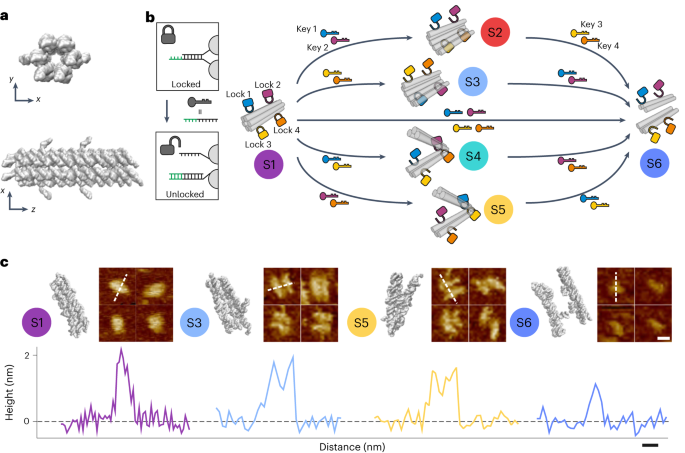
A temporally resolved dna framework state machine in living cells
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The environments in living cells are highly heterogeneous and compartmentalized, posing a grand challenge for the deployment of theranostic agents with spatiotemporal precision.
Despite rapid advancements in creating nanodevices responsive to various cues in cellular environments, it remains difficult to control their operations based on the temporal sequence of
these cues. Here, inspired by the temporally resolved process of viral invasion in nature, we design a DNA framework state machine (DFSM) that can target specific chromatin loci in living
cells in a temporally controllable manner. The DFSM is composed of a six-helix DNA framework with multiple locks that can be opened via DNA strand displacement. The opening of locks at
different locations results in distinct structural configurations of the DFSM. We show that the DFSM can switch among up to six structural states with reversibility, in response to the
temporally ordered molecular inputs, including DNA keys, adenosine triphosphate or nucleolin. By implementing state switching of the DFSM in living cells, we demonstrate temporally
controlled CRISPR–Cas9 targeting towards specific chromatin loci, which sheds light on biocomputing and smart theranostics in complex biological environments. Access through your institution
Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00
per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated
during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS CELLULAR
MACROMOLECULES-TETHERED DNA WALKING INDEXING TO EXPLORE NANOENVIRONMENTS OF CHROMATIN MODIFICATIONS Article Open access 30 March 2021 CONSTRUCTING ARRAYS OF NUCLEOSOME POSITIONING SEQUENCES
USING GIBSON ASSEMBLY FOR SINGLE-MOLECULE STUDIES Article Open access 18 June 2020 DYNAMIC CONTROL OF DNA CONDENSATION Article Open access 01 March 2024 DATA AVAILABILITY The data that
support the findings of this study are available within the paper and its Supplementary Information files. Source data are provided with this paper. CODE AVAILABILITY The code for the
algorithm used for the DNA framework state machine in this work is available in the GitHub repository at https://github.com/HalseyWang/DNA-framework-state-machine ref. 66. REFERENCES *
Whittaker, G. R. Intracellular trafficking of influenza virus: clinical implications for molecular medicine. _Expert Rev. Mol. Med._ 2001, 1–13 (2001). Article Google Scholar *
Brandenburg, B. & Zhuang, X. Virus trafficking-learning from single-virus tracking. _Nat. Rev. Microbiol._ 5, 197–208 (2007). Article Google Scholar * Veneziano, R. et al. Role of
nanoscale antigen organization on B-cell activation probed using DNA origami. _Nat. Nanotechnol._ 15, 716–723 (2020). Article Google Scholar * Kwon, P. S. et al. Designer DNA architecture
offers precise and multivalent spatial pattern-recognition for viral sensing and inhibition. _Nat. Chem._ 12, 26–35 (2020). Article Google Scholar * Kuzyk, A. et al. DNA-based
self-assembly of chiral plasmonic nanostructures with tailored optical response. _Nature_ 483, 311–314 (2012). Article Google Scholar * Fu, J. et al. Multi-enzyme complexes on DNA
scaffolds capable of substrate channelling with an artificial swinging arm. _Nat. Nanotechnol._ 9, 531–536 (2014). Article Google Scholar * Chen, Y. J., Groves, B., Muscat, R. A. &
Seelig, G. DNA nanotechnology from the test tube to the cell. _Nat. Nanotechnol._ 10, 748–760 (2015). Article Google Scholar * Zhang, D. Y. & Seelig, G. Dynamic DNA nanotechnology
using strand-displacement reactions. _Nat. Chem._ 3, 103–113 (2011). Article Google Scholar * Munzar, J. D., Ng, A. & Juncker, D. Comprehensive profiling of the ligand binding
landscapes of duplexed aptamer families reveals widespread induced fit. _Nat. Commun._ 9, 343 (2018). Article Google Scholar * Surana, S., Bhat, J. M., Koushika, S. P. & Krishnan, Y.
An autonomous DNA nanomachine maps spatiotemporal pH changes in a multicellular living organism. _Nat. Commun._ 2, 340 (2011). Article Google Scholar * Tikhomirov, G., Petersen, P. &
Qian, L. Fractal assembly of micrometre-scale DNA origami arrays with arbitrary patterns. _Nature_ 552, 67–71 (2017). Article Google Scholar * Yurke, B., Turberfield, A. J., Mills, A. P.,
Simmel, F. C. & Neumann, J. L. A DNA-fuelled molecular machine made of DNA. _Nature_ 406, 605–608 (2000). Article Google Scholar * Li, T., Lohmann, F. & Famulok, M. Interlocked DNA
nanostructures controlled by a reversible logic circuit. _Nat. Commun._ 5, 4940 (2014). Article Google Scholar * Liu, M. et al. A DNA tweezer-actuated enzyme nanoreactor. _Nat. Commun._
4, 2127 (2013). Article Google Scholar * Martin, T. G. & Dietz, H. Magnesium-free self-assembly of multi-layer DNA objects. _Nat. Commun._ 3, 1103 (2012). Article Google Scholar *
Ranallo, S., Prevost-Tremblay, C., Idili, A., Vallee-Belisle, A. & Ricci, F. Antibody-powered nucleic acid release using a DNA-based nanomachine. _Nat. Commun._ 8, 15150 (2017). Article
Google Scholar * Shibata, T. et al. Protein-driven RNA nanostructured devices that function in vitro and control mammalian cell fate. _Nat. Commun._ 8, 540 (2017). Article Google Scholar
* Woo, S. & Rothemund, P. W. K. Self-assembly of two-dimensional DNA origami lattices using cation-controlled surface diffusion. _Nat. Commun._ 5, 4889 (2014). Article Google Scholar
* Funke, J. J. & Dietz, H. Placing molecules with Bohr radius resolution using DNA origami. _Nat. Nanotechnol._ 11, 47–52 (2016). Article Google Scholar * Maune, H. T. et al.
Self-assembly of carbon nanotubes into two-dimensional geometries using DNA origami templates. _Nat. Nanotechnol._ 5, 61–66 (2010). Article Google Scholar * Modi, S., Nizak, C., Surana,
S., Halder, S. & Krishnan, Y. Two DNA nanomachines map pH changes along intersecting endocytic pathways inside the same cell. _Nat. Nanotechnol._ 8, 459–467 (2013). Article Google
Scholar * Modi, S. et al. A DNA nanomachine that maps spatial and temporal pH changes inside living cells. _Nat. Nanotechnol._ 4, 325–330 (2009). Article Google Scholar * Saha, S.,
Prakash, V., Halder, S., Chakraborty, K. & Krishnan, Y. A pH-independent DNA nanodevice for quantifying chloride transport in organelles of living cells. _Nat. Nanotechnol._ 10, 645–651
(2015). Article Google Scholar * Surana, S., Shenoy, A. R. & Krishnan, Y. Designing DNA nanodevices for compatibility with the immune system of higher organisms. _Nat. Nanotechnol._
10, 741–747 (2015). Article Google Scholar * Cui, Y. & Lieber, C. M. Functional nanoscale electronic devices assembled using silicon nanowire building blocks. _Science_ 291, 851–853
(2001). Article Google Scholar * Ramezani, H. & Dietz, H. Building machines with DNA molecules. _Nat. Rev. Genet._ 21, 5–26 (2020). Article Google Scholar * Li, J., Fan, C., Pei, H.,
Shi, J. & Huang, Q. Smart drug delivery nanocarriers with self-assembled DNA nanostructures. _Adv. Mater._ 25, 4386–4396 (2013). Article Google Scholar * Pei, H., Zuo, X., Zhu, D.,
Huang, Q. & Fan, C. Functional DNA nanostructures for theranostic applications. _Acc. Chem. Res._ 47, 550–559 (2014). Article Google Scholar * Li, S. et al. A DNA nanorobot functions
as a cancer therapeutic in response to a molecular trigger in vivo. _Nat. Biotechnol._ 36, 258–264 (2018). Article Google Scholar * Amir, Y. et al. Universal computing by DNA origami
robots in a living animal. _Nat. Nanotechnol._ 9, 353–357 (2014). Article Google Scholar * Douglas, S. M., Bachelet, I. & Church, G. M. A logic-gated nanorobot for targeted transport
of molecular payloads. _Science_ 335, 831–834 (2012). Article Google Scholar * Groves, B. et al. Computing in mammalian cells with nucleic acid strand exchange. _Nat. Nanotechnol._ 11,
287–294 (2015). Article Google Scholar * Ren, K. et al. A DNA dual lock-and-key strategy for cell-subtype-specific siRNA delivery. _Nat. Commun._ 7, 13580 (2016). Article Google Scholar
* Liu, L. et al. A localized DNA finite-state machine with temporal resolution. _Sci. Adv._ 8, eabm9530 (2022). Article Google Scholar * Cao, S., Wang, F., Wang, L., Fan, C. & Li, J.
DNA nanotechnology-empowered finite state machines. _Nanoscale Horiz._ 7, 578–588 (2022). Article Google Scholar * Kuzuya, A., Wang, R., Sha, R. & Seeman, N. C. Six-helix and
eight-helix DNA nanotubes assembled from half-tubes. _Nano Lett._ 7, 1757–1763 (2007). Article Google Scholar * Liu, P. et al. Charge neutralization drives the shape reconfiguration of DNA
nanotubes. _Angew. Chem. Int. Ed._ 57, 5418–5422 (2018). Article Google Scholar * Chandrasekaran, A. R. Nuclease resistance of DNA nanostructures. _Nat. Rev. Chem._ 5, 225–239 (2021).
Article Google Scholar * Groves, B. et al. Computing in mammalian cells with nucleic acid strand exchange. _Nat. Nanotechnol._ 11, 287–294 (2016). Article Google Scholar * Walsh, A. S.,
Yin, H., Erben, C. M., Wood, M. J. & Turberfield, A. J. DNA cage delivery to mammalian cells. _ACS Nano_ 5, 5427–5432 (2011). Article Google Scholar * Li, J. et al. Self-assembled
multivalent DNA nanostructures for noninvasive intracellular delivery of immunostimulatory CpG oligonucleotides. _ACS Nano_ 5, 8783–8789 (2011). Article Google Scholar * Wang, P. F. et al.
Visualization of the cellular uptake and trafficking of DNA origami nanostructures in cancer cells. _J. Am. Chem. Soc._ 140, 2478–2484 (2018). Article Google Scholar * Bastings, M. M. C.
et al. Modulation of the cellular uptake of DNA origami through control over mass and shape. _Nano Lett._ 18, 3557–3564 (2018). Article Google Scholar * Ye, D. et al. Encapsulation and
release of living tumor cells using hydrogels with the hybridization chain reaction. _Nat. Protoc._ 15, 2163–2185 (2020). Article Google Scholar * Xu, C. F. et al. Rational designs of in
vivo CRISPR–Cas delivery systems. _Adv. Drug. Deliv. Rev._ 168, 3–29 (2021). Article Google Scholar * Sun, W. et al. Self-assembled DNA nanoclews for the efficient delivery of CRISPR–Cas9
for genome editing. _Angew. Chem. Int. Ed._ 54, 12029–12033 (2015). Article Google Scholar * Zuris, J. A. et al. Cationic lipid-mediated delivery of proteins enables efficient
protein-based genome editing in vitro and in vivo. _Nat. Biotechnol._ 33, 73–80 (2015). Article Google Scholar * Lee, B. et al. Nanoparticle delivery of CRISPR into the brain rescues a
mouse model of fragile X syndrome from exaggerated repetitive behaviours. _Nat. Biomed. Eng._ 2, 497–507 (2018). Article Google Scholar * Guo, P., Yang, J., Huang, J., Auguste, D. T. &
Moses, M. A. Therapeutic genome editing of triple-negative breast tumors using a noncationic and deformable nanolipogel. _Proc. Natl Acad. Sci. USA_ 116, 18295–18303 (2019). Article Google
Scholar * Killian, T. et al. Antibody-targeted chromatin enables effective intracellular delivery and functionality of CRISPR/Cas9 expression plasmids. _Nucleic Acids Res._ 47, e55 (2019).
Article Google Scholar * Liang, C. et al. Tumor cell-targeted delivery of CRISPR/Cas9 by aptamer-functionalized lipopolymer for therapeutic genome editing of VEGFA in osteosarcoma.
_Biomaterials_ 147, 68–85 (2017). Article Google Scholar * Deng, W., Shi, X., Tjian, R., Lionnet, T. & Singer, R. H. CASFISH: CRISPR/Cas9-mediated in situ labeling of genomic loci in
fixed cells. _Proc. Natl Acad. Sci. USA_ 112, 11870–11875 (2015). Article Google Scholar * Gao, X. J. & Elowitz, M. B. Precision timing in a cell. _Nature_ 538, 462–463 (2016). Article
Google Scholar * Seelig, G., Soloveichik, D., Zhang, D. Y. & Winfree, E. Enzyme-free nucleic acid logic circuits. _Science_ 314, 1585–1588 (2006). Article Google Scholar * Srinivas,
N., Parkin, J., Seelig, G., Winfree, E. & Soloveiehile, D. Enzyme-free nucleic acid dynamical systems. _Science_ 358, eaal2052 (2017). Article Google Scholar * Xiong, X. W. et al.
Molecular convolutional neural networks with DNA regulatory circuits. _Nat. Mach. Intell._ 4, 625–635 (2022). Article Google Scholar * Piranej, S., Bazrafshan, A. & Salaita, K.
Chemical-to-mechanical molecular computation using DNA-based motors with onboard logic. _Nat. Nanotechnol._ 17, 514–523 (2022). Article Google Scholar * Liu, S. et al. A DNA
nanodevice-based vaccine for cancer immunotherapy. _Nat. Mater._ 20, 421–430 (2021). Article Google Scholar * Shipman, S. L., Nivala, J., Macklis, J. D. & Church, G. M. CRISPR–Cas
encoding of a digital movie into the genomes of a population of living bacteria. _Nature_ 547, 345–349 (2017). Article Google Scholar * Farzadfard, F. & Lu, T. K. Genomically encoded
analog memory with precise in vivo DNA writing in living cell populations. _Science_ 346, 1256272 (2014). Article Google Scholar * Sheth, R. U., Yim, S. S., Wu, F. L. & Wang, H. H.
Multiplex recording of cellular events over time on CRISPR biological tape. _Science_ 358, 1457–1461 (2017). Article Google Scholar * Siuti, P., Yazbek, J. & Lu, T. K. Synthetic
circuits integrating logic and memory in living cells. _Nat. Biotechnol._ 31, 448–452 (2013). Article Google Scholar * Roquet, N., Soleimany, A. P., Ferris, A. C., Aaronson, S. & Lu,
T. K. Synthetic recombinase-based state machines in living cells. _Science_ 353, aad8559 (2016). Article Google Scholar * Perli, S. D., Cui, C. H. & Lu, T. K. Continuous genetic
recording with self-targeting CRISPR-Cas in human cells. _Science_ 353, aag0511 (2016). Article Google Scholar * Schuller, V. J. et al. Cellular immunostimulation by CpG-sequence-coated
DNA origami structures. _ACS Nano_ 5, 9696–9702 (2011). Article Google Scholar * Wang, Y. HalseyWang/DNA-framework-state-machine: DFSM v1.0.1 (v1.0.1). _Zenodo_
https://doi.org/10.5281/zenodo.8144751 (2023). Download references ACKNOWLEDGEMENTS This work was supported by the National Key R&D Program of China (2020YFA0908900 to J.L.), the
National Natural Science Foundation of China (T2188102, 21991134 and 21834007 to C.F.; 22022410 and 82050005 to Y. Zhu), and the New Cornerstone Investigator Program (to C.F.). AUTHOR
INFORMATION Author notes * These authors contributed equally: Yan Zhao, Shuting Cao, Yue Wang, Fan Li. AUTHORS AND AFFILIATIONS * Institute of Materiobiology, Department of Chemistry,
College of Science, Shanghai University, Shanghai, China Yan Zhao, Linjie Guo, Ying Zhu, Lihua Wang & Jiang Li * The Interdisciplinary Research Center, Shanghai Synchrotron Radiation
Facility, Zhangjiang Laboratory, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai, China Yan Zhao, Linjie Guo, Ying Zhu, Lihua Wang & Jiang Li * Institute of
Biomedical Health Technology and Engineering, Shenzhen Bay Laboratory, Shenzhen, China Yan Zhao * Division of Physical Biology, CAS Key Laboratory of Interfacial Physics and Technology,
Shanghai Institute of Applied Physics, Chinese Academy of Sciences, University of Chinese Academy of Sciences, Shanghai, China Shuting Cao, Yue Wang & Lixuan Lin * Xiangfu Laboratory,
Jiashan, China Shuting Cao * Institute of Molecular Medicine, Shanghai Key Laboratory for Nucleic Acid Chemistry and Nanomedicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong
University, Shanghai, China Fan Li & Xiaolei Zuo * School of Chemistry and Chemical Engineering, New Corner Stone Science Laboratory, Frontiers Science Center for Transformative
Molecules, National Center for Translational Medicine, Shanghai Jiao Tong University, Shanghai, China Fei Wang, Xiaolei Zuo & Chunhai Fan * Zhangjiang Laboratory, Shanghai, China Fei
Wang & Lihua Wang * Key Laboratory for Organic Electronics and Information Displays (KLOEID), Institute of Advanced Materials (IAM) and School of Materials Science and Engineering,
Nanjing University of Posts and Telecommunications, Nanjing, China Jie Chao Authors * Yan Zhao View author publications You can also search for this author inPubMed Google Scholar * Shuting
Cao View author publications You can also search for this author inPubMed Google Scholar * Yue Wang View author publications You can also search for this author inPubMed Google Scholar * Fan
Li View author publications You can also search for this author inPubMed Google Scholar * Lixuan Lin View author publications You can also search for this author inPubMed Google Scholar *
Linjie Guo View author publications You can also search for this author inPubMed Google Scholar * Fei Wang View author publications You can also search for this author inPubMed Google
Scholar * Jie Chao View author publications You can also search for this author inPubMed Google Scholar * Xiaolei Zuo View author publications You can also search for this author inPubMed
Google Scholar * Ying Zhu View author publications You can also search for this author inPubMed Google Scholar * Lihua Wang View author publications You can also search for this author
inPubMed Google Scholar * Jiang Li View author publications You can also search for this author inPubMed Google Scholar * Chunhai Fan View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS C.F., J.L. and L.W. directed the research. C.F., J.L., L.W. and Y. Zhao conceived and designed the experiments. Y. Zhao, S.C., Y.W., F.L., L.G.
and L.L. carried out the experiments and analysed data. F.W., J.C., X.Z. and Y. Zhu provided suggestions and technical support on the project. J.L. and C.F. wrote and revised the paper. All
authors discussed the results and commented on the paper. CORRESPONDING AUTHORS Correspondence to Jiang Li or Chunhai Fan. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Machine Intelligence_ thanks the anonymous reviewers for their contribution to the peer review of this work. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 FACS
GATING STRATEGY. Gating strategy to quantify cellular fluorescence in Fig. 5b. A) Gating strategy to sort single cells (P1) according to FSC vs SSC. Then the FITC intensity of cells in P1
was analyzed to determine the cellular fluorescence (taking blank group as an example). B) The same strategy was used to quantify the cellular fluorescence from the DNA nanostructures in
Fig. 5b. EXTENDED DATA FIG. 2 FACS GATING STRATEGY. Gating strategy used for Fig. 6f. Gating strategy to sort single cells (R1) according to aspect ratio vs area. Then the FITC intensity of
cells in R1 was analyzed to determine the cellular EGFP fluorescence (taking DFSM-sgRNA+key group as an example). The cells in R2 (FITC intensity less than 15000) were defined as EGFP
negative cells. The same strategy was used to sort cells in untreated and DFSM-sgRNA groups. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Methods, Figs. 1–18, and Tables
1 and 2. REPORTING SUMMARY SUPPLEMENTARY DATA 1 Source data for supplementary figures. SOURCE DATA SOURCE DATA FIG. 1 Statistical source data. SOURCE DATA FIG. 2 Statistical source data.
SOURCE DATA FIG. 3 Statistical source data. SOURCE DATA FIG. 4 Statistical source data. SOURCE DATA FIG. 5 Statistical source data. SOURCE DATA FIG. 6 Statistical source data. RIGHTS AND
PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s);
author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Zhao, Y., Cao, S., Wang, Y. _et al._ A temporally resolved DNA framework state machine in living cells. _Nat Mach Intell_ 5, 980–990 (2023).
https://doi.org/10.1038/s42256-023-00707-4 Download citation * Received: 24 November 2022 * Accepted: 17 July 2023 * Published: 14 August 2023 * Issue Date: September 2023 * DOI:
https://doi.org/10.1038/s42256-023-00707-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
