Fine scale sampling reveals early differentiation of rhizosphere microbiome from bulk soil in young brachypodium plant roots
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT For a deeper and comprehensive understanding of the composition and function of rhizosphere microbiomes, we need to focus at the scale of individual roots in standardized growth
containers. Root exudation patterns are known to vary along distinct parts of the root even in juvenile plants giving rise to spatially distinct microbial niches. To address this, we
analyzed the microbial community from two spatially distinct zones of the developing primary root (tip and base) in young _Brachypodium distachyon_ grown in natural soil using standardized
fabricated ecosystems known as EcoFABs as well as in more conventional pot and tubes. 16S rRNA based community analysis showed a strong rhizosphere effect resulting in significant enrichment
of several OTUs belonging to Actinobacteria, Bacteroidetes, Firmicutes and Proteobacteria. However, microbial community composition did not differ between root tips and root base or across
different growth containers. Functional analysis of bulk metagenomics revealed significant differences between root tips and bulk soil. The genes associated with different metabolic pathways
and root colonization were enriched in root tips. On the other hand, genes associated with nutrient-limitation and environmental stress were prominent in the bulk soil compared to root
tips, implying the absence of easily available, labile carbon and nutrients in bulk soil relative to roots. Such insights into the relationships between developing root and microbial
communities are critical for judicious understanding of plant-microbe interactions in early developmental stages of plants. SIMILAR CONTENT BEING VIEWED BY OTHERS DIVERSITY, FUNCTION AND
ASSEMBLY OF MANGROVE ROOT-ASSOCIATED MICROBIAL COMMUNITIES AT A CONTINUOUS FINE-SCALE Article Open access 12 November 2020 SOIL DEPTH AND PHYSICOCHEMICAL PROPERTIES INFLUENCE MICROBIAL
DYNAMICS IN THE RHIZOSPHERE OF TWO PERUVIAN SUPERFOOD TREES, CHERIMOYA AND LUCUMA, AS SHOWN BY PACBIO-HIFI SEQUENCING Article Open access 22 August 2024 THE HIERARCHY OF ROOT BRANCHING ORDER
DETERMINES BACTERIAL COMPOSITION, MICROBIAL CARRYING CAPACITY AND MICROBIAL FILTERING Article Open access 19 April 2021 INTRODUCTION Plants exude 20–40% of their photosynthetically fixed
carbon through intact root cells into the surrounding soil [1]. Besides root phenology, root exudates are a key determinant for development of rhizosphere community. These root exudates
contain low-molecular weight organic compounds, and together with mucilage and sloughed off root tissues mainly expelled from root tips, provide a major source of nutrients for the
rhizosphere microbiome [2]. These compounds create an exclusive environment in the rhizosphere that is physiochemically distinct from the surrounding bulk soil and play a key role in
recruiting and selecting relevant beneficial microbes to develop a unique rhizosphere microbiome [3]. Root exudation patterns have been shown to vary spatially along the root system very
early in developing plants, exudates from rapidly dividing root tips differ in composition from exudates released from older sections of the root [4]. While the assembly of the microbial
community along different parts of roots (biogeography) is considered an important parameter in rhizosphere dynamics, systematic and standardized studies probing this deeper are lacking.
Most rhizosphere microbiome studies, where plants are grown in soil, do not compartmentalize the roots based on their length, but rather based on radial distance from the root axis
(rhizosphere, rhizoplane and endosphere). As a result, capturing the effect of spatial differences along the roots is unexplored, causing a gap in understanding how these differences impact
microbial assembly in the rhizoplane in juvenile plants. Furthermore, while few studies have demonstrated influence of plant growth container type on plant morphology [5,6,7,8,9], direct
impacts of growth containers on the rhizosphere microbiome is relatively unexplored under highly controlled experimental conditions. Complex biochemical processes and interactions occur at
microscale dimensions surrounding the root and the ability to interrogate these processes within highly reproduceable and controlled growth containers will propel our understanding of
rhizosphere spatial heterogeneity [10]. In this study, we investigated rhizosphere biogeography from two distinct root zones of young _Brachypodium distachyon_ grown in natural soil, in
three different types of growth containers- conventional pots, tubes and specially fabricated EcoFABs [11] to assess (a) microbiome structure and function across root tips, root base and
bulk soil; and (b) the suitability of standardized growth containers to study plant-microbe interactions at such finer scales in juvenile plants. We also tested these different containers
under open or closed environments (encased within secondary containment). The EcoFABs had demonstrated high value in standardized investigations of plant phenotypic traits and metabolite
production [12], but their applicability to study spatially resolved rhizosphere in juvenile plants had not yet been explored. We used long read 16S rRNA amplicon sequencing and shotgun
metagenomic sequencing to delineate differences between these diverse containers and distinct root zones (root tips, root base). We observed significant differences in microbial structure
and functional potential between root tips and bulk soil even in young developing plant roots. MATERIALS AND METHODS SOIL AND PLANT GROWTH CONDITIONS Soil for plant growth was collected from
the south meadow field site at the Angelo Coast Range Reserve in northern California (39° 44′ 21.4′′ N 123° 37′ 51.0′′ W) in August 2020. The upper layer (0–10 cm) was collected in clean
collection bags, immediately transported on ice and stored at 4 °C until further processing. The collected soil was passed through a 2 mm sieve to remove larger particles like dry roots and
rocks prior to use. In this study, we used three types of containers, EcoFAB, test tubes and plastic pots to grow _B. distachyon_ (Bd21-3 plant line). EcoFABs (_n_ = 11) were fabricated as
reported earlier [13] with slight modifications. Briefly, the oval-shaped polydimethylsiloxane (PDMS) cast measuring 7.7 cm × 5.7 cm × 0.5 cm (height × width × depth) providing a container
volume of 10 mL was held together by metal clamps and screws. Sterile plastic test tubes (_n_ = 14) used to grow plants were 10 cm long with a diameter of 1.5 cm, and had a hole drilled at
the bottom to drain excess water. The pots (_n_ = 14) used were 10 cm × 10 cm squares with a depth of 10.5 cm, tapered from top to bottom. The weight of soil in test tube and EcoFAB was kept
at 15 g each while the pot contained 600 g. The vertical distance between the sown seed to the bottom of the container was 8 cm for EcoFAB and 9 cm for both pot and test tube. Except for
soil, all components were sterilized by UV sterilization or autoclaving. In addition, approximately half of all containers were kept sterile in closed Microbox containers (Sac O2, Belgium)
while others were kept open to the environment. Cold-treated _Brachypodium distachyon_ seeds were de-husked, surface-sterilized in 70% ethanol followed by 50% household bleach for 5 min each
and rinsed thoroughly in sterile water. They were germinated on sterile 0.8% noble agar plates under sunlight at room temperature for two days. Germinated seedlings were transferred into
the containers taking care to place it 0.5 cm below the soil surface, watered once at 100% capacity with sterile water. Subsequent watering was done at 15% holding capacity, every 2 and 4
days for the open and closed containers respectively. The plants were placed in a greenhouse with a 16-h photoperiod, 87.5% relative humidity, and average day and nighttime temperatures of
19.9 °C and 17.9 °C respectively. PLANT PHENOTYPIC MEASUREMENTS Plants were harvested from all containers 14 days after sowing when the primary root had reached bottom of EcoFAB, and key
plant phenotypic characteristics were measured. After excising the roots from the base of plant shoot, dry shoot weight was obtained by oven drying the shoots at 80 °C for 24 h followed by
cooling to room temperature and measuring dry weight [14,15,16]. Shoot length was measured from end of the longest leaf to the point where root starts [17]. Root length was measured from
root base to tip of the primary root. RHIZOSPHERE AND BULK SOIL SAMPLE COLLECTION At the time of harvest, most plants had only one fine primary axile root. The roots were excised carefully
from soil under aseptic conditions and lightly shaken to remove loosely attached bulk soil. Root tip and root base samples were harvested as 2 cm cuttings, measured from tip of the root, and
from base of the plant shoot respectively. Due to complications during sampling resulting in physical damage to the roots, some samples were discarded reducing the number of root samples to
_n_ = 8, _n_ = 11, and _n_ = 7 originating from EcoFAB, test tube, and pot respectively. The loosely-bound rhizosphere soil was obtained by vortexing the root in 5 mM sodium pyrophosphate
for 15 s, three times. The root was then placed in fresh pyrophosphate buffer and sonicated for 5 min to extract tightly-bound fraction. To ensure the complete representation of the
rhizosphere microbiome, both the loosely- and tightly-bound fractions were pooled for subsequent DNA extraction. Bulk soil (0.5 g) was collected from containers at least 1 cm away from the
roots and kept frozen before DNA extraction. DNA EXTRACTION AND SEQUENCING Genomic DNA was extracted using DNeasy PowerLyzer Powersoil kit (Qiagen, US) following the manufacturer’s
instructions and the eluted genomic DNA was quantified using QubitTM dsDNA High Sensitivity assay kit (Thermofisher, US). For bacterial full-length 16S rRNA amplification and sequencing,
genomic DNA from all the available different root locations and bulk soil were sent to Loop Genomics (US). Briefly, the DNA was amplified with indexed forward (5’ CTGCCTAGAACA [Index, F]
AGAGTTTGATCMTGGCTCAG 3’) and reverse primers (5’ TGCCTAGAACAG [Index, R] TACCTTGTTACGACTT 3’) and sequenced using the Illumina sequencing platform via paired end (150 bp X 2) mode followed
by the standard Loop Genomics informatics pipeline that uses short reads to construct synthetic long reads [18]. For metagenomic sequencing, replicates of each sample type (root tip, root
base or bulk soil from each type of container) was pooled to accommodate the 200 ng DNA concentration requirement, resulting in a total of 9 samples. These samples were sent to QB3-Berkeley
Functional Genomics Laboratory (University of California, Berkeley, US) (http://qb3.berkeley.edu/fgl/) for library prep and subsequent sequencing using Illumina 150 bp X 2 paired end reads
with a depth of 20 Gb per sample. 16S RRNA COMMUNITY ANALYSIS 16S amplicon samples which contained less than 1000 reads after demultiplexing were discarded before analysis. We ensured that
there were at least 3 replicate samples for every type of sample under the three variables tested; 1. Container (EcoFAB, pot or test tube), 2. Location (root tip, root base or bulk soil) and
3. Condition (Closed or Open). The demultiplexed data from loop genomics was then clustered into OTUs using usearch (version 11.0.667) for comparative analyses as follows [19]. Briefly,
FASTQ files were 1st trimmed (1400 bps) and quality filtered (maximum expected error cutoff 1.0) before initial clustering and chimera filtering using Unoise 3 command. The resulting OTUs
were further clustered to 97% identity before generating the OTU table, taxonomic assignments and comparative analyses. From the OTUs generated through usearch, DECIPHER v2.0 (r studio
package) was used to obtain taxonomic information based on the SILVA SSU version 138 [20, 21] following default parameters. The generated OTU samples were subjected to Hellinger
transformation using decostand method in vegan R package version 2.5-7 [22] to standardize differences in sequencing depth prior to diversity analysis. Differential abundance of microbial
OTUs across different containers and sample locations were determined using the DESeq2 package (version 1.14.1) in R [23]. Pairwise comparison between sample locations coupled to each
container was carried out using a full DESeq2 model (design = ~Container_Location + Condition). OTUs showing significant log-fold changes (padj < 0.05) in at least one of these
comparisons was further selected and visualized on a phylogenetic tree in iToL [24]. The log fold-change values were tested for correlation using Spearman’s test through custom python
script. Afterwards, pairwise comparisons were repeated with a reduced model (design = ~Location + Container + Condition) to study the effect on sample location while controlling container
and condition variations. Using the transformed data, homogeneity of multivariate dispersions was analyzed for each sample location in each container using betadisper from vegan R package.
METAGENOME ASSEMBLY, ANNOTATION, AND BINNING Shotgun metagenomic sequence for the 9 samples (3 containers * 3 locations) were individually assembled using IDBA-UD v1.1.3 [25] with the
parameters: -pre_correction -mink 20 -maxk 150 -step 10. Following metagenome assembly, all samples were filtered to remove contigs smaller than 1 kb using pullseq
(https://github.com/bcthomas/pullseq). Open reading frames were then predicted on all contigs using Prodigal v2.6.3 [26] with the parameters: -m -p meta. KEGG KO annotations were predicted
using KofamScan [27] using HMM models from release r02_18_2020 using default options. In cases where multiple HMMs matched a protein above threshold, the HMM with the lowest E-value had its
annotation transferred to the protein. Metagenome assemblies were binned into draft genomes using a combination of 4 automated binning methods. Briefly, reads from all 9 samples were mapped
to assembled contigs ≥2.5 kbp using Bowtie2, and a differential coverage profile for each contig across all samples was used as input for the following differential coverage binners:
MaxBin2, CONCOCT, vamb, and MetaBAT [28,29,30,31]. The algorithm DasTool [32], was then used to select the highest quality bins across the 4 binning outputs for each metagenome assembly.
Finally, the full genome set across all samples (_n_ = 146 genomes) was de-replicated at the species level (Average Nucleotide Identity ≥95%) using dRep [33] with the following parameters:
-p 16 -comp 10 -ms 10000 -sa 0.95, resulting in a total of 42 species representative genomes. Species representatives were further selected to have ≥60% completeness and ≤10% contamination
as estimated by checkM [34], this resulted in a final set of 32 species representative genomes meeting the criteria. 16S rRNA sequences were extracted from genomes with ContEst16S tool
available online (https://www.ezbiocloud.net/tools/contest16s, last accessed on August 17, 2022) [35]. These 16S rRNA sequences were compared with the OTUs obtained from amplicon sequencing
using BLAST+ [36] to check for taxonomic consistency. PHYLOGENETIC AND ABUNDANCE ANALYSIS OF GENOME BINS Phylum level taxonomic assignments of 32 de-replicated genome bins and 1 genome (_P_.
calidifontis - GCA000015805) included as an outgroup were inferred using GTDB-Tk v1.5.1 [37] with reference data version r202; phylogenetic relationships between de-replicated genome bins
were inferred using GToTree v1.5.22 based on a set of 25 marker genes, and a phylogenetic tree was produced using FastTree2 [38].The tree was displayed and rooted in Geneious Prime
v2020.2.4. The relative abundance of the 32 genome bins in all samples was assessed by cross mapping reads from each of the 9 samples back to the genome bins using Bowtie2, followed by
quantification of coverage of genomes in each sample using coverM (https://github.com/wwood/CoverM). Differential abundance of genomes between rhizosphere spatial locations was assessed
using the DESeq2 package in R [23]. Detailed steps can be found in Supplementary material. BULK METAGENOME ANALYSIS Phylum level taxonomic composition of bulk metagenomes was assessed
directly from raw sample reads using graftM [39] run with a custom ribosomal protein L6 (rpL6) marker database constructed from the r202 release of the GTDB database. Differentially abundant
KO genes across the different sample locations were determined using the DESeq2 package (version 1.14.1) in R [23]. Pairwise comparison between sample locations was carried out using a
reduced DESeq2 model (design = ~Location). Heatmap of differentially abundant genes were plotted in R using the variance stabilized abundance values. RESULTS CONTAINER TYPE HAS MINIMAL
IMPACT ON PLANT PHENOTYPIC GROWTH We measured three major phenotypes of plant growth, i.e., dry shoot weight, shoot length, root length, to determine container impacts on general plant
growth. The only significant difference was between plants grown in pots in open vs. closed conditions (Supplementary Fig. S1). The microbox used to maintain sterile condition (closed) was
found to trap a visibly higher amount of moisture inside the box and likely created higher water retention promoting plant growth. Regardless, no other significant difference was detected
within or among containers despite differences in container architecture. MICROBIOMES IN ROOT TIPS AND ROOT BASE ARE LARGELY SIMILAR YET DISTINCT FROM THE BULK SOIL _B. distachyon_, a model
grass species for wheat family, was chosen as it produces only one fine primary axile root from the base of the embryo [40] on which the microbial spatial analysis was performed. We analyzed
the rhizosphere microbial community from two different root locations of a 14-day old _B. distachyon_ and the bulk soil using full length 16S rRNA obtained using synthetic long read
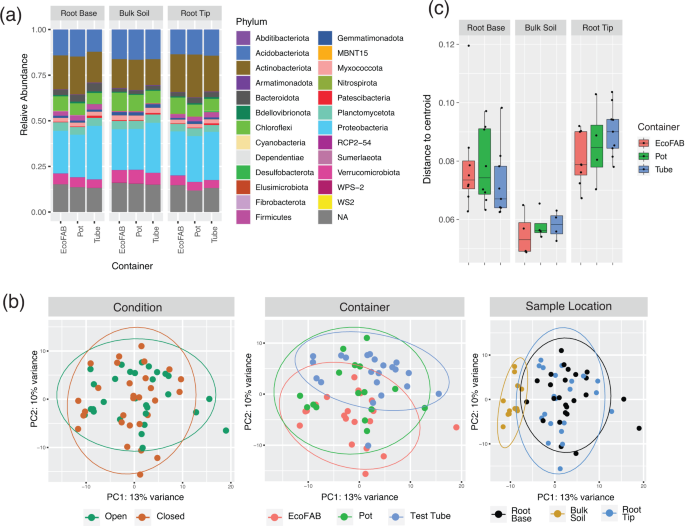
technology. Among the 3674 OTUs obtained after quality filtering, 25 different phyla were identified which corresponded to ~80–87.5% of all reads among the samples. Microbial relative
abundance showed on average a dominance of the bacterial phyla Proteobacteria (22.3–29.3%), Actinobacteriota (14.2–23.5%), Acidobacteriota(12.2–16.5%), Chloroflexi(6.3–10.1%),
Planctomycetota (3.7–4.7%), Verrucomicrobiota (4.2–7.4%), Bacteriodota (1.6–4.5%) and Myxococcota (1.9–2.6%) in all samples (Fig. 1a). Interestingly, phyla Firmicutes had lower relative
abundance in bulk (average - 0.4%) compared to root tip and root base samples (average - 2.6%). Alpha diversity was lower in root tip compared to bulk soil (_p_ < 0.005, Anova and Tukey)
or root base (_p_ < 0.05, Anova and Tukey) in all three diversity metrics analyzed (species number, Shannon and inverse Simpson) (Supplementary Fig. S2). On the other hand, no significant
difference in alpha diversity was observed between root base and bulk soil. When root tip samples were compared between the three containers, there was no significant difference in alpha
diversity (_p_ > 0.05, Anova and Tukey) indicating negligible container impact. Beta diversity analysis was then carried out to investigate the influence of three parameters tested, i.e.,
container type, location on root and open or closed condition. Principal Components Analysis (PCA) of the samples showed no clear separation among the two conditions or among the three
container types whereas a distinct separation was observed between bulk soil samples compared to root base or root tip (Fig. 1b). However, no distinction was seen when comparing root base
and root tip based on ordination analysis. This was supported statistically using MANOVA/_adonis_ which showed the highest dissimilarity contributed by sample location (R2 = 0.10934, _p_ =
9.99e−05) followed by container type (R2 = 0.06336, _p_ = 0.00069) but no significant dissimilarity caused by either open or closed conditions (R2 = 0.02149, _p_ = 0.8119). Next, we examined
whether the homogeneity within samples could be influenced by container type. Overall, the EcoFAB samples exhibited a comparable homogeneity among replicates of the same sample location
compared to the other two conventional containers (Fig. 1c). PAIRWISE COMPARISON BETWEEN SAMPLE LOCATIONS SHOWED THE SAME DIFFERENTIALLY ABUNDANT OTUS REGARDLESS OF CONTAINER TYPE The OTUs
which showed a statistically significant change in any of the pairwise comparisons between sample locations within each container type were selected and visualized using a neighbor-joining
tree (Fig. 2a, Supplementary Table S1–S3). Distinct log-fold changes could be observed for comparisons looking at rhizosphere (root base or root tip) vs. bulk soil; the container type has no
impact on this trend. Only two OTUs had significant abundance difference between root tip vs. root base, indicating that root tip and root base microbiomes may not be very distinct from one
another in young developing plant roots. Further, analysis with Spearman’s correlation coefficient showed that the overall log-fold changes of each OTU were statistically positively
correlated in most comparisons regardless of container (Supplementary Table S4), with the only exception being the root tip vs. root base changes observed in pot vs test tube (rho = −0.02,
_p_ = 0.78). In all three comparisons, results from EcoFAB samples were consistent with the others. Henceforth, we disregard the container variable to focus on spatial differences. Using
comparisons solely based on sample location, the OTUs could be grouped into three distinct clusters (Fig. 2b, Supplementary Table S5). The first and smallest cluster showed the OTUs
exhibiting significant increase in the rhizosphere (root base or root tip) compared to the bulk soil. Among them are multiple OTUs belonging to _Mucilaginibacter_ (Bacteriodota), _Bacillus_
(Firmicutes), _Paenibacillus_ (Firmicutes), and unclassified Oxalobacteraceae (Gammaproteobacteria). The biggest cluster was for OTUs with a large decrease in the rhizosphere which included
the phyla Acidobacteriota, Gemmatimonadota and Chloroflexi. The third cluster contained OTUs with minimal increase or decrease compared within sample locations and contained a mix of phyla.
TAXONOMIC ANALYSIS FROM METAGENOMICS SHOWS SIMILAR COMMUNITY COMPOSITION TO 16S RRNA BASED AMPLICON DATA Read data from shotgun metagenome samples was directly assessed for bulk taxonomic
composition using the ribosomal protein L6 (rpL6) marker gene. The phylum-level relative abundance in all samples showed dominance by the Proteobacteria, Actinobacteriota, Acidobacteriota,
Planctomycetota and Verrucomicrobiota (Supplementary Fig. S3a), similar to the 16S rRNA based community composition (Fig. 1a). A PCA plot also illustrated a clustering of the bulk soil
samples distinctly from the rhizosphere samples as seen earlier in the corresponding 16S amplicon data (Supplementary Fig. S3b). Overall, the metagenomic taxonomy was in correspondence with
the 16S amplicon data and both types of analysis revealed minimal changes contributed by container differences. METAGENOME ASSEMBLED GENOMES (MAGS) REPRESENT A SMALL FRACTION OF THE TOTAL
READS Out of the 32 representative MAGs generated from 9 metagenomes after dereplication and quality filtering (Fig. 3), 11 MAGs belonged to Actinobacteriota; 6 MAGs from
Gammaproteobacteria; 4 MAGs from Acidobacteriota and Alphaproteobacteriota; 3 MAGs each from Chloroflexota; 2 MAGs from Myxococcota and 1 MAG each from Gemmatimonadota and Elusimicrobiota
(Supplementary Table S6). As expected in systems with higher diversity, the total coverage of these genomes was rather low, representing ~3% of the read data. 10 MAGs were identified to be
differentially abundant across sample locations (Fig. 3). It is interesting to note that one Acidobacterial MAG (_Edaphobacter sp_.) had increased abundance in root tip compared to both bulk
and base. Members of _Edaphobacter_ genus are reported to be associated with ectomycorrhizal fungi and are important in their root colonization [41]. Only 6 MAGs had 16S rRNA and all these
sequences had a 97–100% match with OTUs obtained from amplicon sequencing and similar phylogenetic classification. METAGENOME ANALYSIS REVEALS METABOLIC DIFFERENCES BETWEEN ROOT TIP AND BULK
5783 unique KEGG orthology groups (KOs) were annotated in the metagenomes, accounting for ~30% of the total proteins predicted in each metagenome. PCA plot of KEGG Orthology (KO)
composition of samples indicated that samples cluster by location irrespective of the container type (Supplementary Fig. S4) and hence container parameter was excluded from further DESeq
analysis. There were no differentially abundant KOs when root tip was compared to base, in congruence with observations from PCA analysis of OTUs (Section 3.2). Among the 55 differentially
abundant KOs identified (Fig. 4, Supplementary Table S7), 27 were enriched in root tip compared to bulk, while other 27 were decreased in tip vs. bulk and 2 KOs (one KO shared with decreased
tip vs. bulk comparison) increased in bulk over base. KOs involved in different metabolic pathways were over-represented in tip compared to the bulk suggesting an active microbial
population utilizing plant-derived compounds. These KOs, which could be broadly categorized as either enzymes, transcriptional regulators or transporters, play a critical role in substrate
utilization as well as root colonization. Enzymes encoded were peptidases (_ampS, cwlO_), nucleases (_nucS_), kinases (_rsbW, fakA_), and other enzymes involved in fatty acid degradation
(_acd_), lipid storage (_tgs/wax-dgat_), cell wall synthesis (_tagTUV_), and redox regulation (_gshA, fqr_). Transcriptional factors/regulator genes enriched in root tips were involved in
regulation of purine catabolism (_pucR_), arabinogalactan biosynthesis (_embR_), biofilm formation (_sigB_) [42], sulfur utilization (_sutR_) and other functions (_tetR_). The enzyme,
peptidoglycan DL-endopeptidase encoded by _cwlO_, has been shown to regulate biofilm formation and consequently root colonization in plant-beneficial rhizobacterium _Bacillus velezensis_
SQR9 [43]. Interestingly, the anti-sigma factor _rsbW_ and sigma factor _sigB_ were identified as adjacent genes of _sigB_ gene cluster and play important roles in stress resistance, biofilm
formation and root colonization in _Bacillus cereus_ 905 [42].Transporters involved in acquisition of copper (_ycnJ_), amino acid translocation (_rhtA_), ion transport (_nhaA_), and other
nutrients (MFS (_mmr_) and ABC transporters (_mlaD/linD_)) were elevated in root tips. 4 other poorly characterized genes and gene involved in oxidative phosphorylation (_qcrC_) were also
increased in the root tips over bulk metagenomes. Microbes in the bulk soil do not have ready access to the labile carbon and nitrogen compounds in the exudates and hence may have to invest
more in the machinery for nutrient acquisition. Genes involved in heme uptake (_exbBD_ and _tonB_) [44] and nitrogen assimilation/quorum sensing (_rpoN_) [45] were increased in bulk soil. In
addition, KOs involved in glycogen synthesis (_glgA_), lipopolysaccharide export (_lptF_), polysaccharide biosynthesis/export (_wza/gfcE_), maintenance of cellular integrity under acidic
stress (_ompA-ompF_ porin), production of coenzymes (_pqqL_) involved in free-radical scavenging, regulation of exopolysaccharide production (_hprK_), periplasmic divalent cation tolerance
(_cutA_) and osmotic stress genes (_osmY_) may confer resistance to environmental stressors like osmotic stress and desiccation [46, 47] present in bulk soil. KOs corresponding to transfer
RNA biogenesis (_mnmE/trmE, gidA/mnmG_), transcriptional regulation (_rho, ada_), ribosome biogenesis (_rlmI_), and sulfur metabolism (_dmsBC_) were also enriched in the soil. DISCUSSION We
investigated the utility of EcoFABs as a possible alternative to conventional containers such as pots and tubes for studying the spatial microbial biogeography of the rhizosphere. Although
studies have shown that container design parameters such as size, density, depth can affect root growth and basic plant physiological traits during early developmental stages [5,6,7,8,9],
our study with young _Brachypodium_ plants showed that containers had no significant impact. While most of these studies looked at container sizes around 50cm3, they were performed using
woody tree seedlings such as _Pinus sp_. (Pine tree species) and _Quercus sp_. (Oak tree species). Container impacts may not apply to softer plants such as _B. distachyon_ to a discernible
extent, emphasizing the importance of using the correct standardized experimental systems and containers to perform accurate study comparisons for the plant under investigation. Next, we
investigated the impact of microbial community assembly on the root impacted by container differences using both 16S amplicon sequencing and metagenomics. Based on 16S amplicon sequencing
results, microbial community of each location with respect to root showed relatively similar composition across all containers. Differences were observed mostly in root tip or base locations
compared to the bulk soil. At root tips, a decrease of bacterial OTU richness and alpha diversity when compared to bulk soil has been previously reported [3, 48]. This reduction in
microbial diversity in the rhizosphere is commonly observed [49] as the root exudates create a selective environment, recruiting selected microbes from bulk soil. We further observed that
root tips had lower bacterial diversity (richness and evenness) than root base, which concurs with the other studies conducted on _Brachypodium_ roots [50, 51]. Root tip environment appears
to be more stochastic and dynamic compared to the root base as the assembly patterns appear to be more deterministic in older parts of the root [49]. This is partially true in our study as
well, there were a higher number of significant OTUs in the comparison of base vs bulk than comparing tip vs bulk (Fig. 2a). Nonetheless, overall correlations show a significantly positive
correlation which meant that the rhizosphere effect is already developing at the tip even for 2-week old juvenile plants of _Brachypodium_. Usually, microbial composition studies tend to
occur at later stages of _Brachypodium_ growth [50,51,52] because the plant often takes 30 – 35 days to reach maturity [40]. Our study, however, shows that a rhizosphere effect may be
occurring as early as 14 days into the plant growth. Only some of the dominant rhizosphere community members such as Gammaproteobacteria and Bacteriodota matched the observations in a
previous study with _Brachypodium_ rhizosphere [50]. Phyla such as _Betaproteobacteria_, which were highly enriched in a previous study with mature plants [50], were neither abundant nor
showed enrichment in the rhizosphere. Nonetheless, other rhizosphere enriched groups in this study include Actinobacteria, Acidobacteria and Verrucomicrobia which seems to be more of an
effect of the low pH soil characteristic of our field site [53]. Additionally, in that study [50] _Brachypodium_ was grown in sand amended soil which could explain the differences.
Actinobacteria, for instance, is associated with rhizosphere in soils with high organic content [54, 55]. In another study where fine scale sampling of 4-week-old _Brachypodium_ roots was
performed, Firmicutes were more abundant in root tips compared to root base, whereas opposite trend was observed for Verrucomicrobia [51]. Phyla such as Actinobacteria, Proteobacteria and
Bacteriodota were reported to be enriched in wheat rhizosphere [56]. Thus, in line with prior studies, our data also suggests that a combination of root exudates and edaphic factors are
working in tandem to enrich a specific rhizosphere community. Among 150 OTUs which were differentially abundant between different sampling locations, all OTUs belonging to phylum Firmicutes
and Bacteriodota were enriched in rhizosphere over bulk soil. These included genera _Bacillus_ and _Paenibacillus_ (Firmicutes) and _Mucilaginibacter_ (Bacteriodota). Members of
_Paenibacillus_ have been isolated from rhizosphere of wide variety of plants; several of these are capable of fixing-nitrogen [57,58,59]. Similarly, several _Mucilaginibacter_ strains have
been isolated from rhizosphere, and a comparative analysis of various strains in this genus highlighted the presence of diverse carbohydrate active enzymes including cellulose-degrading
enzymes [60]. Impacts of different Bacillus isolates on _Brachypodium_ plants have been characterized previously; _Bacillus_ can accelerate growth, provide drought protection [61], influence
root architecture [61] and can modulate plant hormone homeostasis. Some _Bacillus_, could be classified as r-strategists, and quickly grow in response to nutrient availability in
rhizosphere [51]. Majority of differentially abundant OTUs belonging to Gemmatimonodota, Acidobacteria and Verrucomicrobia had reduced abundance in the rhizosphere compared to bulk. These
bacterial groups are slow-growing and oligotrophic [62,63,64], thus more suited to survive in bulk soil away from the nutrient-rich rhizosphere. OTUs belonging to Actinobacteria,
Gammaproteobacteria and Alphaproteobacteria showed no clear trends. We observed congruence between taxonomic results obtained by 16S rRNA gene sequencing and metagenomics (rpL6 marker gene),
demonstrating reliability of different sequencing methodologies for bacterial profiling (short read Illumina vs. long-read technology). Comparative analysis of metagenomic functional
potential between various sampling locations revealed significant differences between root tips and bulk soil. KO genes involved in different metabolic pathways and root colonization were
over-represented in tip compared to the bulk suggesting an active microbial population capable of utilizing plant-derived exudates and occupying the rhizosphere. KO genes associated with
machinery for nutrient acquisition and stress-tolerance were prominent in bulk soil where readily available substrates are scarce in comparison to the vicinity of roots. These findings are
consistent with other metagenomic studies comparing rhizosphere vs. bulk soil [65] and also in agreement with the results from 16S amplicon sequencing, where rhizosphere is abundant in
fast-growing groups and bacterial assembly in root tips is stochastic, while bulk soil is enriched with groups that are more oligotrophic and adapted to survive in nutrient-limited
conditions. We would also like to highlight a few shortcomings and potential improvements in follow up studies. As a result of low DNA yields from juvenile roots, samples were pooled for
metagenomics which led to low sample numbers. In addition, genome-resolved metagenomics yielded fewer genomes making statistical analysis of genome relative abundance and metabolic potential
analysis challenging. Differences in gene abundance were observed only between root tips and bulk soil, thus differences within rhizosphere compartments (tip vs. base) are unclear which is
probably due to sampling of young plants. Size of current EcoFABs limit how long plants can be grown, but can be addressed with bigger devices in future versions. Thus, in conclusion, we
have demonstrated the influence of developing roots in shaping microbial communities in comparison with bulk soil in 14-day old juvenile _Brachypodium_ plants through 16S rRNA amplicon
sequencing and metagenomic analyses. While we did not observe distinct differences in microbiomes between the two root zones; based on previous work [51] in older plants, there is a need for
high-resolution sampling of rhizosphere to understand biological interactions occurring at finer scales. To further probe into the physiology of root-enriched microbes, we are currently
performing high-throughput enrichment of this rhizobiome on known root exudate compounds to create reduced complexity communities and working on engineering materials that can be integrated
into EcoFABs to enable localized, sub-millimeter scale sampling at different timepoints. DATA AVAILABILITY The 16S rRNA amplicon sequences and metagenome-assembled genomes generated during
the current study are available in the NCBI SRA repository, under the BioProject ID PRJNA902408. The full assemblies for each metagenome sample are publicly available at our in-house
analysis platform, ggKbase (https://ggkbase.berkeley.edu). REFERENCES * Pausch J, Kuzyakov Y. Carbon input by roots into the soil: quantification of rhizodeposition from root to ecosystem
scale. Glob Chang Biol. 2018;24:1–12. Article PubMed Google Scholar * Dennis PG, Miller AJ, Hirsch PR. Are root exudates more important than other sources of rhizodeposits in structuring
rhizosphere bacterial communities? FEMS Microbiol Ecol. 2010;72:313–27. Article CAS PubMed Google Scholar * Bulgarelli D, Schlaeppi K, Spaepen S, Ver Loren van Themaat E, Schulze-Lefert
P. Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol. 2013;64:807–38. Article CAS PubMed Google Scholar * Aufrecht J, Khalid M, Walton CL, Tate K, Cahill
JF, Retterer ST. Hotspots of root-exuded amino acids are created within a rhizosphere-on-a-chip. Lab Chip. 2022;22:954–63. Article CAS PubMed Google Scholar * Howell KD, Harrington TB.
Nursery practices influence seedling morphology, field performance, and cost efficiency of containerized cherrybark oak. Southern J Appl For. 2004;28:152–62. Article Google Scholar * South
DB, Harris SW, Barnett JP, Hainds MJ, Gjerstad DH. Effect of container type and seedling size on survival and early height growth of _Pinus palustris_ seedlings in Alabama, U.S.A. Forest
Ecol Manag. 2005;204:385–98. Article Google Scholar * Tsakaldimi M, Zagas T, Tsitsoni T, Ganatsas P. Root morphology, stem growth and field performance of seedlings of two mediterranean
evergreen oak species raised in different container types. Plant Soil. 2005;278:85–93. Article CAS Google Scholar * Kostopoulou P, Radoglou K, Papanastasi OD, Adamidou C. Effect of
mini-plug container depth on root and shoot growth of four forest tree species during early developmental stages. Turkish J Agric For. 2011;35:379–90. * Chu X, Wang X, Zhang D, Wu X, Zhou Z.
Effects of fertilization and container-type on nutrient uptake and utilization by four subtropical tree seedlings. J For Res. 2020;31:1201–13. Article CAS Google Scholar * Yee MO, Kim P,
Li Y, Singh AK, Northen TR, Chakraborty R. Specialized plant growth chamber designs to study complex rhizosphere interactions. Front Microbiol. 2021;12:625752. Article PubMed PubMed
Central Google Scholar * Zengler K, Hofmockel K, Baliga NS, Behie SW, Bernstein HC, Brown JB, et al. EcoFABs: advancing microbiome science through standardized fabricated ecosystems. Nat
Methods. 2019;16:567–71. Article CAS PubMed PubMed Central Google Scholar * Sasse J, Kant J, Cole BJ, Klein AP, Arsova B, Schlaepfer P, et al. Multilab EcoFAB study shows highly
reproducible physiology and depletion of soil metabolites by a model grass. New Phytol. 2019;222:1149–60. Article CAS PubMed PubMed Central Google Scholar * Gao J, Sasse J, Lewald KM,
Zhalnina K, Cornmesser LT, Duncombe TA, et al. Ecosystem fabrication (EcoFAB) protocols for the construction of laboratory ecosystems designed to study plant-microbe interactions. J Vis Exp.
2018;134:57170. * Barrs HD, Weatherley PE. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci. 1962;15:413. Article Google
Scholar * Darko E, Végh B, Khalil R, Marček T, Szalai G, Pál M, et al. Metabolic responses of wheat seedlings to osmotic stress induced by various osmolytes under iso-osmotic conditions.
PLoS One. 2019;14:e0226151. Article CAS PubMed PubMed Central Google Scholar * Mukami A, Ngetich A, Mweu C, Oduor RO, Muthangya M, Mbinda WM. Differential characterization of
physiological and biochemical responses during drought stress in finger millet varieties. Physiol Mol Biol Plants. 2019;25:837–46. Article CAS PubMed PubMed Central Google Scholar *
Bresolin APS, Dos Santos RS, Wolter RCD, de Sousa RO, da Maia LC, Costa de Oliveira A. Iron tolerance in rice: an efficient method for performing quick early genotype screening. BMC Res
Notes. 2019;12:361. Article CAS PubMed PubMed Central Google Scholar * Callahan BJ, Grinevich D, Thakur S, Balamotis MA, Yehezkel TB. Ultra-accurate microbial amplicon sequencing with
synthetic long reads. Microbiome. 2021;9:130. Article CAS PubMed PubMed Central Google Scholar * Edgar RC, Flyvbjerg H. Error filtering, pair assembly and error correction for
next-generation sequencing reads. Bioinformatics. 2015;31:3476–82. Article CAS PubMed Google Scholar * Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA
ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–6. Article CAS PubMed Google Scholar * Wright ES. Using DECIPHER v2.0
to analyze big biological sequence data in R. R J. 2016;8:352. Article Google Scholar * Jari O. vegan: Community Ecology Package. R package version 1.8-5. http://www.cran.r-project.org
2007. * Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. Article PubMed PubMed Central Google Scholar
* Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49:W293–W296. Article CAS PubMed PubMed
Central Google Scholar * Peng Y, Leung HCM, Yiu SM, Chin FYL. IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics.
2012;28:1420–8. Article CAS PubMed Google Scholar * Hyatt D, Chen G-L, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site
identification. BMC Bioinformatics. 2010;11:119. Article PubMed PubMed Central Google Scholar * Aramaki T, Blanc-Mathieu R, Endo H, Ohkubo K, Kanehisa M, Goto S, et al. KofamKOALA: KEGG
Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics. 2020;36:2251–2. Article CAS PubMed Google Scholar * Wu Y-W, Simmons BA, Singer SW. MaxBin 2.0: an
automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics. 2016;32:605–7. Article CAS PubMed Google Scholar * Alneberg J, Bjarnason BS, de Bruijn
I, Schirmer M, Quick J, Ijaz UZ, et al. Binning metagenomic contigs by coverage and composition. Nat Methods. 2014;11:1144–6. Article CAS PubMed Google Scholar * Nissen JN, Johansen J,
Allesøe RL, Sønderby CK, Armenteros JJA, Grønbech CH, et al. Improved metagenome binning and assembly using deep variational autoencoders. Nat Biotechnol. 2021;39:555–60. Article CAS
PubMed Google Scholar * Kang DD, Froula J, Egan R, Wang Z. MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. PeerJ. 2015;3:e1165.
Article PubMed PubMed Central Google Scholar * Sieber CMK, Probst AJ, Sharrar A, Thomas BC, Hess M, Tringe SG, et al. Recovery of genomes from metagenomes via a dereplication,
aggregation and scoring strategy. Nat Microbiol. 2018;3:836–43. Article CAS PubMed PubMed Central Google Scholar * Olm MR, Brown CT, Brooks B, Banfield JF. dRep: a tool for fast and
accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J. 2017;11:2864–8. Article CAS PubMed PubMed Central Google Scholar *
Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res.
2015;25:1043–55. Article CAS PubMed PubMed Central Google Scholar * Lee I, Chalita M, Ha S-M, Na S-I, Yoon S-H, Chun J. ContEst16S: an algorithm that identifies contaminated prokaryotic
genomes using 16S RNA gene sequences. Int J Syst Evol Microbiol. 2017;67:2053–7. Article CAS PubMed Google Scholar * Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K,
et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. Article PubMed PubMed Central Google Scholar * Chaumeil P-A, Mussig AJ, Hugenholtz P, Parks DH. GTDB-Tk: a
toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics. 2019;36:1925–7. PubMed PubMed Central Google Scholar * Price MN, Dehal PS, Arkin AP. FastTree 2 —
approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. Article PubMed PubMed Central Google Scholar * Boyd JA, Woodcroft BJ, Tyson GW. GraftM: a tool for
scalable, phylogenetically informed classification of genes within metagenomes. Nucleic Acids Res. 2018;46:e59. Article PubMed PubMed Central Google Scholar * Watt M, Schneebeli K, Dong
P, Wilson IW. The shoot and root growth of _Brachypodium_ and its potential as a model for wheat and other cereal crops. Functional Plant Biol. 2009;36:960. Article Google Scholar * Yu
W-Y, Peng M-H, Wang J-J, Ye W-Y, Li Y-L, Zhang T, et al. Microbial community associated with ectomycorrhizal Russula symbiosis and dominated nature areas in southern China. FEMS Microbiol
Lett. 2021;368:fnab028. Article CAS PubMed Google Scholar * Gao T, Li Y, Chai Y, Wang Q, Ding M. SigB regulates stress resistance, glucose starvation, MnSOD production, biofilm
formation, and root colonization in _Bacillus cereus_ 905. Appl Microbiol Biotechnol. 2021;105:5943–57. Article CAS PubMed Google Scholar * Li Q, Li Z, Li X, Xia L, Zhou X, Xu Z, et al.
FtsEX-CwlO regulates biofilm formation by a plant-beneficial rhizobacterium _Bacillus velezensis_ SQR9. Res Microbiol. 2018;169:166–76. Article CAS PubMed Google Scholar * Nienaber A,
Hennecke H, Fischer HM. Discovery of a haem uptake system in the soil bacterium _Bradyrhizobium japonicum_. Mol Microbiol. 2001;41:787–800. Article CAS PubMed Google Scholar * Chuckran
PF, Hungate BA, Schwartz E, Dijkstra P. Variation in genomic traits of microbial communities among ecosystems. _FEMS Microbes_ 2021;2:xtab020. * Feng S, Qiu Y, Huang Z, Yin Y, Zhang H, Zhu
D, et al. The adaptation mechanisms of _Acidithiobacillus caldus_ CCTCC M 2018054 to extreme acid stress: bioleaching performance, physiology, and transcriptomics. Environ Res.
2021;199:111341. Article CAS PubMed Google Scholar * Dsouza M, Taylor MW, Turner SJ, Aislabie J. Genomic and phenotypic insights into the ecology of _Arthrobacter_ from Antarctic soils.
BMC Genomics. 2015;16:36. Article PubMed PubMed Central Google Scholar * Shi S, Nuccio EE, Shi ZJ, He Z, Zhou J, Firestone MK. The interconnected rhizosphere: high network complexity
dominates rhizosphere assemblages. Ecol Lett. 2016;19:926–36. Article PubMed Google Scholar * Rüger L, Feng K, Dumack K, Freudenthal J, Chen Y, Sun R, et al. Assembly patterns of the
rhizosphere microbiome along the longitudinal root axis of maize (_Zea mays_ L.). Front Microbiol. 2021;12:614501. Article PubMed PubMed Central Google Scholar * Kawasaki A, Donn S, Ryan
PR, Mathesius U, Devilla R, Jones A, et al. Microbiome and exudates of the root and rhizosphere of _Brachypodium distachyon_, a model for wheat. PLoS One. 2016;11:e0164533. Article PubMed
PubMed Central Google Scholar * Wei S, Jacquiod S, Philippot L, Blouin M, Sørensen SJ. Spatial analysis of the root system coupled to microbial community inoculation shed light on
rhizosphere bacterial community assembly. Biol Fertil Soils. 2021;57:973–89. Article CAS Google Scholar * Donn S, Kawasaki A, Delroy B, Chochois V, Watt M, Powell JR. Root type is not an
important driver of mycorrhizal colonisation in _Brachypodium distachyon_. Pedobiologia. 2017;65:5–15. Article Google Scholar * Diamond S, Andeer PF, Li Z, Crits-Christoph A, Burstein D,
Anantharaman K, et al. Mediterranean grassland soil C-N compound turnover is dependent on rainfall and depth, and is mediated by genomically divergent microorganisms. Nat Microbiol.
2019;4:1356–67. Article CAS PubMed PubMed Central Google Scholar * Tkacz A, Cheema J, Chandra G, Grant A, Poole PS. Stability and succession of the rhizosphere microbiota depends upon
plant type and soil composition. ISME J. 2015;9:2349–59. Article CAS PubMed PubMed Central Google Scholar * Kopecky J, Kyselkova M, Omelka M, Cermak L, Novotna J, Grundmann GL, et al.
Actinobacterial community dominated by a distinct clade in acidic soil of a waterlogged deciduous forest. FEMS Microbiol Ecol. 2011;78:386–94. Article CAS PubMed Google Scholar * Donn S,
Kirkegaard JA, Perera G, Richardson AE, Watt M. Evolution of bacterial communities in the wheat crop rhizosphere. Environ Microbiol. 2015;17:610–21. Article PubMed Google Scholar * Ripa
FA, Tong S, Cao W-D, Wang ET, Wang T, Liu HC, et al. _Paenibacillus rhizophilus_ _sp. nov._, a nitrogen-fixing bacterium isolated from the rhizosphere of wheat (_Triticum aestivum L._). Int
J Syst Evol Microbiol. 2019;69:3689–95. Article CAS PubMed Google Scholar * Kämpfer P, Busse H-J, McInroy JA, Clermont D, Criscuolo A, Glaeser SP. _Paenibacillus allorhizosphaerae sp.
nov._, from soil of the rhizosphere of _Zea mays_. Int J Syst Evol Microbiol. 2021;71:005051. Article Google Scholar * Li Y, Li Q, Chen S. Diazotroph _Paenibacillus triticisol_i BJ-18
drives the variation in bacterial, diazotrophic and fungal communities in the rhizosphere and root/shoot endosphere of maize. Int J Mol Sci. 2021;22:1460. Article CAS PubMed PubMed
Central Google Scholar * Wang ZY, Wang RX, Zhou JS, Cheng JF, Li YH. An assessment of the genomics, comparative genomics and cellulose degradation potential of _Mucilaginibacter
polytrichastri_strain RG4-7. Bioresour Technol. 2020;297:122389. Article CAS PubMed Google Scholar * Gagné-Bourque F, Mayer BF, Charron J-B, Vali H, Bertrand A, Jabaji S. Accelerated
growth rate and increased drought stress resilience of the model grass _Brachypodium distachyon_ colonized by _Bacillus subtilis_ B26. PLoS One. 2015;10:e0130456. Article PubMed PubMed
Central Google Scholar * Pascault N, Ranjard L, Kaisermann A, Bachar D, Christen R, Terrat S, et al. Stimulation of different functional groups of bacteria by various plant residues as a
driver of soil priming effect. Ecosystems. 2013;16:810–22. Article CAS Google Scholar * Barber NA, Chantos-Davidson KM, Amel Peralta R, Sherwood JP, Swingley WD. Soil microbial community
composition in tallgrass prairie restorations converge with remnants across a 27-year chronosequence. Environ Microbiol. 2017;19:3118–31. Article CAS PubMed Google Scholar * Vieira S,
Sikorski J, Dietz S, Herz K, Schrumpf M, Bruelheide H, et al. Drivers of the composition of active rhizosphere bacterial communities in temperate grasslands. ISME J. 2020;14:463–75. Article
CAS PubMed Google Scholar * Xu J, Zhang Y, Zhang P, Trivedi P, Riera N, Wang Y, et al. The structure and function of the global citrus rhizosphere microbiome. Nat Commun. 2018;9:4894.
Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This research work was funded through the Microbial Community Analysis and Functional Evaluation in
Soils (m-CAFEs) Science Focus Area Program at Lawrence Berkeley National Laboratory funded by the U.S. Department of Energy, Office of Science, Office of Biological & Environmental
Research Awards DE-AC02-05CH11231. AUTHOR INFORMATION Author notes * These authors contributed equally: Shwetha M. Acharya, Mon Oo Yee. AUTHORS AND AFFILIATIONS * Department of Ecology,
Earth & Environmental Sciences Area, Lawrence Berkeley National Laboratory, Berkeley, CA, 94720, USA Shwetha M. Acharya, Mon Oo Yee, Nameera F. Baig, Omolara T. Aladesanmi & Romy
Chakraborty * Department of Earth and Planetary Science, University of California, Berkeley, CA, 94720, USA Spencer Diamond & Jillian F. Banfield * Environmental Genomics and Systems
Biology Division, Lawrence Berkeley National Laboratory, Berkeley, CA, 94720, USA Peter F. Andeer & Trent R. Northen Authors * Shwetha M. Acharya View author publications You can also
search for this author inPubMed Google Scholar * Mon Oo Yee View author publications You can also search for this author inPubMed Google Scholar * Spencer Diamond View author publications
You can also search for this author inPubMed Google Scholar * Peter F. Andeer View author publications You can also search for this author inPubMed Google Scholar * Nameera F. Baig View
author publications You can also search for this author inPubMed Google Scholar * Omolara T. Aladesanmi View author publications You can also search for this author inPubMed Google Scholar *
Trent R. Northen View author publications You can also search for this author inPubMed Google Scholar * Jillian F. Banfield View author publications You can also search for this author
inPubMed Google Scholar * Romy Chakraborty View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Romy Chakraborty.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL MATERIAL SUPPLEMENTAL TABLE S1 SUPPLEMENTAL TABLE S2 SUPPLEMENTAL TABLE S3 SUPPLEMENTAL TABLE
S5 SUPPLEMENTAL TABLE S6 SUPPLEMENTAL TABLE S7 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use,
sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative
Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Acharya, S.M., Yee, M.O., Diamond, S. _et al._ Fine scale sampling reveals early differentiation of rhizosphere microbiome from bulk soil in
young _Brachypodium_ plant roots. _ISME COMMUN._ 3, 54 (2023). https://doi.org/10.1038/s43705-023-00265-1 Download citation * Received: 21 December 2022 * Revised: 18 May 2023 * Accepted: 24
May 2023 * Published: 06 June 2023 * DOI: https://doi.org/10.1038/s43705-023-00265-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
