Multifaceted regulation of siderophore synthesis by multiple regulatory systems in shewanella oneidensis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Siderophore-dependent iron uptake is a mechanism by which microorganisms scavenge and utilize iron for their survival, growth, and many specialized activities, such as
pathogenicity. The siderophore biosynthetic system PubABC in _Shewanella_ can synthesize a series of distinct siderophores, yet how it is regulated in response to iron availability remains
largely unexplored. Here, by whole genome screening we identify TCS components histidine kinase (HK) BarA and response regulator (RR) SsoR as positive regulators of siderophore biosynthesis.
While BarA partners with UvrY to mediate expression of _pubABC_ post-transcriptionally via the Csr regulatory cascade, SsoR is an atypical orphan RR of the OmpR/PhoB subfamily that
activates transcription in a phosphorylation-independent manner. By combining structural analysis and molecular dynamics simulations, we observe conformational changes in OmpR/PhoB-like RRs
that illustrate the impact of phosphorylation on dynamic properties, and that SsoR is locked in the ‘phosphorylated’ state found in phosphorylation-dependent counterparts of the same
subfamily. Furthermore, we show that iron homeostasis global regulator Fur, in addition to mediating transcription of its own regulon, acts as the sensor of iron starvation to increase SsoR
production when needed. Overall, this study delineates an intricate, multi-tiered transcriptional and post-transcriptional regulatory network that governs siderophore biosynthesis. SIMILAR
CONTENT BEING VIEWED BY OTHERS MOLECULAR MECHANISM OF SIDEROPHORE REGULATION BY THE _PSEUDOMONAS AERUGINOSA_ BFMRS TWO-COMPONENT SYSTEM IN RESPONSE TO OSMOTIC STRESS Article Open access 09
March 2024 THE DEOR-LIKE PLEIOTROPIC REGULATOR SCO1897 CONTROLS SPECIALISED METABOLISM, SPORULATION, SPORE GERMINATION, AND PHOSPHORUS ACCUMULATION IN _STREPTOMYCES COELICOLOR_ Article Open
access 07 November 2024 DYNAMIC AND INTRICATE REGULATION BY THE CSR SRNAS IN THE ARCTIC _PSEUDOALTEROMONAS FULIGINEA_ Article Open access 05 March 2025 INTRODUCTION Iron is essential for
virtually all organisms due to its involvement in a range of fundamental biochemical processes such as electron transfer, metabolism, amino acid and nucleoside synthesis, DNA synthesis,
photosynthesis, and gene expression1,2,3. Despite the abundance of iron in the environment, iron acquisition remains a formidable challenge to microorganisms since free iron is readily
oxidized to the ferric state and can form insoluble ferric hydroxide polymers under aerobic conditions4. To overcome this, microbes have evolved sophisticated mechanisms to obtain iron in
various forms from their surroundings, including ferrous (Fe2+), ferric (Fe3+), and iron-containing organic molecules, such as heme. Consistently, transport systems dedicated to iron uptake
are many and diverse3,5. Among them, siderophore-dependent iron acquisition systems are particularly effective in scavenging iron from environmental stocks1,6. Siderophores are a chemically
diverse group of secondary metabolites that bind iron with high affinity, forming soluble Fe3+-siderophore complexes that can be subsequently taken up into the cell7,8. Given the critical
role of siderophores in iron uptake, their biosynthesis and transport are subject to tight regulation. In many bacterial species, the ferric uptake regulator (Fur) is a key player in sensing
intracellular iron levels and modulating gene expression related to siderophore biology1. Additionally, some two-component systems (TCSs) have been implicated in governing the synthesis and
transport of siderophores, such as AlgZ/AlgR and GacS/GacA of _Pseudomonas aeruginosa_9,10. A prototypical TCS contains histidine kinase (HK), which typically is membrane-bound, and soluble
cytoplasmic response regulator (RR). The HK, upon detecting an environmental stimulus, undergoes auto-phosphorylation and subsequently transfers the phosphoryl group to its cognate RR11.
The phosphorylation in the RR at a conserved aspartate residue induces a conformational change, altering the activity of its effecting domain. RRs are most often DNA-binding proteins that
function as a transcriptional regulator12. Apart from TCSs, regulators of other types that play a non-negligible role in the regulation of siderophore pathway have been known in diverse
bacteria, such as sigma factor (e.g., PvdS), sRNA (e.g., RhyB) and RNA chaperone (e.g., Hfq)13,14,15. Many environmental bacteria are renowned for their respiration versatility, which is in
large part due to a vast number of iron-containing proteins, especially hemoproteins16,17. One of the best-studied examples is _Shewanella_, a group of γ-proteobacteria capable of utilizing
numerous compounds as terminal electron acceptors, including oxygen, fumarate, diverse organic and inorganic nitrogen and sulfur compounds, iron, and other metals18,19,20. Conceivably, these
bacteria require iron in substantially larger quantities than model organisms, such as _Escherichia coli_18. Most _Shewanella_, as the genus representative _S. oneidensis_, possess a
three-gene operon (_pubABC_) for the only enzymatic system catalyzing synthesis of three natural macrocyclic hydroxamate siderophores, with putrebactin as the predominant species and
avaroferrin as a robust inhibitor of bacterial swarming behavior21,22,23. Moreover, this PubABC system is rather relaxed in substrate specificity, capable of producing numerous siderophores
if proper synthetic precursors are available24. Despite the importance of the PubABC system in physiology and ecology of _S. oneidensis_ as well as its great potential in biotechnology and
pharmaceutical industry, how the system is regulated at transcriptional levels and beyond remains largely unknown although Fur and SO_2426 have been implicated8,25,26. Here, by using
transposon mutagenesis, we identified TCS components BarA and SO_2426 (renamed as SsoR for siderophore synthesis orphan regulator) as crucial regulatory systems that impact siderophore
production. By partnering with UvrY, BarA mediates siderophore synthesis through two small RNAs (_CrsB1_ and _CrsB2_) and the RNA-binding protein CsrA via post-transcriptional regulation. In
contrast, SsoR functions as an orphan RR, and strikingly its regulatory activity was found to be independent of phosphorylation. Structural analysis and molecular simulations reveal that
SsoR exists in one form only, which mimics the phosphorylated state observed in phosphorylation-dependent RRs. Furthermore, we showed that Fur senses iron levels and regulates transcription
of the _pub_ operon and _ssoR_. In summary, by illustrating a complex and multilayered regulatory network of siderophore synthesis, our results shed light on the evolution of siderophore
production system and its regulation in bacteria. RESULTS BARA AND SSOR ARE POSITIVE REGULATORS OF SIDEROPHORE SYNTHESIS This study aimed to identify potential regulators involved in
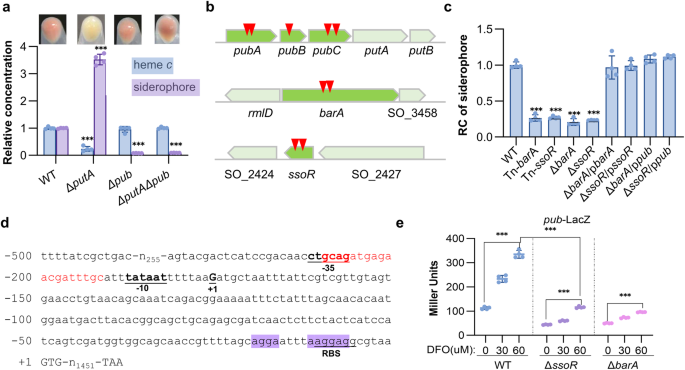
regulation of siderophore synthesis in _Shewanella_. We took advantage of an unexpected color-loss phenotype of Δ_putA_ when grown on LB agar plates, which lacks siderophore receptor PutA27.
Typically, colonies and cell pellets of the _S. oneidensis_ wild-type (WT) strain are reddish-brown, a consequence of the unusually abundant cytochrome _c_ (cyt _c_) proteins (Fig. 1a). On
the contrary, the Δ_putA_ strain loses this signature color, indicative of a significantly reduced cyt _c_ content (Fig. 1a). This phenotypic change is attributed to iron shortage, a result
of the enhanced production, secretion, and extracellular accumulation of siderophore (Fig. 1a)28. Conceivably, the phenotype can be suppressed by the removal of siderophore synthesis operon
_pubABC_. The Δ_putA_Δ_pub_ strain, the same as Δ_pub_ (deleting all three _pub_ genes), has a WT-level cyt _c_ content and consequently regains reddish-brown color (Fig. 1a). To screen for
genes affecting siderophore biosynthesis, a transposon library was constructed from the Δ_putA_ strain and many colonies that recovered reddish-brown color were obtained (Supplementary Fig.
1a). While most of the suppressor mutants carried transposon sequence in the _pub_ operon as expected, several had insertions mapped to _barA_ and _ssoR_ genes multiple times, which encode
the HK of BarA/UvrY TCS and an orphan RR respectively (Fig. 1b). Siderophore assays verified that siderophore production in these isolates was significantly compromised, and this observation
was further substantiated with _barA_ and _ssoR_ in-frame deletion mutants as well as genetic complementation (Fig. 1c, Supplementary Figs. 1b, 2). Influence of BarA and SsoR in vivo on the
expression of the _pub_ operon was then accessed by monitoring the activity of a chromosomally integrated LacZ reporter (_pub_-LacZ) fused to the leader region of _pub_ operon (−500 to the
translation start codon) in cells grown under iron-repleted and iron-depleted conditions (Fig. 1d). While LB broth is used as an iron-repleted medium, desferrioxamine (DFO), which is a
commercially available siderophore that cannot be imported into _S. oneidensis_ cells28, was supplemented to LB to create an iron-depleted medium. As expected, _pub_ expression in the WT
strain was substantially induced, by more than 3-fold, under iron-depleted conditions, and this induction was also observed in the absence of either BarA or SsoR (Fig. 1e). Importantly, the
_pub_ expression in the Δ_barA_ and Δ_ssoR_ strains were significantly lower than that in the WT strain under all conditions tested (Fig. 1e). Moreover, the reduced siderophore production in
the Δ_barA_ and Δ_ssoR_ strains was found to be restored to WT levels by enforced _pub_ expression to proper levels (Fig. 1c, Supplementary Fig. 2). Collectively, these data conclude that
BarA and SsoR act as positive regulators for siderophore synthesis. BARA PARTNERS WITH UVRY BUT NOT WITH SSOR BarA is the HK of a highly conserved TCS called BarA/UvrY (also referred to as
GacS/GacA, BarA/SirA, etc in various species), which has been intensively studied in many Gram-negative bacteria29,30,31,32. BarA is a tripartite HK that has two N-terminal transmembrane
domains followed by a cytoplasmic HAMP domain, a histidine kinase A domain, an ATPase domain, a receiver domain, and a C-terminal histidine phosphotransfer (Hpt) domain (Supplementary Fig.
3)30. Both SsoR and UvrY comprise an N-terminal CheY-like receiver (REC) domain but differ from each other in the C-terminal DNA-binding domain: an OmpR/PhoB type winged helix-turn-helix
(wHTH) in the former versus a LuxR/FixJ type helix-turn-helix (HTH) in the latter (Supplementary Fig. 3)33,34. The phosphorylation residue of _S. oneidensis_ UvrY is Asp54 (D54)30, and its
counterpart within SsoR is highly likely to be Asp52 (D52) according to the annotation of the Uniprot database. BarA in _S. oneidensis_, the same as in all other bacteria hosting the TCS, is
regarded as an orphan HK because it is not in proximity with _uvrY_ on the chromosome. Not surprisingly, cross-talk between BarA and non-cognate RRs (CusR, NarL, NarP, YgeK, RcsB) has been
reported35,36,37. Given that both BarA and SsoR but no other TCS components were identified by transposon screening, we speculated that BarA may function as the HK for SsoR too. To test
this, we expressed C-terminally His6-tagged SsoR and UvrY under the control of isopropyl β-D-1-thiogalactoside (IPTG)-inducible promoter P_tac_ in WT and Δ_barA_. Total proteomes were
extracted from cells grown to the mid-exponential phase and applied to SDS-PAGE containing phosphate-binding tag (Phos-tag), which can associate with the divalent cation of Mn2+ and form a
complex with the phosphorylated proteins, thus retarding migration38. Conventional SDS-PAGE followed by Western blotting revealed single bands for both UvrY and SsoR recombinant proteins
regardless of the strain background, with band intensities correlating with IPTG concentrations (Fig. 2a). However, these two proteins behaved clearly differently in Phos-tag SDS-PAGE and
Western Blotting (Fig. 2b). UvrY proteins existed in both the phosphorylated and unphosphorylated forms in WT, but only in the unphosphorylated form in the absence of BarA. In contrast, SsoR
proteins were always present in both forms. These data indicate that phosphorylation of UvrY but not SsoR in vivo is dependent on BarA, and therefore, BarA/UvrY and SsoR regulate
siderophore synthesis through separate pathways in _S. oneidensis_. BARA/UVRY TCS POSITIVELY REGULATES SIDEROPHORE SYNTHESIS THROUGH THE CSR REGULATORY CASCADE The signals sensed by BarA
have been suggested to be metabolic end products, short-chain fatty acids in particular, such as formate and acetate39. Subsequently, a classical phosphor-relay occurs, resulting in
phosphorylated UvrY (UvrY-P), which in turn activates the transcription of small regulatory RNAs CsrB and CsrC40. These RNAs interact directly with CsrA, a global RNA-binding protein,
influencing its ability to either repress or enhance the expression of its RNA targets41,42, thereby affecting diverse biological processes, including carbon metabolism, biofilm formation,
motility, virulence, and siderophore synthesis31,43,44,45,46,47. The BarA regulatory cascade of _S. oneidensis_ has been proposed to include BarA, UvrY, two regulatory RNAs CsrB1 and CsrB2,
and presumably CsrA30. To unravel how BarA/UvrY/Csr system is linked to siderophore synthesis in _S. oneidensis_, we assessed siderophore levels in mutants lacking each of these components.
It was worth mentioning that the Δ_csrA_ strain showed extremely severe growth defects when grown in LB, which could be completely corrected by moderate expression of _csrA_ in trans
(Supplementary Fig. 4a), echoing that the loss of CsrA has profound and pleiotropic effects on the physiology of _E. coli_48. Like Δ_barA_, the Δ_uvrY_ strain was heavily defective in
siderophore production (Fig. 3a). Similar results were obtained from a _csrB1csrB2_ double knockout (Fig. 3a), which was expected as the expression of _csrB1_ and _csrB2_ depends on
BarA/UvrY (Fig. 3b). Expression of either _csrB1_ or _csrB2_ in trans in Δ_csrB1_Δ_csrB2_ was able to restore siderophore synthesis (Supplementary Fig. 2), indicating that both sRNAs are
functional. Conceivably, similar to the effect of enforced expression of the _pub_ operon, enforced expression of either _csrB1_ or _csrB2_ in the Δ_uvrY_ strain led to a substantial
increase in siderophore production (Fig. 3c, Supplementary Fig. 2). We also observed that siderophore production was inversely correlated to CsrA levels. The _csrA_ deletion drastically
increased siderophore production, and this effect was independent of BarA/UvrY (Fig. 3a, Supplementary Fig. 1b), whereas overexpression of _csrA_ in the Δ_csrA_ strain reduced siderophore
production to the levels below that observed in the WT strain (Fig. 3c, Supplementary Fig. 2). CsrA typically binds to mRNAs containing GGA motif(s) in the 5′ untranslated region (5′UTR),
causing changes in RNA structure, translation, stability, and/or transcription elongation41. To predict the CsrA-binding sites in the region upstream of the _pub_ operon, the transcriptional
start site was determined to be −175 by 5’RACE and the promoter (P_pub_) elements such as −10 and −35 boxes were then proposed (Fig. 1d). Two potential CsrA-binding sites, AGGA that is
located before ribosomal binding site (RBS) and AAGGAG that overlaps with the RBS, were identified in the 5′UTR of the _pub_ transcript (Fig. 1d). These positions coincide with the findings
that CsrA commonly binds to sites overlapping RBS and/or translation initiation region, competing with 30 S ribosomal subunit41. To probe how BarA/UvrY/Csr system regulates the expression of
the _pub_, we examined the transcript levels and translation levels of _pubA_ in relevant strains with qRT-PCR and the _pub_-LacZ reporter respectively. Apparently, mRNA levels of _pubA_ in
each of the mutants under test, including Δ_barA_, Δ_uvrY_, Δ_csrA_, Δ_barA_Δ_csrA_, Δ_uvrY_Δ_csrA_, and Δ_csrB1_Δ_csrB2_, were only slightly different from that in WT: decrease in Δ_barA_,
Δ_uvrY_, and Δ_csrB1_Δ_csrB2_ by about a fifth but increase in any strains lacking CsrA by about a fifth (Fig. 3d). On the contrary, the _pub_-LacZ reporter revealed that the differences in
expression levels between mutants and WT were substantially more pronounced (Fig. 3d). Additionally, a vector expressing PubA with a His6-tag at the C-terminus driven by the entire leader
region upstream of the coding sequence (P_pub_-5’UTR_pub_-_pubA_) was introduced into these strains. By Western blotting, we found that the PubA levels were in excellent agreement with the
_pub_-LacZ data (Fig. 3d). Consistently, repression of overexpressed CsrA on _pubA_ transcription was rather modest, but became much stronger on the protein level (Supplementary Fig. 4b),
supporting the proposal that CsrA inhibits _pub_ expression at the post-transcriptional level in vivo. Interestingly, it seemed that CsrB2 plays a more important role in antagonizing CsrA
activity because CsrB2 was more effective than CsrB1 in elevating PubA protein levels under the same induction conditions (Supplementary Fig. 4b). Moreover, we used the constitutively active
_arcA_ promoter (P_arcA_)49, whose activities were comparable in these strains under experimental conditions (Supplementary Fig. 4c), in place of P_pub_ to drive the expression of the
His6-tagged PubA and similar results were obtained (Fig. 3e). However, when 5’UTR of the _pub_ operon was replaced by 5’UTR of _arcA_ (P_arcA_-5’UTR_arcA_-_pubA_), the PubA protein levels
were no longer responsive to abundance changes of any component of the BarA/UvrY/Csr system (Fig. 3e). To further support that the 5’UTR of the _pub_ transcript contains the regulatory
elements for CsrA, we introduced mutations into the predicted CsrA-binding motif that overlaps the RBS, including GGA-to AGA point mutation and GGA deletion. The results showed that the GGA
deletion abolished expression, which can be readily explained by the removal of the RBS (Fig. 3f). In contrast, the GGA-to AGA point mutation resulted in a significant increase in expression
in WT strain but a negligible change in the Δ_csrA_ strain (Fig. 3f), suggesting that CrsA interacts with the _pub_ transcript at the predicted CsrA-binding motif. Therefore, all of these
data collectively conclude that BarA/UvrY TCS mediates expression of the _pub_ operon via the pathway involving CsrB1, CsrB2, and CsrA. SSOR IS AN ATYPICAL ORPHAN RR IN TERMS OF STRUCTURE
AND PHYLOGENY We next made attempts to identify the possible cognate HK for SsoR. Information on TCSs in _S. oneidensis_ was gathered from multiple sources, such as P2CS (Prokaryotic
2-Component Systems; http://www.p2cs.org)50 and MiST3 (Microbial Signal Transduction database; https://mistdb.com)51. In total, the _S. oneidensis_ genome encodes 103 predicted TCS
components, including 43 HKs, 57 RRs, and 3 Hpts (histidine-containing phosphotransfer proteins) (Supplementary Table 1). Among them, four HK genes (SO_2889, SO_3162, SO_3894, and SO_3999)
and one Hpt (SO_0981) neither are adjacent to an RR gene nor encode proteins belonging to a TCS in which the RR is experimentally confirmed (Supplementary Table 1). To test whether any of
these orphan HKs could phosphorylate SsoR, we generated their in-frame deletion strains, in which His6-tagged recombinant SsoR was examined in terms of the phosphorylation status. The
results showed that SsoR proteins were present in both unphosphorylated and phosphorylated forms in all the mutants as in WT (Fig. 4a), eliminating the possibility that these HKs could act
as the cognate HK for SsoR. Given that some RRs are functional in the unphosphorylated form52,53, we then asked whether SsoR functions independent of phosphorylation. To address this, we
first predicted the structure of SsoR with ColabFold and refined it based on available structures of representative members of the OmpR/PhoB subfamily. SsoR supposedly functions as a dimer,
with each subunit comprising a highly conserved REC domain at the N-terminus, an unusually long flexible linker, and the C-terminal DNA-binding domain (Fig. 4b)54,55,56. While the
DNA-binding domain is composed of a four-stranded β sheet, a wHTH motif, and a β hairpin, the REC domain that consists of five α-helices encircling a central β-sheet of five parallel
strands, arranged in a 21345 topology is responsible for dimerization (Fig. 4b, c)12,57. It has been established that the REC domain alternates between inactive and active allosteric
conformations, with phosphorylation influencing this balance56. The phosphorylation-mediated activation depends on a common dimerization mechanism, called the Y-T coupling that involves a
conserved Thr/Ser (T/S) residue (T83 and S77 in _Ec_PhoB and SsoR respectively) in the phosphorylation pocket influencing the rotameric state of a Tyr/Phe (Y/F) residue (Y102 and F96 in
_Ec_PhoB and SsoR respectively) in the β5 strand, which is also called switch residue (Fig. 4b, c)56,58. Phosphorylation induces a conformational shift in the α4-β5-α5 face, promoting
dimerization, which in turn enhances DNA binding to promoter recognition elements (Fig. 4b)59. Sequence and secondary structure alignments revealed that SsoR retains the conserved and
essential residues of the phosphorylation pocket, including D8, D9, D52, S77, F96 and K99, unlike other characterized RRs that could be active in the non-phosphorylated form such as
_Helicobacter pylori_ HP1043 (Fig. 4c)56,60. We then carried out the analyses of sequences and evolutionary relationships of representative RRs, including SsoR. The Uniref50 sequences of all
OmpR subfamily RRs were retrieved, and five clusters including PhoBs, SsoRs, VbrRs, KdpEs, and CusRs were selected to construct an evolutionary tree for structure alignments (Fig. 5a). For
each cluster, all members have the same genomic backgrounds, either standing alone or next to an HK gene (Supplementary Fig. 5). Apparently, the SsoRs cluster is small, compared to those
made of other RRs (Fig. 5a). Intriguingly, the SsoRs cluster seemed to have emerged at the same time as the PhoBs cluster during evolution, but the VbrRs and KdpEs clusters diverged more
anciently (Fig. 5a). The CusRs cluster, composed of the homologs of _H. pylori_ HP1043, separated from other RRs in the tree even earlier (Fig. 5a). In the OmpR/PhoB subfamily RRs, the
switch residue (Fig. 4b, c) could work as an indicator of an RR’s status56,61,62. Indeed, the REC domains of the OmpR/PhoB subfamily RRs from the PDB database share highly similar structures
but vary in the orientation of the switch residues (Fig. 5b, c). To further verify this, we generated a structural similarity dendrogram with the _Ec_PhoB PDB structures and predicted
structures as AlphaFold2 can generate various conformations that exist naturally, even with identical input protein sequences, which are equivalent to multiple same sequences63. The REC
domain structures of 55 proteins sharing identical sequences with _Ec_PhoB were collected from AlphaFold Protein Structure Database for dendrogram construction using DALI (Supplementary Fig.
6a), which summarizes the occurrence frequency of all possible conformations, that is, the orientation of the switch residue (Fig. 5c). The switch residues in the predicted structures were
found to be in one of four major different orientations, inner (active, phosphorylated), outer (inactive, non-phosphorylated), and two intermediate states, which are less frequent under
native conditions (Fig. 5c). Importantly, from identical PhoB proteins, the conformations isolated by the orientation of the switch residues tend to cluster into distinct groups on the
dendrogram, supporting that the predicted structures are consistent with those obtained experimentally (Supplementary Fig. 6a–c). Then, the predicted REC domain structures of the members in
the evolutionary tree were gathered from the AlphaFold Protein Structure Database, aligned in PyMol, and the numbers of all varying states were counted (Fig. 5c). The switch residues in two
groups of phosphorylation-dependent RRs, PhoBs and VbrRs, could be active, inactive, and intermediate states. In contrast, the proportion of switch residue orientations in the SsoRs and
CusRs clusters notably differs from that of the PhoBs and VbrRs clusters. Specifically, the switch residue of the phosphorylation-independent CusRs cluster exclusively exhibits inward
orientations (Fig. 5d), consistent with observations in the structure of the CusRs cluster member HP1043 (PDB: 2PLN)60, and a similar scenario was found with the SsoRs cluster members. More
importantly, the lack of the occurrence of outer state in these two groups of RRS indicates that they could not exist in unphosphorylated inactive form (Fig. 5d), strongly supporting that
SsoR possibly is active independent of phosphorylation. SSOR REGULATES TRANSCRIPTION IN A PHOSPHORYLATION-INDEPENDENT MANNER To address that SsoR probably employs a
phosphorylation-independent activation mechanism, we compared the regulation activity of two SsoR variants carrying mutations at the phosphorylation residue (D52), SsoRD52N and SsoRD52E.
Both variants are in the non-phosphorylated form, but the Asp to Glu mutation is phosphomimetic64, that is, SsoRD52E would be constitutively active. Unlike SsoR, both SsoRD52N and SsoRD52E
migrated on SDS-PAGE as a single band independent of Phos-tag (Fig. 6a), validating that they exist in the non-phosphorylated form only. To assess the regulatory activity, these SsoR
variants were expressed in the Δ_ssoR_ strain and siderophore production and _pub_ expression were examined. When expressed at the same levels, both SsoRD52N and SsoRD52E behaved
indistinguishably from SsoR (Fig. 6b, c, Supplementary Fig. 2), indicating that they are functional. In addition, His6-tagged recombinant SsoR, SsoRD52N, and SsoRD52E proteins were expressed
and purified from _E. coli_ (Supplementary Fig. 7), and direct interaction between the purified proteins and the _pub_ promoter region were analyzed by EMSA. Apparently, all SsoR variants
bound well to the DNA fragment in comparison with negative control P16s (the promoter sequence of the 16 s rRNA gene) (Fig. 6d). Altogether, these data allow us to conclude that SsoR
directly regulates transcription of the _pub_ operon in a phosphorylation-independent manner. CONFORMATIONAL OCCURRENCE OF THE SWITCH RESIDUE IN SSOR To unravel the mechanism underlying the
phosphorylation-independent regulation of SsoR, comparative analyses of SsoR and phosphorylation-dependent _Vp_VbrR (VbrR from _Vibrio parahaemolyticus_) were conducted with all-atoms
molecular dynamics (MD) simulations. The systems were built on the phosphorylation mimic VbrRD-RD-D51E (PDB: 7E90) dimer, the non-phosphorylated VbrRD-RD-D51N dimer, and the monomers
(VbrRM-RD-D51N and VbrRM-RD-D51E) extracted65. In parallel, we constructed systems for SsoRD-RD-D52E, SsoRD-RD-D52N, SsoRM-RD-D52E, and SsoRM-RD-D52N. Each system underwent a 3 μs MD
simulation, and the resulting trajectories were generated and analyzed, each consisting of 3000 frames (1 ns per frame). According to the Chi1 angle of the switch residue and the distance
between the backbone N of SsoR98T or VbrR99T and the CZ atom of SsoR96F or VbrR97Y switch residue, the frames were categorized into four dynamics states as described in Fig. 5c
(Supplementary Movie 1). In fact, the conformational changes among “inner state”, “intermediate state A”, and “intermediate state B” are continuous processes, encompassing a large number of
intermediate states. A statistical analysis of the distribution of the four states in the trajectories was then conducted (Fig. 7a-h). It was observed that the outer state only exists in
VbrRD-RD-D51N and VbrRM-RD-D51N (Fig. 7f, h) but not VbrRD-RD-D51E and VbrRM-RD-D51E (Fig. 7e, g), indicating that the mimicked phosphorylation in phosphorylation site blocks conformational
transition from inner state to outer site. The distribution patterns of these states differ between the monomeric and dimeric forms. The intermediate state B is notably more prominent in
VbrRM-RD-D51E (Fig. 7d) compared to VbrRM-RD-D51N (Fig. 7h), but the trend is opposite in dimers (Fig. 7b, f). Moreover, in the dimeric form, a higher occurrence of intermediate state A is
observed in VbrRD-RD-D51N (Fig. 7f). Furthermore, the distribution ratio of inner and outer states is notably higher in VbrRD-RD-D51N (Fig. 7f) than that of VbrRM-RD-D51N (Fig. 7h),
demonstrating that a tendency exists in the inner state when in the dimeric configuration. In addition, it is worth mentioning that the MD simulation results of VbrRM-RD-D51N are consistent
with the alignment results of VbrRs (Figs. 5d and 7f). The conformational occurrences of the switch residues in SsoRD-RD-D52E (Fig. 7a), SsoRM-RD-D52E (Fig. 7c), SsoRD-RD-D52N (Fig. 7e), and
SsoRM-RD-D52N (Fig. 7g) are notably uniform, with the inner state being predominant, and only a small number of the outer states being observed in SsoRM-RD-D52N (Fig. 7g). Although the
occurrences of the inner state between SsoRM-RD-D52E (Fig. 7c) and SsoRM-RD-D52N (Fig. 7g) exhibit some differences as the switch residue is more stable in SsoRM-RD-D52E (RMSF of switch
residue = 0.111 in SsoRM-RD-D52E, 0.136 in SsoRM-RD-D52N) and outer state appears in SsoRM-RD-D52N but not SsoRM-RD-D52E (Fig. 7c, g), the MD simulations overwhelmingly support that the
state of the phosphorylation site has almost no effect on SsoR. By combining physiological and biochemical data presented above, we conclude that SsoR represents a group of OmpR/PhoB
subfamily RRs that are unique in that they function in a phosphorylation-independent manner despite retaining a phosphorylation pocket and the popular ‘Y-T coupling’ mechanism. FUR ACTS AS
AN IRON SENSOR TO REGULATE TRANSCRIPTION OF THE _PUB_ OPERON BOTH DIRECTLY AND VIA SSOR A Fur-binding site (gcagatgagaacgatttgc, −210 to −192 relative to the start codon) partially
overlapping the −35 box of the _pub_ promoter was reported before66, implying that Fur likely acts as a transcriptional repressor for the _pub_ operon (Fig. 1e). By using EMSA, we first
substantiated the direct interaction between purified His6-tagged Fur and the _pub_ promoter sequence (Fig. 8a). Then the repressing effect of Fur on transcription of the _pub_ operon was
confirmed by qRT-PCR and Western blot: the Fur loss resulted in substantially increased transcription, and this elevation was no longer responsive to the changes in iron levels (Fig. 8b). In
addition, we found that Fur is also responsible for sensing iron in the absence of SsoR with a _fur ssoR_ double mutant, in which the _pub_ expression was found not to be altered
significantly upon changes in iron levels (Fig. 8b). As a phosphorylation-independent transcriptional regulator, SsoR likely enhances expression of its target genes by increasing its own
abundance. Our previous proteomic analyses revealed a 4.32-fold increase in SsoR protein levels in the Δ_fur_ strain compared to WT, suggesting that Fur represses SsoR expression67. In line
with this, a Fur-binding motif was identified in the _ssoR_ promoter region, which partially overlaps with the −10 box and the transcription start site (Fig. 8c). With LacZ reporter and
Western blotting, we found that the expression of _ssoR_ was induced inversely proportional to iron levels in the WT strain but became constitutive at significantly higher levels in the
Δ_fur_ strain (Fig. 8d). Moreover, SsoR is subject to self-regulation. The _ssoR-_LacZ reporter assay showed that the absence of _ssoR_ enhanced β‐galactosidase activity considerably
compared to that of the WT strain grown under the same conditions (Fig. 8d). Although the additional removal of Fur abolished the response to iron levels, the repressing effect of SsoR on
its own expression was still observable (Fig. 8d). Furthermore, we substantiated that SsoR proteins in either phosphorylated or non-phosphorylated form were able to bind with the _ssoR_
promoter fragment (Fig. 8e), strengthening that SsoR functions independently of phosphorylation. All these data indicate that in _S. oneidensis_, Fur is the primary, if not exclusive, iron
sensor and, by sensing changes in intracellular iron levels, influences siderophore biosynthesis both directly and indirectly. In cells grown under iron-repleted conditions, Fur is
sufficient to repress the transcription of the _pub_ operon, but when iron is scarce, Fur-mediated repression is relieved, and transcription is activated by SsoR. We envision that
self-regulation of SsoR offers an additional safeguarding mechanism to prevent this activity-unconstrained regulator from overproduction. DISCUSSION _Shewanella_ are found in a wide range of
ecological niches and play a critical role in global element cycles because of their unparallel respiration versatility. This capacity is largely based on iron proteins, and, therefore
_Shewanella_ usually has high iron demand, which relies on multiple strategies for iron uptake16,17,18. One of the unique features of most _Shewanella_ is the presence of a single enzyme
system for biosynthesis of an array of siderophores8,23. Importantly, some of the siderophores have additional activities, such as inhibition of motility and biofilm formation, and would
conceivably have a profound ecological impact on shaping local community7,8. However, our understanding of the regulatory mechanisms behind siderophore synthesis in _Shewanella_ is still
limited. In this work, we identified two TCSs, along with Fur, that modulate the siderophore production at multiple levels. While BarA/UvrY relies on an sRNA-dependent cascade, SsoR is an RR
that functions in a phosphorylation-independent manner (Fig. 9). Identification of these two regulators was enabled by the unexpected color-loss phenotype of the siderophore-overproducing
strain Δ_putA_27,28. Disruption of either _barA_ or _ssoR_ by transposon insertion compromises siderophore production, restoring the signature colony color. Although it is attractive to
speculate that BarA and SsoR may belong to the same regulatory pathway, BarA does not affect the phosphorylation state of SsoR in vivo. Instead, BarA constitutes a TCS with UvrY as in many
other bacteria hosting this system30,32, affecting the expression of the siderophore biosynthesis system PubABC via the post-transcriptional regulatory mechanism (Fig. 9). Our study shows
that the BarA/UvrY system of _S. oneidensis_, in line with its counterparts in other γ-proteobacteria such as _E. coli_ and _P. aeruginosa_, employs Csr/Rsm cascade to regulate siderophore
synthesis9,68. At least two sRNAs, CsrB1 and CsrB2, and RNA chaperone CsrA were identified to play critical roles in transducing signals perceived by BarA and relayed by UvrY to control
siderophore biosynthesis. Multiple lines of evidence were presented to support that CsrA directly interacts with the _pub_ transcript to block translation. This effect is antagonized by
CsrB1 and CsrB2, whose transcription is activated by UvrY upon phosphorylation. However, given that the BarA/UvrY/Csr regulatory network is rather complex, featuring autoregulatory circuitry
and the involvement of various factors like cAMP-CRP and RpoE42,69,70, further investigation is needed to identify other factors that influence siderophore synthesis through the
BarA/UvrY/Csr pathway in _Shewanella_. Since the physiological stimulus for BarA has been suggested to be metabolic end products39,42,69,70, we speculate that perhaps shifts in carbon
metabolism or some secondary metabolite processes trigger the response, thereby putting siderophore biosynthesis under the control of cellular metabolic status. Coupling carbon metabolism
and iron uptake may be particularly important for _Shewanella_ as this group of bacteria encodes a vast number of iron-containing proteins, many of which are involved in metabolism as
enzymes and electron carriers71,72. RRs of the OmpR/PhoB subfamily are thought to become activated through phosphorylation, which triggers an allosteric change to enable homodimerization and
enhance DNA binding56,59. However, some are able to bind DNA without phosphorylation to exert different functions not observed from their phosphorylated counterparts52,53,73. Hence, one of
the most striking findings in this study is that as an RR of the OmpR/PhoB subfamily, SsoR regulates siderophore synthesis in a phosphorylation-independent manner. By examining the
phosphorylation state of SsoR in vivo, we eliminated the possibility that the orphan HKs that phosphorylate SsoR are present. Results of both in vivo gene expression analysis and in vitro
EMSA assay support that regulation of the _pub_ operon transcription by SsoR is not dependent on phosphorylation, providing a case for noncanonical functioning modes of the OmpR/PhoB
subfamily. In contrast to atypical RRs reported before that do not require phosphorylation for activity60,64, SsoR has key conserved sites for phosphorylation-dependent regulation, and in
the evolutionary tree, the SsoRs cluster is closely related to the typical PhoB RRs. By aligning AlpahFold2 predicted structures, we found distinctive behaviors of the F/Y switch residue of
SsoRs from those of the phosphorylation-dependent RRs, such as PhoBs and VbrRs. Importantly, MD simulations further reveal the contrasting effects of phosphorylation on the conformational
occurrences of the switch residues in typical VbrRs and atypical SsoRs. The switch residue in phosphorylation-dependent RRs could be in any state, but its counterpart in SsoR is locked in
the active inner state only. Nevertheless, SsoRs belong to the OmpR/PhoB subfamily as they retain all conserved features observed from the phosphorylation-dependent members, the ‘Y-T
coupling’ mechanism in particular56. This is in sharp contrast to CusRs (HP1043), whose independence of phosphorylation is due to the lack of the phosphorylation residue (Fig. 4c). Thus,
SsoRs represent a unique group of OmpR/PhoB subfamily RRs that evolve out a phosphorylation-independent activating mechanism from the conventional phosphorylation-dependent chassis. How this
occurs can be addressed by more in-depth structural analysis and MD simulations, which are underway. Although phosphorylation is not required for functionality, SsoR retains the ability to
be phosphorylated because of a highly conserved phosphorylation pocket56,74. As a result, a portion of SsoR appears to be constitutively phosphorylated in the cell, indicating the presence
of phosphate donors. Given that none of the orphan HKs are found to be the exclusive cognate HK for SsoR, candidate phosphate donors should be alternative orphan kinases, non-cognate
kinases, and/or small-molecule high-energy phosphodonors, such as phosphoramidate and acetyl phosphate75,76. In addition to BarA/UvrY TCS and SsoR, Fur regulates the expression of the _pub_
operon both directly and indirectly in response to iron availability. Under iron-repleted conditions, Fur binds to the Fur-boxes in the promoter region of the _ssoR_ gene and the _pub_
operon, which overlap the RNA polymerase binding site, to repress transcription. Under iron-depleted conditions, Fur falls off, allowing transcription of both _pubABC_ and _ssoR_. SsoR seems
to be produced in needed quantity upon the Fur removal, which in turn provides additional activation for _pubABC_ transcription. In addition, SsoR represses its own expression, preventing
overproduction. Clearly, only when SsoR and Fur work together, _S. oneidensis_ cells are capable of rapidly upregulating the siderophore synthesis when faced with iron-depleted condition.
Some atypical phosphorylation-independent RRs which lack HK adopt alternative strategies, such as post-translational acetylation, to regulate their own activity56. Our research has revealed
a unique paradigm, SsoR is not phosphorylation-dependent but dose-dependent to regulate the transcription of its regulon. Here, Fur acts as the sensor for SsoR. Fur’s regulation and
self-regulation together prevent SsoR from being constitutively active. We suggest that perhaps accidental loss of the HK gene occurred first, forcing SsoR to select another type of sensory
partner and evolve a phosphorylation-independent activation capacity, or perhaps Fur regulation occurred first, leading to redundancy of HK. Either way, this merits further investigation.
Overall, this study suggests that through the orchestrated regulatory network, different signals, i.e., iron availability or central metabolic state, are integrated into the multilayered
regulation of siderophore synthesis, providing more insights into the current understanding of already complex regulatory mechanisms for siderophore production in bacteria. METHODS BACTERIAL
STRAINS, PLASMIDS, AND CULTURE CONDITIONS Bacterial strains and plasmids used in this study are listed in Supplementary Table 2. Information for primers used in this study is given in
Supplementary Table 3. Chemicals were obtained from Sigma‐Aldrich (Shanghai, China) unless otherwise noted. _E. coli_ and _S. oneidensis_ strains were grown under aerobic conditions in
Lennox LB (Difco, Beijing, China) under aerobic conditions at 37 and 30 °C for genetic manipulation. When needed, the growth medium was supplemented with chemicals at the following
concentrations: 2,6‐diaminopimelic acid (DAP), 0.3 mM; ampicillin sodium, 50 μg/ml; kanamycin sulfate, 50 μg/ml; and gentamycin sulfate; 15 μg/ml. IN-FRAME MUTANT CONSTRUCTION AND GENETIC
COMPLEMENTATION In‐frame deletion strains for _S. oneidensis_ were constructed using the _att_‐based fusion PCR method77. In brief, two fragments flanking the gene of interest were amplified
and then joined together by a second round of PCR. The resulting fusion fragment was introduced into suicide plasmid pHGM01 by site‐specific recombination using the BP Clonase (Invitrogen,
Carlsbad, CA) and the resulting mutagenesis vectors were maintained in _E. coli_ DAP-auxotroph WM3064. The vectors were then transferred from _E. coli_ into the relevant _S. oneidensis_
strain by conjugation. Integration of the mutagenesis construct into the chromosome was selected by gentamycin resistance and confirmed by PCR. For genetic complementation of the mutants and
inducible gene expression, genes of interest generated by PCR were cloned into pHGEN‐Ptac under the control of IPTG-inducible promoter P_tac_78. After verification by sequencing, the
resultant vectors in _E. coli_ WM3064 were transferred into the relevant strains via conjugation. SITE-DIRECTED MUTAGENESIS Site-directed mutagenesis was performed to generate SsoR proteins
carrying point mutations (D52N and D52E) using a QuikChange II XL site-directed mutagenesis kit (Agilent, Beijing, China). The _ssoR_ gene within pHGEN-Ptac and pET-28a(+) was subjected to
modification, and the resulting products were digested by DpnI at 37 °C for 6 h and subsequently transformed into _E. coli_ WM3064. The vectors carrying the intended mutations, which were
verified by sequencing, were transferred into the relevant _S. oneidensis_ and _E. coli_ strains by conjugation. TRANSPOSON MUTAGENESIS A random mutation library for the Δ_putA_ strain,
which forms white colonies on LB agar plates, was constructed with mariner-based plasmid pFAC79,80. A total of ~15,000 random mutants were screened for reddish-brown colonies on LB agar
plates supplemented with gentamycin. To identify the transposon insertion sites in these isolates, arbitrary PCR was employed81. HEME _C_ ASSAYS Cultures of _S. oneidensis_ strains grown in
liquid LB to the early stationary phase were centrifuged, and the pellets were photographed. The cytochrome _c_ abundance of strains was first estimated by the color intensity of the cell
pellets. Subsequently, the pellets were suspended in phosphate-buffered saline (PBS, pH 7.0), adjusted to the same OD600 values, and the cells from the same-volume aliquots were disrupted.
All proteins were precipitated by trichloroacetic acid precipitation82 and assayed for heme _c_ levels with the QuantiChrom heme assay kit (BioAssay Systems, CA, USA) according to the
manufacturer’s instructions. SIDEROPHORE MEASUREMENT To visualize siderophores, _S. oneidensis_ strains grown on LB agar plates were subjected to Chrome Azurol S (CAS) plate assay using CAS
and Hexadecyltrimethylammonium bromide (HDTMA) as indicators. Siderophores with higher iron affinity scavenge iron from the Fe-CAS-HDTMA complex, and subsequent release of the CAS dye
results in a color shift from blue to orange83. Ten microliters of cultures of the mid-exponential phase (OD600, ~0.6, the same throughout the study) were dropped and incubated on LB agar
plates containing 30 mM DFO for 24 h, followed by pouring in CAS reagent to completely cover the entire plate. The formation of chelated halos was observed and photographed three hours
later. To quantify total siderophores, _S. oneidensis_ strains were grown in liquid LB to the stationary phase, and cell-free culture supernatants were obtained by centrifugation.
Siderophore concentrations within the supernatants were determined using the liquid CAS assay83. SDS-PAGE, MN(II)-PHOS-TAG SDS-PAGE, AND WESTERN BLOTTING ASSAYS Cells entering the
mid-exponential growth phase were harvested by centrifugation, washed with Tris/HCl (pH 7.0) buffer containing phosphatase inhibitors (Solarbio, Beijing, China), resuspended in the same
buffer, and sonicated. Throughout this study, the total protein concentration of the cell lysates was determined by the bicinchoninic acid assay using bovine serum albumin (BSA) as a
standard or using a GE NanoVue Spectrophotometer for fast assessment. Conventional SDS-PAGE was performed using slab gels consisting of a 10% acrylamide separating gel, and a 5% stacking
gel. Mn(II)-Phos-tag SDS-PAGE was used to separate SsoR and UvrY proteins in different phosphorylation states. Fifty μM acrylamide-pendant Phos-tag ligand (Wako Pure Chemical, Osaka, Japan)
and 100 μM MnCl2 were added to a 10% separating gel before polymerization according to the instructions provided by the Phos-tag Consortium84. After electrophoresis, Phos-tag acrylamide gels
were washed with transfer buffer (50 mM Tris, 384 mM glycine, 20% methanol) containing 1 mM EDTA for 10 min with gentle shaking and then with transfer buffer without EDTA for 10 min to
remove Mn2+. Proteins on the PAGE gels were then electrophoretically transferred to PVDF membrane (Millipore, Bedford, MA) according to the manufacturer’s instructions (Bio-Rad, Hercules,
CA, USA). Tris Buffered Saline with 0.1% Tween containing 5% BSA was used to block the membrane. The membrane was probed with a 1:5000 dilution of a mouse monoclonal his-tag antibody
(Abbkine, Shanghai, China), followed by a 1:10,000 dilution of Goat anti-mouse IgG-HRP (horseradish peroxidase) (Beyotime, Beijing, China) and the signal was detected using a
chemiluminescence Western blotting kit (Roche, Basel, Switzerland). Images were visualized with ChemiScope 6000 Imaging System (Clinx, Shanghai, China). LACZ REPORTER ASSAY Expression of
target genes was assessed using a single‐copy integrative LacZ reporter system85. Briefly, fragments containing the sequence upstream of the target operons (−500 to +1 relative to the
translation start codon) were amplified, cloned into the reporter vector pHGEI01, and transformed into _E. coli_ WM3064 and verified by sequencing. The correct vector was then transferred by
conjugation into relevant _S. oneidensis_ strains, which it integrated into the chromosome. Cells of the mid-exponential phase under test conditions were harvested by centrifugation, washed
with PBS, and lyzed with the lysis buffer (0.25 M Tris/HCl, pH 7.5, 0.5% Triton X-100). The resulting soluble protein was collected after centrifugation and used for enzyme assay by adding
the aliquot of the o-nitrophenyl-β-d-galactopyranoside (4 mg/ml). β‐galactosidase activity was determined by monitoring color development at 420 nm using a Synergy 2 Pro200 Multi‐Detection
Microplate Reader (Tecan, Männedorf, Switzerland), and results were presented as Miller units. QUANTITATIVE RT-PCR (QRT-PCR) Total RNAs were extracted using a Trizol reagent (Invitrogen,
Carlsbad, CA, USA) following the manufacturer’s instructions. The extracted RNAs were purified using an RNeasy Mini Kit and RNase-Free DNase Set (Qiagen, Valencia, CA, USA). The QuantiTect
Reverse Transcription Kit (Qiagen, Valencia, CA, USA) was used to synthesize cDNA.RT-qPCR was performed using 2xSYBR Green PCR Mastermix (Solarbio, Beijing, China) and monitored in CFX Opus
Real-time PCR System (Bio-Rad, Hercules, CA, USA). The cycle threshold (CT) values for each gene of interest were averaged and normalized against the CT value of the _arcA_ gene, whose
abundance was constant during the exponential phase. The relative abundance (RA) of each gene compared with that of _arcA_ was calculated using the 2−ΔΔCT method86. The expression of each
gene was determined from four biological replicates, and in a single qRT-PCR experiment, three replicates were measured. DETECTION OF PROTEIN LEVELS IN VIVO The fragments containing the
natural or recombinant leader region and open reading frame of _pubA_ and _ssoR_ genes with His6-tag at the C-terminus were amplified, cloned into the promoterless and low-copy plasmid
pHG10187, and transformed into _E. coli_ WM3064 and verified by sequencing. The correct vector was then transferred by conjugation into relevant _S. oneidensis_ strains. To detect protein
levels in vivo, cells were cultured under relevant conditions, and proteins were extracted and subjected to SDS-PAGE and Western blotting. RECOMBINANT PROTEIN EXPRESSION AND PURIFICATION AND
EMSA _E. coli_ BL21(DE3) and the pET-28a(+) plasmid were used for the production of recombinant SsoR and Fur with His6-tag at the N-terminus66. Expression of SsoR and Fur in _E. coli_ BL21
cells was induced with 0.2 mM IPTG from the mid-exponential phase at 16 °C overnight. The cells were grown to saturation and then collected by centrifugation resuspended in lysis buffer (50
mM Tris/HCl, pH 7.5, 500 mM NaCl, 1 mM PMSF, 5 μg/ml DNaseI), and broken by passage twice through a French press. Soluble Fur proteins were included in the clarified bacterial supernatant.
The resulting SsoR inclusion body pellets were solubilized with 20 mM Tris/HCl (pH 7.0), 8 M urea and 200 mM NaCl. SsoR or Fur proteins were further purified by using nickel-ion affinity
column (GE Healthcare, Chicago, IL, USA) under denaturing or non-denaturing conditions according to manufacturer instructions. The eluted fractions containing Fur proteins were collected and
then concentrated by ultrafiltration (10-kDa cutoff) and exchanged into 20 mM Tris-HCl (pH 8.0) containing 150 mM NaCl. To renature the SsoR protein, the eluted fractions containing SsoR
were diluted into 2 M urea, 20 mM Tris/HCl (pH 7.0), 1 mM EDTA by sequential dilutions and then dialyzed against 20 mM Tris/HCl (pH 7.0) overnight. Purified SsoR and Fur proteins were
determined by SDS-PAGE and Coomassie brilliant blue staining. EMSA was performed with the instructions provided in the LightShift Chemiluminescent EMSA Kit (Thermo Fisher Scientific,
Rockford, USA). Binding reactions were performed with 40 nmol biotin end-labeled probes, and various amounts of protein in 12 μl binding buffer containing 100 mM Tris/HCl (pH 7.4), 20 mM
KCl, 10 mM MgCl2, 2 mM DTT, 40-nmol poly(dI·dC) and 10% glycerol at 15 °C for 60 min. Samples were loaded onto a 6% non-denaturing polyacrylamide gel and subjected to electrophoresis at 80 V
for 2 h and then transferred to a nylon membrane (Amersham, Thermo Fisher Scientific, Rockford, USA) in 0.5× TBE at 130 V for 60 min. After UV cross-linking, the probe-protein complexes on
the membrane were detected using the Chemiluminescence Nucleic Acid Detection Module Kit (Thermo Fisher Scientific, Rockford, USA). PHYLOGENETIC TREE CONSTRUCTION A number of UniRef50
representative proteins, including _E. coli_ PhoB (_Ec_PhoB), SsoR, _Ec_KdpE, VbrR from _V. parahaemolyticus_ (PDB: 7E90), and HP1043 of _H. pylori_ (PDB: 2PLN), and their
high-sequence-similarity homologs were selected for the analysis. A neighbor-joining phylogenetic tree was constructed using the Clustal W alignment method in MEGA788. The bootstrap
consensus tree inferred from 1000 replicates represented the evolutionary history. The EFI Genome-Neighborhood Tool89 was employed to assay the visualized genomic context of the members in
the phylogenetic tree. ALIGNMENT OF THE ALPHAFOLD2 PREDICTED AND CRYSTAL/NMR STRUCTURES The AlphaFold2-predicted structures of the proteins included in the phylogenetic tree were obtained
from the AlphaFold Protein Structure Database90. The number of predicted structures in each cluster in the phylogenetic tree was reduced to no >70 by removing redundant sequences using
Jalview91. The receiver domains (REC, residue 1–120) of the predicted structures and 69 PDB structures (with each polymer retaining a monomer at random) were aligned in PyMol. STRUCTURAL
SIMILARITY DENDROGRAM BUILDING Structural similarity dendrogram was built to illustrate the changes in conformations. The method was validated with 55 proteins, which are identical to
_Ec_PhoB in sequence, obtained from the AlphaFold Protein Structure Database90. The REC domains of these proteins were compared with crystal structures of _Ec_PhoB from the PDB (including
1B00, 1ZES, 2IYN, 2JB9, and 2JBA, with polymers split into monomers). Both AlphaFold2 predicted and PDB monomers exhibited multiple states, with inner, outer, intermediate state A, and
intermediate state B as the major states. A structural similarity dendrogram was generated using “All against all” structure comparisons in the DALI server92, and the resulting dendrogram
was visualized using iTOL93. Dendrograms for other proteins were generated with AlphaFold2-predicted structures used for structural alignment. MD SIMULATIONS The dimer complexes of the REC
domains of AlphaFold2-predicted SsoR and _Vp_VbrR (PDB: 7E90) were refined by ColabFold94, and then were applied to MD simulations using CHARMM-GUI95. For total eight systems (two mutated
dimers and two monomers for SsoR and _Vp_VbrR), a rectangular water box with at least 1 nm edge distance from the protein(s) was used to solve the systems with 150 mM NaCl ions electrolyte
to pH 7.0. The periodic boundary conditions were generated for PME FFT by CHARMM-GUI automatically95. All-atom CHARMM36m force field was used for ions, protein(s) and TIP3P water and all
unbiased simulations were performed using GROMACS-v202396. Before MD production, an energy minimization and the equilibration in the NVT ensemble at a temperature of 310 K using mdp files
from CHARMM-GUI were executed sequentially to equilibrate the simulation box. A series of MD simulations were conducted in the NPT ensemble at a temperature of 310 K and a pressure of 1 bar
for a total of 3000 ns for each system. Temperature and pressure were coupled using the velocity-rescale method (time constant of 1 ps) and isotropic pressure coupling with the
Parrinello-Rahman algorithm (time constant of 5 ps), respectively. Frames from MD simulation trajectories were processed and extracted using GROMACS, one frame per nanosecond. Structures of
different states were grouped based on the relative positions of the switch residue (96 F of SsoR or 98Yof _Vp_VbrR). The positions were described using Chi1 angle of switch residue and the
distance between CZ atom of the switch residue and backbone N atom of 99 T (in _Vp_VbrR) or 98 T (in SsoR). The relative positions of the switch residues were analyzed using Plumed-v2.9.0
(developed by PLUMED consortium to promote transparency and reproducibility in enhanced molecular simulations)97, and RMSF calculations were performed using GROMACS. The 3D structure models
and movies were processed and rendered using PyMol. PROMOTER PREDICTION The multiple promoter prediction tools (BPROM, bTSSfinder, BacPP, and iPromoter-2L) were used to analyze the promoters
of the indicated genes98. STATISTICS AND REPRODUCIBILITY Most analyses were based on a minimum of four independent experiments, yielding biological replicates. Data were shown for either
all replicates or presented as mean ± standard error of the mean (SEM). Pairwise comparisons were conducted using Student’s _t_ test, with a _P_ value below 0.05 considered statistically
significant. Graphics and statistical analysis were performed using the Prism v9.5.1 software (GraphPad Software LLC, San Diego, CA, USA), completing the statistical test indicated in the
text and figure legends. REPORTING SUMMARY Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY The data
supporting the findings of this study are available in the article and its supplementary information files. All of the uncropped images in western blotting were shown in Supplementary Fig.
8. The source data underlying the graphs in the paper can be found in Supplementary Data. Supplementary Movie shows the switch residue as it transitions. The raw data for MD are available at
https://doi.org/10.5281/zenodo.10924978. REFERENCES * Andrews, S. C., Robinson, A. K. & Rodríguez-Quiñones, F. Bacterial iron homeostasis. _FEMS Microbiol. Rev._ 27, 215–237 (2003).
Article CAS PubMed Google Scholar * Chandrangsu, P., Rensing, C. & Helmann, J. D. Metal homeostasis and resistance in bacteria. _Nat. Rev. Microbiol._ 15, 338–350 (2017). Article
CAS PubMed PubMed Central Google Scholar * Wandersman, C. & Delepelaire, P. Bacterial iron sources: from siderophores to hemophores. _Annu. Rev. Microbiol._ 58, 611–647 (2004).
Article CAS PubMed Google Scholar * Melton, E. D., Swanner, E. D., Behrens, S., Schmidt, C. & Kappler, A. The interplay of microbially mediated and abiotic reactions in the
biogeochemical Fe cycle. _Nat. Rev. Microbiol._ 12, 797–808 (2014). Article CAS PubMed Google Scholar * Lau, C. K., Krewulak, K. D. & Vogel, H. J. Bacterial ferrous iron transport:
the Feo system. _FEMS Microbiol. Rev._ 40, 273–298 (2016). Article CAS PubMed Google Scholar * Wilson, B. R., Bogdan, A. R., Miyazawa, M., Hashimoto, K. & Tsuji, Y. Siderophores in
iron metabolism: from mechanism to therapy potential. _Trends Mol. Med._ 22, 1077–1090 (2016). Article CAS PubMed PubMed Central Google Scholar * Kramer, J., Özkaya, Ö. & Kümmerli,
R. Bacterial siderophores in community and host interactions. _Nat. Rev. Microbiol._ 18, 152–163 (2020). Article CAS PubMed Google Scholar * Liu, L., Wang, W., Wu, S. & Gao, H.
Recent advances in the siderophore biology of Shewanella. _Front. Microbiol._ 13, 823758 (2022). Article PubMed PubMed Central Google Scholar * Frangipani, E. et al. The Gac/Rsm and
cyclic-di-GMP signalling networks coordinately regulate iron uptake in Pseudomonas aeruginosa. _Environ. Microbiol._ 16, 676–688 (2014). Article CAS PubMed Google Scholar * Little, A. S.
et al. Pseudomonas aeruginosa AlgR phosphorylation status differentially regulates pyocyanin and pyoverdine production. _mBio_ 9, e02318–02317 (2018). Article CAS PubMed PubMed Central
Google Scholar * Mitrophanov, A. Y. & Groisman, E. A. Signal integration in bacterial two-component regulatory systems. _Genes Dev._ 22, 2601–2611 (2008). Article CAS PubMed PubMed
Central Google Scholar * Galperin, M. Y. Structural classification of bacterial response regulators: diversity of output domains and domain combinations. _J. Bacteriol._ 188, 4169–4182
(2006). Article CAS PubMed PubMed Central Google Scholar * Chareyre, S. & Mandin, P. Bacterial iron homeostasis regulation by sRNAs. _Microbiol. Spectr._ 6, RWR-0010–RWR-2017
(2018). Article Google Scholar * Cornelis, P. et al. High affinity iron uptake by pyoverdine in Pseudomonas aeruginosa involves multiple regulators besides Fur, PvdS, and FpvI. _Biometals_
36, 255–261 (2023). Article CAS PubMed Google Scholar * Jaworska, K. et al. Interplay between the RNA chaperone Hfq, small RNAs and transcriptional regulator OmpR modulates iron
homeostasis in the enteropathogen Yersinia enterocolitica. _Int. J. Mol. Sci._ 24, 11157 (2023). Article CAS PubMed PubMed Central Google Scholar * Imlay, J. A. The mismetallation of
enzymes during oxidative stress. _J. Biol. Chem._ 289, 28121–28128 (2014). Article CAS PubMed PubMed Central Google Scholar * Liu, J. et al. Metalloproteins containing cytochrome,
iron-sulfur, or copper redox centers. _Chem. Rev._ 114, 4366–4469 (2014). Article CAS PubMed PubMed Central Google Scholar * Daly, M. J. et al. Accumulation of Mn(II) in Deinococcus
radiodurans facilitates gamma-radiation resistance. _Science_ 306, 1025–1028 (2004). Article CAS PubMed Google Scholar * Fredrickson, J. K. et al. Towards environmental systems biology
of Shewanella. _Nat. Rev. Microbiol._ 6, 592–603 (2008). Article CAS PubMed Google Scholar * Shirodkar, S., Reed, S., Romine, M. & Saffarini, D. The octahaem SirA catalyses
dissimilatory sulfite reduction in Shewanella oneidensis MR-1. _Environ. Microbiol._ 13, 108–115 (2011). Article CAS PubMed Google Scholar * Codd, R. et al. The chemical biology and
coordination chemistry of putrebactin, avaroferrin, bisucaberin, and alcaligin. _J. Biol. Inorg. Chem._ 23, 969–982 (2018). Article CAS PubMed Google Scholar * Kadi, N., Arbache, S.,
Song, L., Oves-Costales, D. & Challis, G. L. Identification of a gene cluster that directs putrebactin biosynthesis in Shewanella species: PubC catalyzes cyclodimerization of
N-hydroxy-N-succinylputrescine. _J. Am. Chem. Soc._ 130, 10458–10459 (2008). Article CAS PubMed Google Scholar * Wang, S. et al. Promiscuous enzymes cause biosynthesis of diverse
siderophores in Shewanella oneidensis. _Appl. Environ. Microbiol._ 86, e00030–00020 (2020). CAS PubMed PubMed Central Google Scholar * Rütschlin, S., Gunesch, S. & Böttcher, T. One
enzyme to build them all: ring-size engineered siderophores inhibit the swarming motility of vibrio. _ACS Chem. Biol._ 13, 1153–1158 (2018). Article PubMed Google Scholar * Henne, K. L.,
Wan, X. F., Wei, W. & Thompson, D. K. SO2426 is a positive regulator of siderophore expression in Shewanella oneidensis MR-1. _BMC Microbiol._ 11, 125 (2011). Article CAS PubMed
PubMed Central Google Scholar * Yang, Y. et al. Snapshot of iron response in Shewanella oneidensis by gene network reconstruction. _BMC Genomics_ 10, 131 (2009). Article PubMed PubMed
Central Google Scholar * Dong, Z., Guo, S., Fu, H. & Gao, H. Investigation of a spontaneous mutant reveals novel features of iron uptake in Shewanella oneidensis. _Sci. Rep._ 7, 11788
(2017). Article PubMed PubMed Central Google Scholar * Liu, L., Li, S., Wang, S., Dong, Z. & Gao, H. Complex iron uptake by the putrebactin-mediated and Feo systems in Shewanella
oneidensis. _Appl. Environ. Microbiol._ 84, e01752–01718 (2018). Article CAS PubMed PubMed Central Google Scholar * Altier, C., Suyemoto, M. & Lawhon, S. D. Regulation of Salmonella
enterica serovar typhimurium invasion genes by csrA. _Infect. Immun._ 68, 6790–6797 (2000). Article CAS PubMed PubMed Central Google Scholar * Binnenkade, L., Lassak, J. &
Thormann, K. M. Analysis of the BarA/UvrY two-component system in Shewanella oneidensis MR-1. _PLoS One_ 6, e23440 (2011). Article CAS PubMed PubMed Central Google Scholar * Brencic, A.
et al. The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. _Mol.
Microbiol._ 73, 434–445 (2009). Article CAS PubMed PubMed Central Google Scholar * Pernestig, A. K., Melefors, O. & Georgellis, D. Identification of UvrY as the cognate response
regulator for the BarA sensor kinase in Escherichia coli. _J. Biol. Chem._ 276, 225–231 (2001). Article CAS PubMed Google Scholar * Martínez-Hackert, E. & Stock, A. M. The
DNA-binding domain of OmpR: crystal structures of a winged helix transcription factor. _Structure_ 5, 109–124 (1997). Article PubMed Google Scholar * Baikalov, I. et al. Structure of the
Escherichia coli response regulator NarL. _Biochemistry_ 35, 11053–11061 (1996). Article CAS PubMed Google Scholar * Huynh, T. N., Lin, H.-Y., Noriega, C. E., Lin, A. V. & Stewart,
V. Cross talk inhibition nullified by a receiver domain missense substitution. _J. Bacteriol._ 197, 3294–3306 (2015). Article CAS PubMed PubMed Central Google Scholar * Salvail, H.
& Groisman, E. A. The phosphorelay BarA/SirA activates the non-cognate regulator RcsB in Salmonella enterica. _PLos Genet._ 16, e1008722 (2020). Article CAS PubMed PubMed Central
Google Scholar * Yamamoto, K. et al. Functional characterization in vitro of all two-component signal transduction systems from Escherichia coli. _J. Biol. Chem._ 280, 1448–1456 (2005).
Article CAS PubMed Google Scholar * Kinoshita, E., Kinoshita-Kikuta, E., Takiyama, K. & Koike, T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. _Mol. Cell.
Proteomics_ 5, 749–757 (2006). Article CAS PubMed Google Scholar * Chavez, R. G., Alvarez, A. F., Romeo, T. & Georgellis, D. The physiological stimulus for the BarA sensor kinase.
_J. Bacteriol._ 192, 2009–2012 (2010). Article CAS PubMed Google Scholar * Martínez, L. C. et al. Integration of a complex regulatory cascade involving the SirA/BarA and Csr global
regulatory systems that controls expression of the Salmonella SPI-1 and SPI-2 virulence regulons through HilD. _Mol. Microbiol._ 80, 1637–1656 (2011). Article PubMed PubMed Central Google
Scholar * Romeo, T. & Babitzke, P. Global regulation by CsrA and Its RNA antagonists. _Microbiol. Spectr._ 6, RWR-0009–RWR-2017 (2018). Article Google Scholar * Pourciau, C., Lai, Y.
J., Gorelik, M., Babitzke, P. & Romeo, T. Diverse mechanisms and circuitry for global regulation by the RNA-binding protein CsrA. _Front. Microbiol._ 11, 601352 (2020). Article PubMed
PubMed Central Google Scholar * Schachterle, J. K. et al. Yersinia pseudotuberculosis BarA-UvrY two-component regulatory system represses biofilms via CsrB. _Front. Cell. Infect.
Microbiol._ 8, 323 (2018). Article PubMed PubMed Central Google Scholar * Jackson, D. W. et al. Biofilm formation and dispersal under the influence of the global regulator CsrA of
Escherichia coli. _J. Bacteriol._ 184, 290–301 (2002). Article CAS PubMed PubMed Central Google Scholar * Dubey, A. K. et al. CsrA regulates translation of the Escherichia coli carbon
starvation gene, cstA, by blocking ribosome access to the cstA transcript. _J. Bacteriol._ 185, 4450–4460 (2003). Article CAS PubMed PubMed Central Google Scholar * Teplitski, M.,
Al-Agely, A. & Ahmer, B. M. M. Contribution of the SirA regulon to biofilm formation in Salmonella enterica serovar Typhimurium. _Microbiol. (Read.)_ 152, 3411–3424 (2006). Article CAS
Google Scholar * Fineran, P. C., Slater, H., Everson, L., Hughes, K. & Salmond, G. P. C. Biosynthesis of tripyrrole and beta-lactam secondary metabolites in Serratia: integration of
quorum sensing with multiple new regulatory components in the control of prodigiosin and carbapenem antibiotic production. _Mol. Microbiol._ 56, 1495–1517 (2005). Article CAS PubMed
Google Scholar * Timmermans, J. & Van Melderen, L. Conditional essentiality of the csrA gene in Escherichia coli. _J. Bacteriol._ 191, 1722–1724 (2009). Article CAS PubMed Google
Scholar * Gao, H. et al. Physiological roles of ArcA, Crp, and EtrA and their interactive control on aerobic and anaerobic respiration in Shewanella oneidensis. _PLoS One_ 5, e15295 (2010).
Article CAS PubMed PubMed Central Google Scholar * Ortet, P., Whitworth, D. E., Santaella, C., Achouak, W. & Barakat, M. P2CS: updates of the prokaryotic two-component systems
database. _Nucleic Acids Res._ 43, D536–D541 (2015). Article CAS PubMed Google Scholar * Gumerov, V. M., Ortega, D. R., Adebali, O., Ulrich, L. E. & Zhulin, I. B. MiST 3.0: an
updated microbial signal transduction database with an emphasis on chemosensory systems. _Nucleic Acids Res._ 48, D459–d464 (2020). Article CAS PubMed Google Scholar * Desai, S. K. &
Kenney, L. J. To ~P or Not to ~P? Non-canonical activation by two-component response regulators. _Mol. Microbiol._ 103, 203–213 (2017). Article CAS PubMed Google Scholar *
Gomez-Arrebola, C., Solano, C. & Lasa, I. Regulation of gene expression by non-phosphorylated response regulators. _Int. Microbiol._ 24, 521–529 (2021). Article CAS PubMed Google
Scholar * Kenney, L. J. Structure/function relationships in OmpR and other winged-helix transcription factors. _Curr. Opin. Microbiol._ 5, 135–141 (2002). Article CAS PubMed Google
Scholar * Itou, H. & Tanaka, I. The OmpR-family of proteins: insight into the tertiary structure and functions of two-component regulator proteins. _J. Biochem._ 129, 343–350 (2001).
Article CAS PubMed Google Scholar * Gao, R., Bouillet, S. & Stock, A. M. Structural basis of response regulator function. _Annu. Rev. Microbiol._ 73, 175–197 (2019). Article CAS
PubMed Google Scholar * Kenney, L. J. & Anand, G. S. EnvZ/OmpR two-component signaling: an archetype system that can function noncanonically. _EcoSal Plus_ 9, SP-0001-2019 (2020). *
Cho, H. S. et al. NMR structure of activated CheY. _J. Mol. Biol._ 297, 543–551 (2000). Article CAS PubMed Google Scholar * Barbieri, C. M., Wu, T. & Stock, A. M. Comprehensive
analysis of OmpR phosphorylation, dimerization, and DNA binding supports a canonical model for activation. _J. Mol. Biol._ 425, 1612–1626 (2013). Article CAS PubMed PubMed Central Google
Scholar * Hong, E. et al. Structure of an atypical orphan response regulator protein supports a new phosphorylation-independent regulatory mechanism. _J. Biol. Chem._ 282, 20667–20675
(2007). Article CAS PubMed Google Scholar * Zhu, X., Rebello, J., Matsumura, P. & Volz, K. Crystal structures of CheY mutants Y106W and T87I/Y106W. CheY activation correlates with
movement of residue 106. _J. Biol. Chem._ 272, 5000–5006 (1997). Article CAS PubMed Google Scholar * Bachhawat, P., Swapna, G. V. T., Montelione, G. T. & Stock, A. M. Mechanism of
activation for transcription factor PhoB suggested by different modes of dimerization in the inactive and active states. _Structure_ 13, 1353–1363 (2005). Article CAS PubMed PubMed
Central Google Scholar * Wayment-Steele, H. K. et al. Predicting multiple conformations via sequence clustering and AlphaFold2. _Nature_ 625, 832-839 (2023). * Lin, W. et al. Atypical
OmpR/PhoB subfamily response regulator GlnR of actinomycetes functions as a homodimer, stabilized by the unphosphorylated conserved Asp-focused charge interactions. _J. Biol. Chem._ 289,
15413–15425 (2014). Article CAS PubMed Google Scholar * Cho, S. Y. & Yoon, S.-I. Structural analysis of the activation and DNA interactions of the response regulator VbrR from Vibrio
parahaemolyticus. _Biochem. Biophys. Res. Commun._ 555, 102–108 (2021). Article CAS PubMed Google Scholar * Fu, H., Liu, L., Dong, Z., Guo, S. & Gao, H. Dissociation between iron
and heme biosyntheses is largely accountable for respiration defects of shewanella oneidensis fur mutants. _Appl. Environ. Microbiol._ 84, e00039–00018 (2018). Article CAS PubMed PubMed
Central Google Scholar * Liu, L. et al. Free rather than total iron content is critically linked to the fur physiology in Shewanella oneidensis. _Front. Microbiol._ 11, 593246 (2020).
Article PubMed PubMed Central Google Scholar * Rehm, N. et al. Two polyketides intertwined in complex regulation: posttranscriptional CsrA-mediated control of colibactin and
yersiniabactin synthesis in Escherichia coli. _mBio_ 13, e0381421 (2021). Article PubMed Google Scholar * Suzuki, K. et al. Regulatory circuitry of the CsrA/CsrB and BarA/UvrY systems of
Escherichia coli. _J. Bacteriol._ 184, 5130–5140 (2002). Article CAS PubMed PubMed Central Google Scholar * Pannuri, A. et al. Circuitry linking the catabolite repression and Csr global
regulatory systems of Escherichia coli. _J. Bacteriol._ 198, 3000–3015 (2016). Article CAS PubMed PubMed Central Google Scholar * Zhou, G. et al. Combined effect of loss of the caa3
oxidase and Crp regulation drives Shewanella to thrive in redox-stratified environments. _ISME J._ 7, 1752–1763 (2013). Article CAS PubMed PubMed Central Google Scholar * Guo, K. et al.
NapB Restores cytochrome c biosynthesis in bacterial dsbD-deficient mutants. _Commun. Biol._ 5, 87 (2022). Article CAS PubMed PubMed Central Google Scholar * Nguyen, M. P., Yoon, J.
M., Cho, M. H. & Lee, S. W. Prokaryotic 2-component systems and the OmpR/PhoB superfamily. _Can. J. Microbiol._ 61, 799–810 (2015). Article CAS PubMed Google Scholar * Bourret, R. B.
Receiver domain structure and function in response regulator proteins. _Curr. Opin. Microbiol._ 13, 142–149 (2010). Article CAS PubMed PubMed Central Google Scholar * Lukat, G. S.,
McCleary, W. R., Stock, A. M. & Stock, J. B. Phosphorylation of bacterial response regulator proteins by low molecular weight phospho-donors. _Proc. Natl Acad. Sci. USA_ 89, 718–722
(1992). Article CAS PubMed PubMed Central Google Scholar * Wolfe, A. J. Physiologically relevant small phosphodonors link metabolism to signal transduction. _Curr. Opin. Microbiol._ 13,
204–209 (2010). Article CAS PubMed PubMed Central Google Scholar * Jin, M. et al. Unique organizational and functional features of the cytochrome c maturation system in Shewanella
oneidensis. _PLoS One_ 8, e75610 (2013). Article CAS PubMed PubMed Central Google Scholar * Meng, Q., Sun, Y. & Gao, H. Cytochromes c constitute a layer of protection against nitric
oxide but not nitrite. _Appl. Environ. Microbiol._ 84, e01255–01218 (2018). Article CAS PubMed PubMed Central Google Scholar * Fu, H. et al. Crp-dependent cytochrome bd oxidase confers
nitrite resistance to Shewanella oneidensis. _Environ. Microbiol._ 15, 2198–2212 (2013). Article CAS PubMed Google Scholar * Wong, S. M. & Mekalanos, J. J. Genetic footprinting with
mariner-based transposition in Pseudomonas aeruginosa. _Proc. Natl Acad. Sci. USA_ 97, 10191–10196 (2000). Article CAS PubMed PubMed Central Google Scholar * Das, S., Noe, J. C., Paik,
S. & Kitten, T. An improved arbitrary primed PCR method for rapid characterization of transposon insertion sites. _J. Microbiol. Methods_ 63, 89–94 (2005). Article CAS PubMed Google
Scholar * Sivaraman, T., Kumar, T. K., Jayaraman, G. & Yu, C. The mechanism of 2,2,2-trichloroacetic acid-induced protein precipitation. _J. Protein Chem._ 16, 291–297 (1997). Article
CAS PubMed Google Scholar * Schwyn, B. & Neilands, J. B. Universal chemical assay for the detection and determination of siderophores. _Anal. Biochem._ 160, 47–56 (1987). Article CAS
PubMed Google Scholar * Kinoshita, E., Kinoshita-Kikuta, E. & Koike, T. Separation and detection of large phosphoproteins using Phos-tag SDS-PAGE. _Nat. Protoc._ 4, 1513–1521 (2009).
Article CAS PubMed Google Scholar * Fu, H., Jin, M., Ju, L., Mao, Y. & Gao, H. Evidence for function overlapping of CymA and the cytochrome bc1 complex in the Shewanella oneidensis
nitrate and nitrite respiration. _Environ. Microbiol._ 16, 3181–3195 (2014). Article CAS PubMed Google Scholar * Pfaffl, M. W. A new mathematical model for relative quantification in
real-time RT-PCR. _Nucleic Acids Res._ 29, e45 (2001). Article CAS PubMed PubMed Central Google Scholar * Wu, L., Wang, J., Tang, P., Chen, H. & Gao, H. Genetic and molecular
characterization of flagellar assembly in Shewanella oneidensis. _PLoS One_ 6, e21479 (2011). Article CAS PubMed PubMed Central Google Scholar * Kumar, S., Stecher, G. & Tamura, K.
MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. _Mol. Biol. Evol._ 33, 1870–1874 (2016). Article CAS PubMed PubMed Central Google Scholar * Oberg, N.,
Zallot, R. & Gerlt, J. A. EFI-EST, EFI-GNT, and EFI-CGFP: enzyme function initiative (EFI) web resource for genomic enzymology tools. _J. Mol. Biol._ 435, 168018 (2023). Article CAS
PubMed Google Scholar * Varadi, M. et al. AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. _Nucleic
Acids Res._ 50, D439–D444 (2022). Article CAS PubMed Google Scholar * Waterhouse, A. M., Procter, J. B., Martin, D. M. A., Clamp, M. & Barton, G. J. Jalview Version 2–a multiple
sequence alignment editor and analysis workbench. _Bioinformatics_ 25, 1189–1191 (2009). Article CAS PubMed PubMed Central Google Scholar * Holm, L., Laiho, A., Törönen, P. &
Salgado, M. DALI shines a light on remote homologs: one hundred discoveries. _Protein Sci._ 32, e4519 (2023). Article CAS PubMed PubMed Central Google Scholar * Letunic, I. & Bork,
P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. _Nucleic Acids Res._ 49, W293–W296 (2021). Article CAS PubMed PubMed Central Google
Scholar * Mirdita, M. et al. ColabFold: making protein folding accessible to all. _Nat. Methods_ 19, 679–682 (2022). Article CAS PubMed PubMed Central Google Scholar * Jo, S., Kim, T.,
Iyer, V. G. & Im, W. CHARMM-GUI: a web-based graphical user interface for CHARMM. _J. Comput. Chem._ 29, 1859–1865 (2008). Article CAS PubMed Google Scholar * Abraham, M. J. et al.
GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. _SoftwareX_ 1-2, 19–25 (2015). Article Google Scholar * Tribello, G. A.,
Bonomi, M., Branduardi, D., Camilloni, C. & Bussi, G. PLUMED 2: New feathers for an old bird. _Comput. Phys. Commun._ 185, 604–613 (2014). Article CAS Google Scholar * Cassiano, M. H.
A. & Silva-Rocha, R. Benchmarking bacterial promoter prediction tools: potentialities and limitations. _mSystems_ 5, e00439–00420 (2020). Article CAS PubMed PubMed Central Google
Scholar Download references ACKNOWLEDGEMENTS This research was supported by the National Natural Science Foundation of China (31930003), the National Key R&D Program of China
(2018YFA0901300), and the Natural Science Foundation of Zhejiang Province (LZ23C010002). AUTHOR INFORMATION Author notes * These authors contributed equally: Peilu Xie, Yuanyou Xu. AUTHORS
AND AFFILIATIONS * Institute of Microbiology and College of Life Sciences, Zhejiang University, Hangzhou, Zhejiang, 310058, China Peilu Xie, Yuanyou Xu, Jiaxin Tang, Shihua Wu & Haichun
Gao Authors * Peilu Xie View author publications You can also search for this author inPubMed Google Scholar * Yuanyou Xu View author publications You can also search for this author
inPubMed Google Scholar * Jiaxin Tang View author publications You can also search for this author inPubMed Google Scholar * Shihua Wu View author publications You can also search for this
author inPubMed Google Scholar * Haichun Gao View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Peilu Xie and Yuanyou Xu, investigation,
validation, writing—original draft, formal analysis; Jiaxin Tang, investigation; Shihua Wu and Haichun Gao, writing—review and editing, conceptualization, funding acquisition, project
administration, and supervision. CORRESPONDING AUTHORS Correspondence to Shihua Wu or Haichun Gao. ETHICS DECLARATIONS COMPETING INTERESTS Haichun Gao is an Editorial Board Member for
_Communications Biology_, but was not involved in the editorial review of, nor the decision to publish this article. PEER REVIEW PEER REVIEW INFORMATION _Communications Biology_ thanks Yoko
Eguchi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Tobias Goris. A peer review file is available. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION PEER REVIEW FILE
SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA SUPPLEMENTARY VIDEO REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Xie, P., Xu, Y., Tang, J. _et al._ Multifaceted regulation of
siderophore synthesis by multiple regulatory systems in _Shewanella oneidensis_. _Commun Biol_ 7, 498 (2024). https://doi.org/10.1038/s42003-024-06193-7 Download citation * Received: 15
January 2024 * Accepted: 15 April 2024 * Published: 25 April 2024 * DOI: https://doi.org/10.1038/s42003-024-06193-7 SHARE THIS ARTICLE Anyone you share the following link with will be able
to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
