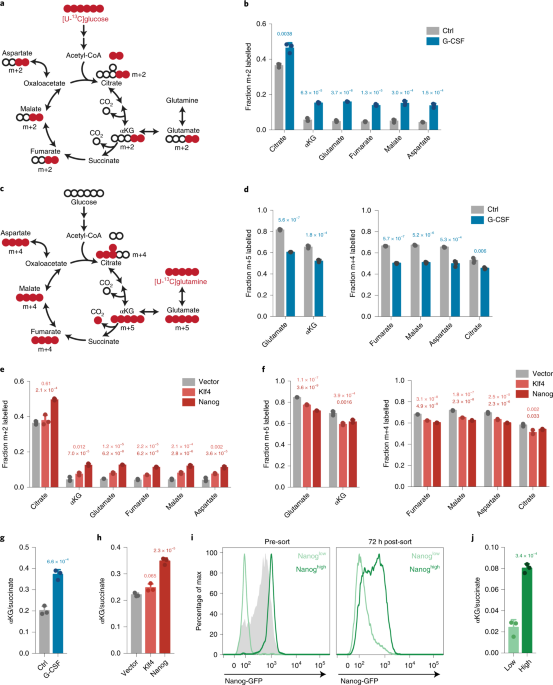
Glutamine independence is a selectable feature of pluripotent stem cells
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Most rapidly proliferating mammalian cells rely on the oxidation of exogenous glutamine to support cell proliferation. We previously found that culture of mouse embryonic stem cells
in the presence of inhibitors against mitogen-activated protein kinase kinase and glycogen synthase kinase 3 beta to maintain pluripotency reduces cellular reliance on glutamine for
tricarboxylic acid cycle anaplerosis, enabling embryonic stem cells to proliferate in the absence of exogenous glutamine. Here we show that reduced dependence on exogenous glutamine is a
generalizable feature of pluripotent stem cells. Enhancing self-renewal, through either overexpression of pluripotency-associated transcription factors or altered signal transduction,
decreases the use of glutamine-derived carbons in the tricarboxylic acid cycle. As a result, cells with the highest potential for self-renewal can be enriched by transient culture in
glutamine-deficient media. During pluripotent cell culture or reprogramming to pluripotency, transient glutamine withdrawal selectively leads to the elimination of non-pluripotent cells.
These data reveal that reduced dependence on glutamine anaplerosis is an inherent feature of self-renewing pluripotent stem cells and reveal a simple, non-invasive mechanism to select for
mouse and human pluripotent stem cells within a heterogeneous population during both embryonic stem cell passage and induced pluripotent cell reprogramming. Access through your institution
Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00
per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated
during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS IMPROVING THE
DIFFERENTIATION POTENTIAL OF PLURIPOTENT STEM CELLS BY OPTIMIZING CULTURE CONDITIONS Article Open access 19 August 2022 EPIGENETIC AND TRANSCRIPTIONAL REGULATIONS PRIME CELL FATE BEFORE
DIVISION DURING HUMAN PLURIPOTENT STEM CELL DIFFERENTIATION Article Open access 25 January 2023 DISSECTION OF TWO ROUTES TO NAÏVE PLURIPOTENCY USING DIFFERENT KINASE INHIBITORS Article Open
access 25 March 2021 DATA AVAILABILITY The data that support the findings of this study are available from the corresponding author upon reasonable request. Source data for all
gas-chromatography–mass spectrometry data are provided in Supplementary Table 1. CODE AVAILABILITY The MATLAB code that supports the findings of this study is also available from the
corresponding author upon reasonable request. REFERENCES * DeBerardinis, R. J., Lum, J. J., Hatzivassiliou, G. & Thompson, C. B. The biology of cancer: metabolic reprogramming fuels cell
growth and proliferation. _Cell Metab._ 7, 11–20 (2008). Article CAS Google Scholar * Palm, W. & Thompson, C. B. Nutrient acquisition strategies of mammalian cells. _Nature_ 546,
234–242 (2017). Article CAS Google Scholar * Vander Heiden, M. G. & DeBerardinis, R. J. Understanding the intersections between metabolism and cancer biology. _Cell_ 168, 657–669
(2017). Article CAS Google Scholar * Schvartzman, J. M., Thompson, C. B. & Finley, L. W. S. Metabolic regulation of chromatin modifications and gene expression. _J. Cell Biol._ 217,
2247–2259 (2018). Article CAS Google Scholar * Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. _Cell_ 168, 960–976 (2017). Article CAS Google
Scholar * Su, X., Wellen, K. E. & Rabinowitz, J. D. Metabolic control of methylation and acetylation. _Curr. Opin. Chem. Biol._ 30, 52–60 (2016). Article CAS Google Scholar * Lu, C.
& Thompson, C. B. Metabolic regulation of epigenetics. _Cell Metab._ 16, 9–17 (2012). Article CAS Google Scholar * Carey, B. W., Finley, L. W., Cross, J. R., Allis, C. D. &
Thompson, C. B. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. _Nature_ 518, 413–416 (2015). Article CAS Google Scholar * Gu, W. et al. Glycolytic
metabolism plays a functional role in regulating human pluripotent stem cell state. _Cell Stem Cell_ 19, 476–490 (2016). Article CAS Google Scholar * Tohyama, S. et al. Glutamine
oxidation is indispensable for survival of human pluripotent stem cells. _Cell Metab._ 23, 663–674 (2016). Article CAS Google Scholar * Zhang, H. et al. Distinct metabolic states can
support self-renewal and lipogenesis in human pluripotent stem cells under different culture conditions. _Cell Rep._ 16, 1536–1547 (2016). Article CAS Google Scholar * Hwang, I. Y. et al.
Psat1-dependent fluctuations in α-ketoglutarate affect the timing of ESC differentiation. _Cell Metab._ 24, 494–501 (2016). Article CAS Google Scholar * Moussaieff, A. et al.
Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. _Cell Metab._ 21, 392–402 (2015). Article CAS Google Scholar *
TeSlaa, T. et al. α-Ketoglutarate accelerates the initial differentiation of primed human pluripotent stem cells. _Cell Metab._ 24, 485–493 (2016). Article CAS Google Scholar * Chambers,
I. et al. Nanog safeguards pluripotency and mediates germline development. _Nature_ 450, 1230–1234 (2007). Article CAS Google Scholar * Filipczyk, A. et al. Biallelic expression of nanog
protein in mouse embryonic stem cells. _Cell Stem Cell_ 13, 12–13 (2013). Article CAS Google Scholar * Ying, Q. L. et al. The ground state of embryonic stem cell self-renewal. _Nature_
453, 519–523 (2008). Article CAS Google Scholar * Chisolm, D. A. et al. CCCTC-binding factor translates interleukin 2- and α-ketoglutarate-sensitive metabolic changes in T cells into
context-dependent gene programs. _Immunity_ 47, 251–267.e7 (2017). Article CAS Google Scholar * Liu, P. S. et al. α-Ketoglutarate orchestrates macrophage activation through metabolic and
epigenetic reprogramming. _Nat. Immunol._ 18, 985–994 (2017). Article CAS Google Scholar * Yang, Q. et al. AMPK/α-ketoglutarate axis dynamically mediates DNA demethylation in the _Prdm16_
promoter and brown adipogenesis. _Cell Metab._ 24, 542–554 (2016). Article CAS Google Scholar * Burdon, T., Stracey, C., Chambers, I., Nichols, J. & Smith, A. Suppression of SHP-2
and ERK signalling promotes self-renewal of mouse embryonic stem cells. _Dev. Biol._ 210, 30–43 (1999). Article CAS Google Scholar * van Oosten, A. L., Costa, Y., Smith, A. & Silva,
J. C. JAK/STAT3 signalling is sufficient and dominant over antagonistic cues for the establishment of naive pluripotency. _Nat. Commun._ 3, 817 (2012). Article Google Scholar * Martello,
G., Bertone, P. & Smith, A. Identification of the missing pluripotency mediator downstream of leukaemia inhibitory factor. _EMBO J._ 32, 2561–2574 (2013). Article CAS Google Scholar *
Chambers, I. et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. _Cell_ 113, 643–655 (2003). Article CAS Google Scholar * Mitsui, K.
et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. _Cell_ 113, 631–642 (2003). Article CAS Google Scholar * Zhang, P., Andrianakos,
R., Yang, Y., Liu, C. & Lu, W. Kruppel-like factor 4 (Klf4) prevents embryonic stem (ES) cell differentiation by regulating _Nanog_ gene expression. _J. Biol. Chem._ 285, 9180–9189
(2010). Article CAS Google Scholar * Festuccia, N. et al. _Esrrb_ is a direct Nanog target gene that can substitute for Nanog function in pluripotent cells. _Cell Stem Cell_ 11, 477–490
(2012). Article CAS Google Scholar * Shi, W. et al. Regulation of the pluripotency marker _Rex-1_ by Nanog and Sox2. _J. Biol. Chem._ 281, 23319–23325 (2006). Article CAS Google Scholar
* Cheng, T. et al. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. _Proc. Natl Acad. Sci. USA_ 108, 8674–8679 (2011). Article CAS Google Scholar *
Faddah, D. A. et al. Single-cell analysis reveals that expression of nanog is biallelic and equally variable as that of other pluripotency factors in mouse ESCs. _Cell Stem Cell_ 13, 23–29
(2013). Article CAS Google Scholar * Boroviak, T., Loos, R., Bertone, P., Smith, A. & Nichols, J. The ability of inner-cell-mass cells to self-renew as embryonic stem cells is
acquired following epiblast specification. _Nat. Cell Biol._ 16, 516–528 (2014). Article CAS Google Scholar * Karwacki-Neisius, V. et al. Reduced Oct4 expression directs a robust
pluripotent state with distinct signaling activity and increased enhancer occupancy by Oct4 and Nanog. _Cell Stem Cell_ 12, 531–545 (2013). Article CAS Google Scholar * Silva, J. et al.
Nanog is the gateway to the pluripotent ground state. _Cell_ 138, 722–737 (2009). Article CAS Google Scholar * Nichols, J. et al. Formation of pluripotent stem cells in the mammalian
embryo depends on the POU transcription factor Oct4. _Cell_ 95, 379–391 (1998). Article CAS Google Scholar * Hochedlinger, K. & Jaenisch, R. Induced pluripotency and epigenetic
reprogramming. _Cold Spring Harb. Perspect. Biol._ 7, a019448 (2015). Article Google Scholar * Silva, J. et al. Promotion of reprogramming to ground state pluripotency by signal
inhibition. _PLoS Biol._ 6, e253 (2008). Article Google Scholar * Stadtfeld, M., Maherali, N., Borkent, M. & Hochedlinger, K. A reprogrammable mouse strain from gene-targeted embryonic
stem cells. _Nat. Methods_ 7, 53–55 (2010). Article CAS Google Scholar * Dunn, S. J., Martello, G., Yordanov, B., Emmott, S. & Smith, A. G. Defining an essential transcription factor
program for naïve pluripotency. _Science_ 344, 1156–1160 (2014). Article CAS Google Scholar * Buganim, Y. et al. The developmental potential of iPSCs is greatly influenced by
reprogramming factor selection. _Cell Stem Cell_ 15, 295–309 (2014). Article CAS Google Scholar * Weinberger, L., Ayyash, M., Novershtern, N. & Hanna, J. H. Dynamic stem cell states:
naive to primed pluripotency in rodents and humans. _Nat. Rev. Mol. Cell Biol._ 17, 155–169 (2016). Article CAS Google Scholar * Choi, J. et al. Prolonged Mek1/2 suppression impairs the
developmental potential of embryonic stem cells. _Nature_ 548, 219–223 (2017). Article CAS Google Scholar * Yagi, M. et al. Derivation of ground-state female ES cells maintaining
gamete-derived DNA methylation. _Nature_ 548, 224–227 (2017). Article CAS Google Scholar * Huang, K., Maruyama, T. & Fan, G. The naive state of human pluripotent stem cells: a
synthesis of stem cell and preimplantation embryo transcriptome analyses. _Cell Stem Cell_ 15, 410–415 (2014). Article CAS Google Scholar * Wang, J. et al. Isolation and cultivation of
naive-like human pluripotent stem cells based on HERVH expression. _Nat. Protoc._ 11, 327–346 (2016). Article CAS Google Scholar * Warrier, S. et al. Direct comparison of distinct naive
pluripotent states in human embryonic stem cells. _Nat. Commun._ 8, 15055 (2017). Article CAS Google Scholar * Carbognin, E., Betto, R. M., Soriano, M. E., Smith, A. G. & Martello, G.
Stat3 promotes mitochondrial transcription and oxidative respiration during maintenance and induction of naive pluripotency. _EMBO J._ 35, 618–634 (2016). Article CAS Google Scholar *
Ficz, G. et al. FGF signaling inhibition in ESCs drives rapid genome-wide demethylation to the epigenetic ground state of pluripotency. _Cell Stem Cell_ 13, 351–359 (2013). Article CAS
Google Scholar * Habibi, E. et al. Whole-genome bisulfite sequencing of two distinct interconvertible DNA methylomes of mouse embryonic stem cells. _Cell Stem Cell_ 13, 360–369 (2013).
Article CAS Google Scholar * Galonska, C., Ziller, M. J., Karnik, R. & Meissner, A. Ground state conditions induce rapid reorganization of core pluripotency factor binding before
global epigenetic reprogramming. _Cell Stem Cell_ 17, 462–470 (2015). Article CAS Google Scholar * Bauer, D. E. et al. Cytokine stimulation of aerobic glycolysis in hematopoietic cells
exceeds proliferative demand. _FASEB J._ 18, 1303–1305 (2004). Article CAS Google Scholar * Frauwirth, K. A. et al. The CD28 signaling pathway regulates glucose metabolism. _Immunity_ 16,
769–777 (2002). Article CAS Google Scholar * Utsunomiya-Tate, N., Endou, H. & Kanai, Y. Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid
transporter. _J. Biol. Chem._ 271, 14883–14890 (1996). Article CAS Google Scholar * Kammen, H. O. & Hurlbert, R. B. Amination of uridine nucleotides to cytidine nucleotides by soluble
mammalian enzymes; role of glutamine and guanosine nucleotides. _Biochim. Biophys. Acta_ 30, 195–196 (1958). Article CAS Google Scholar * Marsboom, G. et al. Glutamine metabolism
regulates the pluripotency transcription factor OCT4. _Cell Rep._ 16, 323–332 (2016). Article CAS Google Scholar * Alexander, P. B., Wang, J. & McKnight, S. L. Targeted killing of a
mammalian cell based upon its specialized metabolic state. _Proc. Natl Acad. Sci. USA_ 108, 15828–15833 (2011). Article CAS Google Scholar * Finley, L. W. S. et al. Pluripotency
transcription factors and Tet1/2 maintain Brd4-independent stem cell identity. _Nat. Cell Biol._ 20, 565–574 (2018). Article CAS Google Scholar * Shi, Z. D. et al. Genome editing in hPSCs
reveals _GATA6_ haploinsufficiency and a genetic interaction with _GATA4_ in human pancreatic development. _Cell Stem Cell_ 20, 675–688.e6 (2017). Article CAS Google Scholar * Millard,
P., Letisse, F., Sokol, S. & Portais, J. C. IsoCor: correcting MS data in isotope labeling experiments. _Bioinformatics_ 28, 1294–1296 (2012). Article CAS Google Scholar * Lengner, C.
J. et al. _Oct4_ expression is not required for mouse somatic stem cell self-renewal. _Cell Stem Cell_ 1, 403–415 (2007). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS
We thank the Finley laboratory for discussion and A. Intlekofer for critical feedback. We thank A. Smith (University of Cambridge) for the gift of the chimeric LIF receptor and R. Jaenisch
(Whitehead Institute for Biomedical Research) for the gift of the Nanog-GFP ESCs. S.A.V. is a Parker Fellow with the Parker Institute of Cancer Immunotherapy. B.P.R. was supported by an
National Institutes of Health (NIH) T32 Training Grant in Molecular and Cellular Biology (no. T32GM008539). L.W.S.F. is a Searle Scholar and was a Dale F. Frey-William Raveis Charitable Fund
Scientist supported by the Damon Runyon Cancer Research Foundation (no. DFS-23-17). This work was additionally supported by the Concern Foundation and the Anna Fuller Fund (to L.W.S.F.),
The Starr Foundation (no. I11-0039 to L.W.S.F.), a Pathway to Independence Award from the NIH (no. R00 CA191021 to C.C.-F.), NIH/National Institute of Diabetes and Digestive and Kidney
Diseases (no. R01DK096239 to D.H.) and the Memorial Sloan Kettering Cancer Center Support Grant no. P30 CA008748. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Cancer Biology and Genetics
Program, Memorial Sloan Kettering Cancer Center, New York, NY, USA Santosha A. Vardhana & Craig B. Thompson * Center for Epigenetics Research, Memorial Sloan Kettering Cancer Center, New
York, NY, USA Santosha A. Vardhana, Paige K. Arnold, Yanyang Chen, Craig B. Thompson & Lydia W. S. Finley * Cell Biology Program, Memorial Sloan Kettering Cancer Center, New York, NY,
USA Paige K. Arnold, Yanyang Chen & Lydia W. S. Finley * Louis V. Gerstner, Jr., Graduate School of Biomedical Sciences, Memorial Sloan Kettering Cancer Center, New York, NY, USA Paige
K. Arnold * Developmental Biology Program, Memorial Sloan Kettering Cancer Center, New York, NY, USA Bess P. Rosen & Danwei Huangfu * Laboratory of Chromatin Biology and Epigenetics, The
Rockefeller University, New York, NY, USA Bryce W. Carey * Center for Genomics & Systems Biology, Department of Biology, New York University, New York, NY, USA Carlos Carmona-Fontaine
Authors * Santosha A. Vardhana View author publications You can also search for this author inPubMed Google Scholar * Paige K. Arnold View author publications You can also search for this
author inPubMed Google Scholar * Bess P. Rosen View author publications You can also search for this author inPubMed Google Scholar * Yanyang Chen View author publications You can also
search for this author inPubMed Google Scholar * Bryce W. Carey View author publications You can also search for this author inPubMed Google Scholar * Danwei Huangfu View author publications
You can also search for this author inPubMed Google Scholar * Carlos Carmona-Fontaine View author publications You can also search for this author inPubMed Google Scholar * Craig B.
Thompson View author publications You can also search for this author inPubMed Google Scholar * Lydia W. S. Finley View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS S.A.V. and L.W.S.F. conceived the study. S.A.V., P.K.A and L.W.S.F. performed all the experiments with assistance from Y.C. B.W.C. assisted with the
reprogramming experiments. B.P.R. and D.H. performed the human ESC experiments. C.C.-F. performed the immunofluorescence and image analysis. C.B.T. provided additional work in study
conception and guidance. S.A.V. and L.W.S.F. wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Lydia W. S. Finley. ETHICS DECLARATIONS COMPETING INTERESTS C.B.T. is a founder of
Agios Pharmaceuticals and a member of its scientific advisory board. He also previously served on the board of directors of Merck and Charles River Laboratories. ADDITIONAL INFORMATION PEER
REVIEW INFORMATION: Primary Handling Editor: Ana Mateus PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–6 REPORTING SUMMARY SUPPLEMENTARY TABLE 1 Source data for all gas chromatography–mass spectrometry
experiments SUPPLEMENTARY TABLE 2 Calculation of reprogramming efficiency, related to Fig. 4c RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Vardhana,
S.A., Arnold, P.K., Rosen, B.P. _et al._ Glutamine independence is a selectable feature of pluripotent stem cells. _Nat Metab_ 1, 676–687 (2019). https://doi.org/10.1038/s42255-019-0082-3
Download citation * Received: 10 August 2018 * Accepted: 06 June 2019 * Published: 08 July 2019 * Issue Date: July 2019 * DOI: https://doi.org/10.1038/s42255-019-0082-3 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative
