic seizures. Herein, we observed the behaviors of _Ngly1_−/− mice by video monitoring and found that these mice exhibited seizure-like behaviors spontaneously.
Gene expression analyses and enzyme immunoassay revealed significant decreases in oxytocin in the hypothalamus of _Ngly1_−/− mice. The effects of intranasal oxytocin administration on the
seizure-like behaviors in _Ngly1_−/− mice were evaluated to confirm the involvement of oxytocin with seizure-like behaviors in _Ngly1_−/− mice. RESULTS SPONTANEOUS SEIZURE-LIKE BEHAVIORS IN
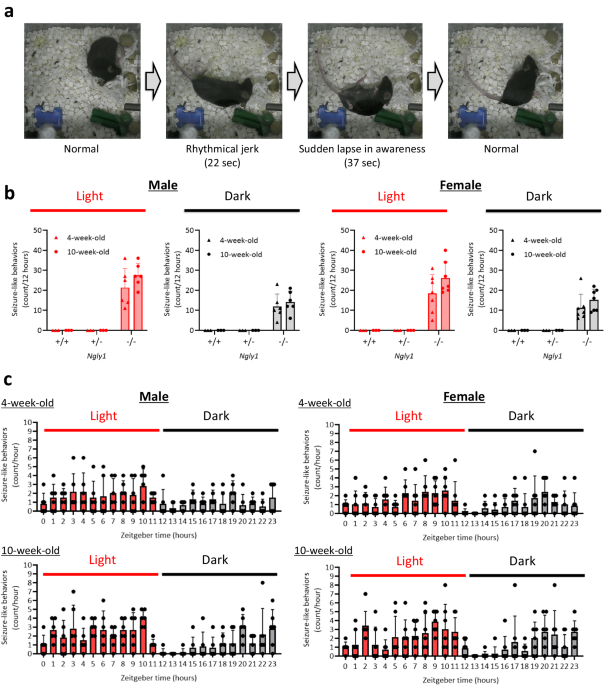
_NGLY1_ −/− MICE To clarify whether _Ngly1_−/− mice exhibit epileptic seizures, we observed the behaviors of male and female _Ngly1_−/− mice by video monitoring for 24 h. As shown in the
representative figures and video of female _Ngly1_−/− mice, these mice exhibited several seizure-like behaviors spontaneously (Fig. 1a, Supplementary Movie 1). These behaviors seemed to be
tonic-clonic or absence seizures, characterized by body stiffness and rhythmical jerking of the limbs or a sudden lapse in awareness. The average frequencies of seizure-like behaviors in 4
and 10-week-old male _Ngly1_−/− mice were 21 and 28 episodes per 12-hour light phase, 12 and 14 episodes per 12-hour dark phase, respectively. Those of female _Ngly1_−/− mice were 18 and 26
episodes per 12 h-light phase, 11 and 15 episodes per 12-hour dark phase, respectively. _Ngly1_+/+ and _Ngly1_+/− mice did not exhibit any seizure-like behavior in both light and dark phases
at 4 and 10-week-old (Fig. 1b). Although the time zone of occurrence varied individually, almost all mice did not exhibit seizure-like behaviors at the beginning of the dark phase
(zeitgeber time 12–13) when they began to move actively (Fig. 1c). TRANSCRIPTOME ANALYSES IN THE BRAIN AND SPINAL CORD OF MALE _NGLY1_ −/− MICE To elucidate the disease mechanism of
seizure-like behaviors in _Ngly1_−/− mice, we analyzed the differences in gene expression profiles in the cerebellum, hippocampus, thalamus, striatum, cerebral cortex, and spinal cord of 4
and 10-week-old male _Ngly1_−/− and _Ngly1_+/+ mice. Principal component analysis (PCA) of the transcriptome data depicted the differences between these tissues, whereas differences between
ages or _Ngly1_−/− and _Ngly1_+/+ mice were unclear in the PCA plot (Fig. 2a). However, volcano plots of the transcriptome data revealed significant decreases in oxytocin transcripts in the
thalami of 4-week-old _Ngly1_−/− mice (Fig. 2b). Significant decreases in oxytocin transcripts were also detected in the striata of 4-week-old _Ngly1_−/− mice and the thalami of 10-week-old
_Ngly1_−/− mice (Fig. 2c). Although 159 Gene Ontology terms were obtained from the enrichment analysis of differentially expressed genes in _Ngly1_−/− mice, NGLY1-related terms, such as
proteasome and autophagy, were not included among the 159 terms. Instead, 21 neurotransmission-related terms were included among the 159 terms, suggesting an abnormal neurotransmission in
_Ngly1_−/− mice (Supplementary Table 1). SPECIFIC DOWN-REGULATION OF OXYTOCIN TRANSCRIPTS IN THE HYPOTHALAMUS OF _NGLY1_ −/− MICE The expression levels of oxytocin transcripts were also
measured using quantitative PCR (Supplementary Tables 2 and 3) in the hypothalamus of 10-week-old male and female _Ngly1_−/− mice because the hypothalamus is the main production site of
oxytocin. The decreased rates of oxytocin transcripts in _Ngly1_−/− mice were 56% (male) and 58% (female) compared to those of _Ngly1_+/+ mice. Oxytocin levels were not significantly changed
in male and female _Ngly1_+/− mice compared to those of _Ngly1_+/+ mice (Fig. 3a). Transcripts of other major hypothalamic neuropeptides, i.e., vasopressin and corticotropin-releasing
hormone, were not significantly changed in male and female _Ngly1_−/− mice compared to those of _Ngly1_+/+ mice, suggesting that among the hypothalamic neuropeptides, the down-regulation was
specific to oxytocin (Fig. 3b, c). OXYTOCIN PEPTIDE LEVELS IN THE HYPOTHALAMUS, PITUITARY GLAND, AND PLASMA OF _NGLY1_ −/− MICE To confirm oxytocin down-regulation at peptide levels, we
quantified oxytocin peptides in the hypothalamus of 10-week-old male and female _Ngly1_−/− mice. The decrease rates of oxytocin peptides in _Ngly1_−/− mice were 78% (male) and 66% (female)
compared to those of _Ngly1_+/+ mice (Fig. 4a). Down-regulation of oxytocin peptides was also detected in the pituitary gland of _Ngly1_−/− mice, which is the secretory tissue of oxytocin.
The decrease rates were 79% (male) and 76% (female) compared to those of _Ngly1_+/+ mice (Fig. 4b). At that timing, down-regulation of oxytocin peptides was not detected in the plasma of
_Ngly1_−/− mice, probably due to the diurnal variation and rapid clearance of plasma oxytocin (Supplementary Fig. 1). Therefore, plasma oxytocin levels were repeatedly measured every 27 h
four times in 7-week-old male and female _Ngly1_−/− mice. Although statistical significance was not detected at most intervals, plasma oxytocin levels showed a tendency to be decreased in
_Ngly1_−/− mice compared to _Ngly1_+/+ mice (Fig. 4c). Down-regulation of oxytocin peptides was further confirmed in the hypothalamus and pituitary gland of 29-week-old male _Ngly1_−/− mice
to increase the credibility of oxytocin down-regulation (Supplementary Fig. 2). In all experiments, oxytocin levels were not significantly changed in _Ngly1_+/− mice compared to _Ngly1_+/+
mice. OXYTOCIN LEVELS IN _NGLY1_ −/− MICE AFTER INTRANASAL ADMINISTRATION OF OXYTOCIN Delivery of intranasal oxytocin to the central nervous system was confirmed using 17 to 18-week-old
female _Ngly1_−/− mice. Dosing was initiated at 10 or 100 mg/kg (6000 or 60,000 IU/kg) due to a lack of effect on seizure-like behaviors in _Ngly1_−/− mice at 1 and 3 mg/kg in our
preliminary study. Five minutes after the single intranasal administration, oxytocin levels were increased in the plasma, hippocampus, striatum, cerebral cortex, and spinal cord of
_Ngly1_−/− mice, suggesting that this regimen could be applied when evaluating the effects of oxytocin on seizure-like behaviors in _Ngly1_−/− mice (Fig. 5a–f). An increasing trend was also
observed in the thalamus, whereas it was not observed in the hypothalamus, probably because the endogenous oxytocin levels were relatively high compared to that in other tissues (Fig. 5g,
h). TRANSIENT SUPPRESSION OF SEIZURE-LIKE BEHAVIORS BY INTRANASAL OXYTOCIN IN _NGLY1_ −/− MICE Effects of intranasal oxytocin administration on seizure-like behaviors in _Ngly1_−/− mice were
evaluated in crossover studies using 12-week-old male and female _Ngly1_−/− mice (Fig. 6a). Seizure-like behaviors in male and female _Ngly1_−/− mice were transiently suppressed by
intranasal oxytocin administration. The differences of mean episodes per 4 h-light phase (and two-sided 95% confidence interval) compared to control group (0 mg/kg) were −3.3 (−10.2, 3.7) at
10 mg/kg, −2.5 (−9.1, 4.1) at 30 mg/kg, −8.5 (−14.8, −2.2) at 100 mg/kg in male _Ngly1_−/− mice and −9.0 (−17.1, −0.9) at 10 mg/kg, −11.0 (−21.4, −0.6) at 30 mg/kg, −14.5 (−22.8, −6.2) at
100 mg/kg in female _Ngly1_−/− mice, respectively (Fig. 6b). DISCUSSION Although epileptic seizure is one of the most severe symptoms in patients with NGLY1 deficiency, preclinical studies
have not been carried out due to the lack of animal models for epileptic seizures in NGLY1 deficiency. Here we firstly clarified that _Ngly1_−/− mice exhibited several seizure-like behaviors
spontaneously (Fig. 1, Supplementary Movie 1). This finding enabled us to analyze the disease mechanism of epileptic seizure in NGLY1 deficiency and explore treatment options. Significant
decreases in oxytocin transcripts were detected in the thalami of male _Ngly1_−/− mice (Fig. 2). Oxytocin is a hypothalamic neuropeptide known for its role in parturition18,19, lactation20,
and social interaction21. Oxytocin is also reported to play an important role in the maintenance of brain homeostasis by regulating stress-related hormones, such as corticotropin-releasing
hormone, adrenocorticotropic hormone and corticosterone, in the hypothalamic–pituitary–adrenal (HPA) axis22,23,24,25. Dysregulation of these hormones in HPA axis is a risk factor for
epileptic seizure26. Therefore, it was speculated that the downregulation of oxytocin in _Ngly1_−/− mice could cause the dysregulation of HPA axis and led to seizure-like behaviors (Fig. 1).
The facts that seizure-like behaviors were not detected in _Ngly1_+/− mice (Fig. 1) and that oxytocin was not significantly decreased in _Ngly1_+/− mice (Figs. 3 and 4) also reinforced our
hypothesis. To evaluate the effect of oxytocin on seizure-like behaviors in _Ngly1_−/− mice, oxytocin must be delivered to the central nervous system (CNS) of _Ngly1_−/− mice. Delivery of
oxytocin to the CNS had already been demonstrated by intranasal administration in mice27, rats28, rhesus macaques29, and patients with autism spectrum disorder30. Therefore, we selected
intranasal administration of oxytocin and confirmed its delivery to the CNS in _Ngly1_−/− mice (Fig. 5). Seizure-like behaviors in _Ngly1_−/− mice were transiently suppressed by single
intranasal administration of oxytocin (Fig. 6). The safety of single or chronic intranasal oxytocin administration has been investigated in several clinical studies. For example, no
side-effect was detected in school-age children (8–12 years old) 90 min and 24 h after a single administration31; chronic administration (twice daily for four weeks) was well-tolerated in
generally healthy older men (55–95 years old)32. Based on these clinical studies, oxytocin has the potential to be administered to patients with NGLY1 deficiency by carefully optimizing the
dosing regimen. This study has some limitations. While the down-regulation of oxytocin in the hypothalamus of _Ngly1_−/− mice was clarified by bulk RNA-seq analyses, the detailed mechanism
of oxytocin down-regulation by _Ngly1_ deletion could not be clarified by our subsequent single-nucleus RNA-seq analyses in the hypothalamus of _Ngly1_−/− mice. Other approaches are required
to clarify this mechanism. In conclusion, we observed spontaneous seizure-like behaviors in _Ngly1_−/− mice and the down-regulation of oxytocin in the hypothalamus of _Ngly1_−/− mice. Our
results also demonstrated the suppressive effects of intranasal oxytocin administration on seizure-like behaviors in _Ngly1_−/− mice. These findings suggest the therapeutic potential of
oxytocin for epileptic seizures in patients with NGLY1 deficiency and would contribute to the clarification of the disease mechanism. METHODS APPROVAL OF ANIMAL EXPERIMENTS All animal care
procedures and experiments conformed to the Association for Assessment and Accreditation of Laboratory Animal Care guidelines and were approved by the Institutional Animal Care and Use
Committee of Takeda Pharmaceutical Co., Ltd. (Kanagawa, Japan). ESTABLISHMENT AND BREEDING OF _NGLY1_ −/− MICE Homozygous _Ngly1_ knockout (_Ngly1_−/−) mice, heterozygous _Ngly1_ knockout
(_Ngly1_+/−) mice, and wild-type (_Ngly1_+/+) littermates were established previously by mating C57BL/6 background _Ngly1_+/− mice with Japanese fancy mouse 1 background _Ngly1_+/− mice to
rescue the embryonic lethality of _Ngly1_−/− mice15. These mice were bred and provided by Axcelead Drug Discovery Partners (Kanagawa, Japan). VIDEO ANALYSIS OF SEIZURE-LIKE BEHAVIORS IN
_NGLY1_ −/− MICE Mice were habituated to single housing for one day in clear acrylic cages (W15 cm, D15 cm, H25 cm) with vents and water insertion slots. The next day, videos were recorded
for 24 h from the top of the cage using an infrared camera. Seizure-like behaviors were manually counted using HDWriterAE5.4 software (Panasonic, Osaka, Japan) in a blinded manner by Orizuru
Therapeutics (Kanagawa, Japan). All types of seizure-like behaviors were counted as one regardless of the degree and duration. These video analyses were repeated using the same mice at 4
and 10 weeks old. TRANSCRIPTOME ANALYSES IN THE BRAIN TISSUES AND SPINAL CORD OF _NGLY1_ −/− MICE The cerebellum, hippocampus, thalamus, striatum, cerebral cortex, and spinal cord were
collected from 4- and 10-week-old male _Ngly1_+/+ and _Ngly1_−/− mice (_N_ = 3). Total RNA was extracted from each tissue using an RNeasy Mini QIAcube kit (QIAGEN, Hilden, Germany). RNA
sequencing was performed on the DNBSEQ platform in Azenta. Transcriptome data was analyzed by Axcelead Drug Discovery Partners. Differentially expressed genes were identified using the edgeR
(3.1.1) and limma (3.46.0) R packages. In brief, gene expression counts were initially transformed to counts per million (CPM). Subsequently, genes with low expression, where the minimum
log-CPM was less than or equal to zero, were removed. Data were then subjected to the trimmed mean of M-values normalization. QUANTIFICATION OF OXYTOCIN AND OTHER TRANSCRIPTS USING REAL-TIME
PCR Total RNAs were extracted from the hypothalamus of 10-week-old male and female _Ngly1_+/+, _Ngly1_+/− and _Ngly1_−/− mice using the RNeasy Mini QIAcube kit (_N_ = 7, combined two
experiments). The RNAs were reverse-transcribed using the High-Capacity RNA to cDNA kit (ThermoFisher Scientific, Tokyo, Japan). Transcripts of oxytocin, vasopressin, CRH, and MAP2 were
quantified using a 7900HT Fast Real-Time PCR system (ThermoFisher Scientific). Primers, probes, and standard oligos for quantitative PCR were designed using Primer ExpressTM software v3.0.1
(ThermoFisher Scientific), and their sequences are listed in Supplementary Tables 2 and 3. MEASUREMENT OF OXYTOCIN PEPTIDES USING ENZYME IMMUNOASSAY Plasma was collected every 27 h four
times from the left ventricles of 7-week-old male and female _Ngly1_+/+, _Ngly1_+/−, and _Ngly1_−/− mice (_N_ = 3) under anesthesia with 2.5% isoflurane. The hypothalamus and pituitary gland
were extracted after euthanasia from 10-week-old male and female _Ngly1_+/+, _Ngly1_+/−, and _Ngly1_−/− mice (_N_ = 5). Oxytocin levels were measured by a chemiluminescent enzyme
immunoassay in ASKA Pharma Medical Co., Ltd. (Kanagawa, Japan). INTRANASAL ADMINISTRATION OF OXYTOCIN Oxytocin peptide (CAS number 50-56-6) was purchased from AA Blocks (San Diego, CA, USA)
and dissolved in saline at a maximum concentration of 200 mg/mL. Female _Ngly1_−/− mice were anesthetized with 2.5% isoflurane and intranasally administered with oxytocin using a manual
pipette at a volume of 0.5 ml/kg. Oxytocin solutions were prepared in Protein LoBind Tubes (Eppendorf, Hamburg, Germany) just before the intranasal administration. CROSSOVER STUDIES TO
EVALUATE THE EFFECTS OF OXYTOCIN ON SEIZURE-LIKE BEHAVIORS Oxytocin (0, 10, 30, 100 mg/kg in saline) was intranasally administered to 12-week-old male and female _Ngly1_−/− mice.
Seizure-like behaviors were counted just after the single administration for 4 h, as previously described. This assessment was carried out with a crossover design with washout periods of 20
h. STATISTICAL ANALYSES Unless otherwise specified, the results were analyzed using EXSUS2014 software version 8.0, SAS 9.3 TS Levle1M2 (SAS Institute, Inc., NC). Limma package, with its
voom method33, was used to identify differentially expressed genes. Thresholds for identification were a _p_ value less than 0.05 and an absolute log-FC (fold change) greater than 1. Bayes
algorithm was used to test the statistical significance of differences between _Ngly1_+/+ and _Ngly1_−/− mice. The estimated differences in seizure-like behaviors between oxytocin-treated
groups (10, 30, 100 mg/kg) and the control group (0 mg/kg) and their adjusted two-sided 95% confidence intervals were calculated to evaluate effects of oxytocin on seizure-like behaviors in
male and female _Ngly1_−/− mice. The sample size was chosen based on our preliminary studies with similar methods. REPORTING SUMMARY Further information on research design is available in
the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY All data supporting the findings described in this manuscript are available in the Supplementary Data and
from the corresponding author upon reasonable request. _Ngly1_−/− mice, _Ngly1_+/− mice, and _Ngly1_+/+ littermates will be available upon reasonable request under MTA. CODE AVAILABILITY
Transcriptome data were analyzed using a publicly available algorithm. https://www.bioconductor.org/packages/devel/workflows/vignettes/RNAseq123/inst/doc/limmaWorkflow.html. REFERENCES *
Suzuki, T., Park, H., Hollingsworth, N. M., Sternglanz, R. & Lennarz, W. J. PNG1, a yeast gene encoding a highly conserved peptide:N-glycanase. _J. Cell Biol._ 149, 1039–1052 (2000).
Article CAS PubMed PubMed Central Google Scholar * Suzuki, T., Huang, C. & Fujihira, H. The cytoplasmic peptide: N-glycanase (NGLY1) - structure, expression and cellular functions.
_Gene_ 577, 1–7 (2016). Article CAS PubMed Google Scholar * Suzuki, T., Park, H., Till, E. A. & Lennarz, W. J. The PUB domain: a putative protein-protein interaction domain
implicated in the ubiquitin-proteasome pathway. _Biochem. Biophys. Res. Commun._ 287, 1083–1087 (2001). Article CAS PubMed Google Scholar * Suzuki, T., Kwofie, M. A. & Lennarz, W. J.
Ngly1, a mouse gene encoding a deglycosylating enzyme implicated in proteasomal degradation: expression, genomic organization, and chromosomal mapping. _Biochem. Biophys. Res. Commun._ 304,
326–332 (2003). Article CAS PubMed Google Scholar * Lehrbach, N. J., Breen, P. C. & Ruvkun, G. Protein sequence editing of SKN-1A/Nrf1 by peptide: N-glycanase controls proteasome
gene expression. _Cell_ 177, 737–750.e715 (2019). Article CAS PubMed PubMed Central Google Scholar * Tomlin, F. M. et al. Inhibition of NGLY1 inactivates the transcription factor Nrf1
and potentiates proteasome inhibitor cytotoxicity. _ACS Cent. Sci._ 3, 1143–1155 (2017). Article CAS PubMed PubMed Central Google Scholar * Ge, H. et al. Two novel compound heterozygous
mutations in NGLY1as a cause of congenital disorder of deglycosylation: a case presentation. _BMC Med. Genet._ 21, 135 (2020). Article CAS PubMed PubMed Central Google Scholar * Enns,
G. M. et al. Mutations in NGLY1 cause an inherited disorder of the endoplasmic reticulum-associated degradation pathway. _Genet. Med._ 16, 751–758 (2014). Article CAS PubMed PubMed
Central Google Scholar * Need, A. C. et al. Clinical application of exome sequencing in undiagnosed genetic conditions. _J. Med. Genet._ 49, 353–361 (2012). Article CAS PubMed Google
Scholar * Pandey, A. & Jafar-Nejad, H. Tracing the NGLY1 footprints: insights from drosophila. _J. Biochem._ 171, 153–160 (2022). Article CAS PubMed Google Scholar * Levy, R. J.,
Frater, C. H., Gallentine, W. B., Phillips, J. M. & Ruzhnikov, M. R. Delineating the epilepsy phenotype of NGLY1 deficiency. _J. Inherit. Metab. Dis._ 45, 571–583 (2022). Article CAS
PubMed Google Scholar * Stanclift, C. R. et al. NGLY1 deficiency: estimated incidence, clinical features, and genotypic spectrum from the NGLY1 registry. _Orphanet J. Rare Dis._ 17, 440
(2022). Article PubMed PubMed Central Google Scholar * Nolan, D. K., Pastore, M. T. & McBride, K. L. Expanding the NGLY1 deficiency phenotype: case report of an atypical patient.
_Eur. J. Med. Genet._ 65, 104558 (2022). Article CAS PubMed Google Scholar * Asahina, M. et al. Ngly1 -/- rats develop neurodegenerative phenotypes and pathological abnormalities in
their peripheral and central nervous systems. _Hum. Mol. Genet._ 29, 1635–1647 (2020). Article CAS PubMed PubMed Central Google Scholar * Asahina, M. et al. JF1/B6F1 Ngly1(-/-) mouse as
an isogenic animal model of NGLY1 deficiency. _Proc. Jpn. Acad. Ser. B Phys. Biol. Sci._ 97, 89–102 (2021). Article CAS PubMed PubMed Central Google Scholar * Asahina, M. et al.
Correction to: reversibility of motor dysfunction in the rat model of NGLY1 deficiency. _Mol. Brain_ 14, 127 (2021). Article PubMed PubMed Central Google Scholar * Zhu, L. et al.
AAV9-NGLY1 gene replacement therapy improves phenotypic and biomarker endpoints in a rat model of NGLY1 deficiency. _Mol. Ther. Methods Clin. Dev._ 27, 259–271 (2022). Article CAS PubMed
PubMed Central Google Scholar * Ott, I. & Scott, J. C. The action of infundibulin upon the mammary secretion. _Proc. Soc. Exp. Biol. Med._ 8, 48–49 (1910). Article Google Scholar *
Sharpey-Schafer, E. A. & Mackenzie, K. The action of animal extracts on milk secretion. _Proc. Roy. Soc. London. Ser. B_ 84, 16–22 (1911). Article Google Scholar * Dale, H. H. On some
physiological actions of ergot. _J. Physiol._ 34, 163–206 (1906). Article PubMed PubMed Central Google Scholar * Uvnäs-Moberg, K. Oxytocin may mediate the benefits of positive social
interaction and emotions. _Psychoneuroendocrinology_ 23, 819–835 (1998). Article PubMed Google Scholar * Windle, R. J., Shanks, N., Lightman, S. L. & Ingram, C. D. Central oxytocin
administration reduces stress-induced corticosterone release and anxiety behavior in rats. _Endocrinology_ 138, 2829–2834 (1997). Article CAS PubMed Google Scholar * Amico, J., Mantella,
R., Vollmer, R. & Li, X. Anxiety and stress responses in female oxytocin deficient mice. _J. Neuroendocrinol._ 16, 319–324 (2004). Article CAS PubMed Google Scholar * Takahashi, T.
Sensory stimulation of oxytocin release is associated with stress management and maternal care. _Front. Psychol._ 11, 588068 (2020). Article PubMed Google Scholar * Zinni, M., Colella,
M., Batista Novais, A. R., Baud, O. & Mairesse, J. Modulating the oxytocin system during the perinatal period: a new strategy for neuroprotection of the immature brain? _Front. Neurol._
9, 229 (2018). Article PubMed PubMed Central Google Scholar * van Campen, J. S., Jansen, F. E., de Graan, P. N., Braun, K. P. & Joels, M. Early life stress in epilepsy: a seizure
precipitant and risk factor for epileptogenesis. _Epilepsy Behav._ 38, 160–171 (2014). Article PubMed Google Scholar * Smith, A. S., Korgan, A. C. & Young, W. S. Oxytocin delivered
nasally or intraperitoneally reaches the brain and plasma of normal and oxytocin knockout mice. _Pharmacol. Res._ 146, 104324 (2019). Article CAS PubMed PubMed Central Google Scholar *
Yeomans, D. C. et al. Nasal oxytocin for the treatment of psychiatric disorders and pain: achieving meaningful brain concentrations. _Transl. Psychiatry_ 11, 388 (2021). Article CAS PubMed
PubMed Central Google Scholar * Lee, M. R. et al. Labeled oxytocin administered via the intranasal route reaches the brain in rhesus macaques. _Nat. Commun._ 11, 2783 (2020). Article
CAS PubMed PubMed Central Google Scholar * Guastella, A. J. et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. _Biol. Psychiatry_ 67,
692–694 (2010). Article CAS PubMed Google Scholar * Verhees, M. et al. No side-effects of single intranasal oxytocin administration in middle childhood. _Psychopharmacology_ 235,
2471–2477 (2018). Article CAS PubMed Google Scholar * Rung, J. M. et al. Safety and tolerability of chronic intranasal oxytocin in older men: results from a randomized controlled trial.
_Psychopharmacology_ 238, 2405–2418 (2021). Article CAS PubMed PubMed Central Google Scholar * Law, C. W., Chen, Y., Shi, W. & Smyth, G. K. voom: precision weights unlock linear
model analysis tools for RNA-seq read counts. _Genome Biol._ 15, R29 (2014). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We are deeply grateful to
Drs. Shinya Yamanaka and Yasushi Kajii of the T-CiRA program for supervising this project and the T-CiRA members for the fruitful discussions. We also thank Tomoyuki Kakizume of Takeda
Pharmaceutical Company for their helpful suggestions in the statistical analyses and design of crossover trials, Masahiro Ide and Moe Nomura of RABICS LTD. for supporting the animal
experiments, Noriyasu Sano and Koji Murakami of Axcelead Drug Discovery Partners for the preliminary analysis of seizure-like behaviors, Akira Mitsui and Katsuyuki Nakanishi of Axcelead Drug
Discovery Partners for the transcriptome analyses, and Ayami Onodera of Orizuru Therapeutics for quantifying the seizure-like behaviors. Finally, we express our deep gratitude to the
patients, parents, caregivers, and researchers of Grace Science Foundation. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Takeda-CiRA Joint Program, 26-1, Muraoka-Higashi 2-chome, Fujisawa,
Kanagawa, 251-8555, Japan Yukimasa Makita, Makoto Asahina & Hiroshi Yukitake * Global Advanced Platform, R&D Research, Takeda Pharmaceutical Co., Ltd. 26-1, Muraoka-Higashi 2-chome,
Fujisawa, Kanagawa, 251-8555, Japan Yukimasa Makita, Makoto Asahina, Reiko Fujinawa, Hiroshi Yukitake & Tadashi Suzuki * Glycometabolic Biochemistry Laboratory, Cluster for Pioneering
Research, RIKEN, 2-1 Hirosawa, Wako Saitama, 351-0198, Japan Reiko Fujinawa & Tadashi Suzuki Authors * Yukimasa Makita View author publications You can also search for this author
inPubMed Google Scholar * Makoto Asahina View author publications You can also search for this author inPubMed Google Scholar * Reiko Fujinawa View author publications You can also search
for this author inPubMed Google Scholar * Hiroshi Yukitake View author publications You can also search for this author inPubMed Google Scholar * Tadashi Suzuki View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.M., H.Y. and T.S. conceptualized the research. Y.M. designed the experiments, collected and analyzed the data, wrote
the main manuscript text, and prepared all figures and tables. M.A. and R.F. assisted with data collection. All authors reviewed and approved the manuscript. CORRESPONDING AUTHOR
Correspondence to Tadashi Suzuki. ETHICS DECLARATIONS COMPETING INTERESTS Y.M., H.Y. and M.A. are employees of Takeda Pharmaceutical Company, Ltd. and own stocks or stock options. The other
authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Communications Biology_ thanks Rebecca Levy and Slobodan Janković for their contribution to the peer review of
this work. Primary Handling Editor: Benjamin Bessieres. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY MATERIALS SUPPLEMENTARY MOVIE 1 SUPPLEMENTARY DATA 1 REPORTING SUMMARY
RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes
were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Makita, Y., Asahina, M., Fujinawa, R. _et al._ Intranasal oxytocin suppresses seizure-like behaviors in a mouse model of NGLY1 deficiency. _Commun Biol_ 7, 460 (2024).
https://doi.org/10.1038/s42003-024-06131-7 Download citation * Received: 05 December 2023 * Accepted: 01 April 2024 * Published: 22 April 2024 * DOI:
https://doi.org/10.1038/s42003-024-06131-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
