Exploration of first onsets of mania, schizophrenia spectrum disorders and major depressive disorder in perimenopause
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Although the relationship between perimenopause and changes in mood has been well established, knowledge of risk of a broad spectrum of psychiatric disorders associated with
reproductive aging is limited. Here we investigate whether the perimenopause (that is, the years around the final menstrual period (FMP)) is associated with increased risk of developing
psychiatric disorders compared with the late reproductive stage. Information on menopausal timing and psychiatric history was obtained from nurse-administered interviews and online
questionnaires from 128,294 female participants within UK Biobank. Incidence rates of psychiatric disorders during the perimenopause (4 years surrounding the FMP) were compared with the
reference premenopausal period (6–10 years before the FMP). The rates were calculated for major depressive disorder (MDD), mania, schizophrenia spectrum disorders and other diagnoses.
Overall, of 128,294 participants, 753 (0.59%) reported their first onset of a psychiatric disorder during the late reproductive stage (incidence rate 1.53 per 1,000 person-years) and 1,133
(0.88%) during the perimenopause (incidence rate 2.33 per 1,000 person-years). Compared with the reference reproductive period, incidence rates of psychiatric disorders significantly
increased during the perimenopause (incidence rate ratio (RR) of 1.52, 95% confidence interval (CI) 1.39–1.67) and decreased back down to that observed in the premenopausal period in the
postmenopause (RR of 1.09 (95% CI 0.98–1.21)). The effect was primarily driven by increased incidence rates of MDD, with an incidence RR of 1.30 (95% CI 1.16–1.45). However, the largest
effect size at perimenopause was observed for mania (RR of 2.12 (95% CI 1.30–3.52)). No association was found between perimenopause and incidence rates of schizophrenia spectrum disorders
(RR of 0.95 (95% CI 0.48–1.88)). In conclusion, perimenopause was associated with an increased risk of developing MDD and mania. No association was found between perimenopause and first
onsets of schizophrenia spectrum disorders. SIMILAR CONTENT BEING VIEWED BY OTHERS PERIMENOPAUSE SYMPTOMS, SEVERITY, AND HEALTHCARE SEEKING IN WOMEN IN THE US Article Open access 25 February
2025 EPIDEMIOLOGIC AND GENETIC ASSOCIATIONS OF FEMALE REPRODUCTIVE DISORDERS WITH DEPRESSION OR DYSTHYMIA: A MENDELIAN RANDOMIZATION STUDY Article Open access 12 March 2024 KEY
SUBPHENOTYPES OF BIPOLAR DISORDER ARE DIFFERENTIALLY ASSOCIATED WITH POLYGENIC LIABILITIES FOR BIPOLAR DISORDER, SCHIZOPHRENIA, AND MAJOR DEPRESSIVE DISORDER Article Open access 14 February
2024 MAIN There are estimated to be greater than 945 million women and those assigned female at birth aged between 40 and 60 years in the world1. During perimenopause (the years around the
final menstrual period (FMP)), approximately 80% of people develop neuropsychiatric symptoms, most commonly hot flushes, cognitive dysfunction, sleep disturbances and mood-related symptoms2.
It has been suggested that perimenopause is also a high-risk period for the onset or exacerbation of psychiatric disorders, including major depressive disorder (MDD), schizophrenia spectrum
disorders and bipolar disorder; although, research thus far has predominately measured only depressive symptoms3,4,5,6,7. A two- to four-times greater risk of a depressive episode during
perimenopause compared with the reproductive stage has been observed by the limited studies that have investigated MDD, even after controlling for other predictors and perimenopausal
symptoms8,9,10. However, failure to account for multiple testing, retrospective design and focus on mild symptomatology have limited the generalizability of the results. Very little research
has been conducted on the association between perimenopause and onset of disorders such as schizophrenia and bipolar disorder. Observations thus far have been limited to risk of recurrence
in women with preexisting disorders and have shown that midlife in women is associated with worsening bipolar symptoms11, as well as an increased risk of hospitalizations for psychosis
compared with men of the same age12. A major limitation of researching the association between reproductive aging and psychiatric disorders is related to the difficulties in obtaining
reliable evaluations of ovarian aging, especially in epidemiological studies, with most studies using age as a proxy for age at FMP13. However, there is an over 20-year range variation in
age at menopause, with research advocating that chronological age is not a valid proxy for menopausal status14,15,16. The UK Biobank represents a unique opportunity to study the effects of
reproductive aging, with information on menopausal timing and deep longitudinal data collected on over 200,000 female participants recruited at 40–69 years of age17. Here, we focus on ‘late’
first onsets, because studies on the effect of reproductive aging on first-onset severe mental illness (that is, schizophrenia spectrum disorders and bipolar disorder) are lacking. This is
likely due to the low prevalence and, therefore, difficulty to detect any effect in epidemiological studies specifically designed to study menopause. In this article, we aim to exploit a
unique and specific asset of the UK Biobank: the combination of questions on first-onset psychiatric illness and age at FMP in an epidemiological study large enough to detect less prevalent,
devastating outcomes, such as first incidence of bipolar disorder/schizophrenia, at a population level. The aim of this study is to test the hypothesis that perimenopause is a time of
increased rate of first-onset psychiatric disorders, including MDD, mania and schizophrenia spectrum disorders, compared with the late reproductive stage. RESULTS A total of 128,294
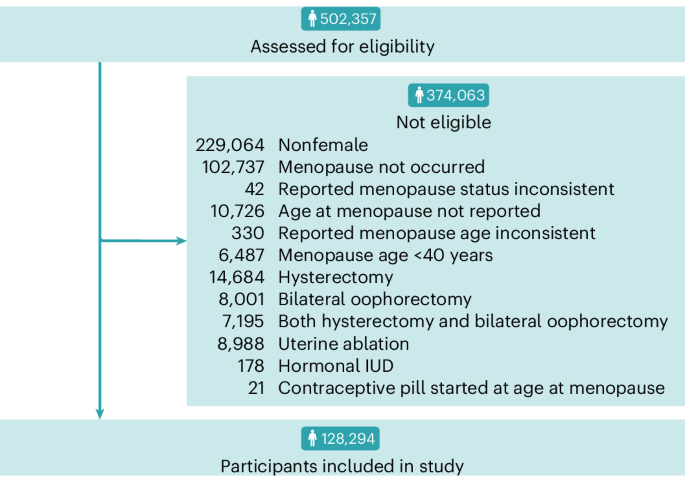
participants from the UK Biobank met inclusion criteria, as shown in Fig. 1. The mean age at recruitment into this study was 59.6 years (standard deviation (s.d.) of 5.69 years) with an
average follow-up period of 2.98 years (s.d. of 3.92 years) and mean age at menopause of 50.6 years of age (range of 40–68 years; s.d. of 4.00 years), as displayed in Supplementary Fig. 1.
Demographic characteristics of female participants, including self-reported ethnicity, are presented in Table 1. As presented in Table 2, a total of 753 participants (0.59%) had a first
onset of a psychiatric disorder 6–10 years before the FMP (premenopause period), 1,133 participants (0.88%) between 2 years prior and 2 years following FMP (perimenopause) and 637
participants (0.50%) 6–10 years following the FMP (postmenopause), with incidence rates per 1,000 person-years of 1.53, 2.33 and 1.66, respectively. Trends in the number of new onsets of
each psychiatric disorder for both female and male participants are displayed in Fig. 2. The perimenopause was associated with a significant increase in the incidence rates of psychiatric
disorders compared with the premenopause period (incidence rate ratio (RR) of 1.52 (95% confidence interval (CI) 1.39–1.67); Table 2). During the postmenopause, the incidence rate decreased
back down to that observed in the premenopause period (RR of 1.09 (95% CI 0.98–1.21)). DISORDER-SPECIFIC ANALYSES MDD was found to have a higher incidence rate during the perimenopause
compared with the premenopause stage, with a RR of 1.30 (95% CI 1.16–1.45) (Fig. 3 and Table 2). The incidence rate of MDD became significantly lower in the postmenopause compared with the
premenopause (RR of 0.68 (95% CI 0.59–0.78)). The incidence rate of mania significantly increased in the perimenopause compared with the premenopause (RR of 2.12 (95% CI 1.30–3.52)), to then
return to the premenopause level during the postmenopause (RR of 0.98 (95% CI 0.51–1.86)). Perimenopause was not found to be significantly associated with a change in incidence rate of
schizophrenia spectrum disorders compared with the premenopause stage (RR of 0.95 (95% CI 0.48–1.88)), though rates were found to be lower during the postmenopause (RR of 0.37 (95% CI
0.12–0.96)). Both perimenopause and postmenopause were found to be significantly associated with an increased incidence rate of other diagnoses compared with the premenopause, with incidence
RRs of 2.10 (95% CI 1.78–2.49) and 2.08 (95% CI 1.74–2.49), respectively. SENSITIVITY ANALYSIS Sensitivity analyses did not detect any effect of potential confounders on results. Compared
with the premenopause, an association between perimenopause and an increased incidence rate of psychiatric disorders was observed in those with low and high Townsend Deprivation Indexes,
those of healthy, preobese and obese body mass index (BMI) categories, across all six alcohol intake frequency categories and in both previous and never smokers (Supplementary Table 1).
Perimenopause was not found to be significantly associated with an increase in incidence rate of psychiatric disorders compared with the premenopause in those with underweight BMI or current
smokers. ANALYSIS OF MALE PARTICIPANTS Demographic characteristics of male participants are available in Supplementary Table 2. No individual disorder had any significant peak of incidence
at the interval period that matched ‘perimenopause’ (Extended Data Fig. 1). First onsets of MDD had a similar pattern in male and female participants; although, this did not reach
statistical significance in the male sample during the ‘perimenopause’ proxy (RR of 1.13 (95% CI 1.00–1.29); Extended Data Fig. 2 and Supplementary Table 3). In male participants, no
increase in incidence rate was observed during the ‘perimenopause’ proxy compared with the ‘premenopause’ proxy for mania (RR of 0.88 (95% CI 0.55–1.41)) nor schizophrenia spectrum disorders
(RR of 0.57 (95% CI 0.26–1.22); Supplementary Table 3). Similarly to the female sample, both the perimenopause and postmenopause proxies in male participants were associated with an
increased rate of other diagnoses compared with the premenopause proxy, with incidence RRs of 1.44 (95% CI 1.20–1.74) and 1.36 (95% CI 1.11–1.67), respectively. DISCUSSION Our results show
that the perimenopause is a period of increased risk of first-onset psychiatric disorders, with compelling evidence for a specific link with mania. We found that participants without
previous history of mania were over twice as likely to develop mania for the first time in the perimenopause than in the late reproductive stage. The increased risk was specific for the
perimenopause, as the rates of first-onset mania returned to premenopause levels in the postmenopause. MAIN FINDINGS Our results highlight the importance of considering the FMP rather than
chronological age in both clinical practice and research concerning mental health and reproductive aging. Previous studies using chronological age had not been able to detect the effect of
perimenopause on mania/bipolar disorder18. Given the 20-year range variation in age at FMP14, inferring age at menopause on solely chronological age can lead to errors and, in research, to
false negatives. The disease-specific and narrow time window (4 years) of increased risk for mania also suggests that specific changes associated with the perimenopause may trigger mania in
people without previous psychiatric history of mania. The lack of any association in male participants corroborates the idea that sex-specific factors are associated with the first
occurrence of mania at the time of the perimenopause. For bipolar disorder and mania, evidence suggests a trimodal distribution of age of onset, reflecting possible biological
heterogeneity19. By selecting a period of 4–10 years prior the FMP as reference, our analyses focused on the late onset group, providing for the first time direct evidence of a possible link
between mania and the hormonal fluctuations of the perimenopause. Intriguingly, one of the most robust pieces of evidence in psychiatry is that of the association between mania and the
first few weeks after childbirth, with a 23 times increased risk of first-onset mania in the 30 days postpartum compared with 12 months after giving birth20. Although the effect we found for
perimenopause is much smaller than that observed for childbirth, our results support the theory that there is a link between mania/bipolar disorder and reproductive events beyond
childbirth. It has been suggested that menopausal mood disorders could be predicted by sensitivity to estradiol, with 27% of individuals sensitive to either withdrawal (7%) or absolute
change (20%) in estradiol levels21. As the postpartum period is associated with a sharp decline in estradiol levels, it may be that individuals with an estradiol-sensitive predisposition may
have already developed an onset of mania during postpartum, reducing the number of first onsets at perimenopause. Contrary to mania, we did not find any specific association between
schizophrenia spectrum disorders and the perimenopause. While the lack of effect may be due to the small sample size, we did find that the incidence rate of schizophrenia spectrum disorder
was significantly lower in the postmenopause compared with the premenopausal stage. Our findings, therefore, do not support the widely discussed hypothesis that hypoestrogenism may trigger
the first onset of schizophrenia but are in line with those of a large collaborative study on over 130,000 incident cases of schizophrenia, showing a decline in new onsets in women after the
age of 40 years22. Our results corroborate findings from previous studies that have observed an increased risk of depression in the perimenopause3,4,8,9,10. The effect was smaller than that
for mania, but the CIs were narrower, as there were more people who developed major depression than people who developed mania. Interestingly, while rates of first-onset MDD decreased in
the postmenopause, rates of first-onset depressive symptoms remained high in the postmenopause (Supplementary Table 4). The mechanisms underpinning the link between first-onset depressive
symptoms and reproductive aging may, therefore, be complex and include not only hormonal changes associated with the perimenopause, but also biopsychosocial challenges associated with aging.
Depression is likely an umbrella term for several conditions with heterogeneous disease pathways. It is then possible that onsets at different reproductive stages are linked to different
mechanisms. Given that our study ascertained age at first diagnosis by a health care professional, it is also possible that some participants who experienced depressive symptoms during the
perimenopause delayed seeking help or that their symptoms had become severe enough to seek help only in the postmenopause. Curiously, although the male analysis revealed a similar trend of
depression risk, upon closer inspection of the number of onsets around the FMP proxy (Fig. 2), they lack the sharp peak present in the female analysis. The present study observed a decline
in risk of a first onset of MDD during the postmenopausal period compared with premenopause in both the female and male proxy analysis. Although older populations often have high prevalence
of depression23, with the course of MDD becoming poorer with age24, our results are in line with previous studies which observe a decline in first-ever onsets of depression in the decade
following the average age at FMP (~50 years of age)25,26,27,28. As such, it may be that while the onset of depression occurs during perimenopause or earlier in life, some individuals may
remain depressed during the postmenopause, leading to a high prevalence. STRENGTHS AND LIMITATIONS Using data from the UK Biobank has allowed us to use a reported age at menopause to
determine reproductive timing and, thus, estimate time periods to capture the pre-, peri- and postmenopausal life stages. This is a major strength over many previous studies, which have
often used chronological age as a proxy for menopause despite the large variability in reproductive timing between individuals14. However, the UK Biobank does not include additional
information on the menstrual cycle, which would provide more fine grain information on the stages of reproductive aging and menopausal symptoms. Aware of this UK Biobank limitation and of
the variability in the length of the early menopause transition, we focused our analyses on the late menopausal transition and the early postmenopause, as: (1) they have less variability in
their length29 and (2) previous studies have suggested that the late menopause transition is the period of highest risk of new-onset depression9. We then chose the reference period of 6–10
years before the FMP, as only very few people will experience a late menopausal transition longer than 6 years29. Another unique strength of the UK Biobank is that the large sample size, the
collection on information on FMP and the cohort design allow inference on a range of psychiatric disorders, including less prevalent and more severe ones, such as mania and psychosis.
However, due to the particularly low prevalence of schizophrenia spectrum disorders, we were not able to run fine-grained analyses considering the different disorders separately. Further
replication of our results should be carried out before definitive conclusions can be drawn. Additionally, representativeness of UK Biobank has been an area of controversy, with diverging
views on the validity of its measures of association30,31. Our sensitivity analyses show the robustness of our findings across several socioeconomic, health and lifestyle characteristics.
The increased incidence of psychiatric conditions at perimenopause were present across different levels of the Townsend Deprivation Index, BMI categories, alcohol intake frequencies and in
previous and never smokers. Additionally, we note that our study design selects for those without a diagnosis of severe mental illness before 10 years before the FMP and, thus, will be
‘healthier’ by definition as is the design of the study, rather than due to an unintended selection bias. We emphasize that the incidence and prevalence estimates of our study are not the
focus of the paper and should not be taken as representative of the UK population as a whole. Moreover, our study focused on the effect of the perimenopause on the first incidence of
psychiatric disorders. By using as a reference group people 10 years before their FMP, by design, we selected a population that has reached the middle age without any psychiatric diagnosis.
This study design has the advantage of excluding the well-established peak of severe psychiatric disorders in early adulthood, which would mask the effect of the perimenopause. DIRECTIONS
FOR FUTURE WORK Our study focused on the first onset of psychiatric disorders during the perimenopause. The study of recurrence of preexisting psychiatric disorders was beyond the scope of
this paper and would have required a different study design. Further research focusing on large cohorts of people with previous history of mental illness is necessary to improve their risk
prediction and mitigation associated with reproductive aging. CONCLUSIONS Our results highlight the importance of diagnostic accuracy in the assessment of psychiatric phenomena associated
with aging. First, research and clinicians should consider interpersonal variability in reproductive aging rather than use demographic age as a proxy. Second, differential diagnosis is key,
and not all psychiatric symptoms with onset at the perimenopause should be considered depressive symptoms etiologically related to the perimenopause. Mechanistic research needs to
differentiate between clinical manifestation that are mostly epiphenomena from those that may be triggered by the physiological changes associated with reproductive aging. Clinically, the
link with mania was particularly striking and significant: although first-onset bipolar disorder is usually associated with younger age, the perimenopause represents a period of increased
risk of manic onset in those without previous psychiatric history of mania. Primary care physicians should be aware of this late onset presentation of bipolar disorder to avoid the risks
associated with a delayed diagnosis and misdiagnosis with depression. Antidepressants without mood stabilizers can in fact precipitate manic episodes in those with bipolar diathesis32.
METHODS STUDY DESIGN AND PARTICIPANTS The initial sample consisted of 502,357 individuals from the UK Biobank17. The UK Biobank is a large-scale biomedical database designed to enable
research into genetic and environmental determinants of disease. Participants were recruited at 40–69 years of age between 2006 and 2010 and undertook extensive data collection. Measurements
included assessment center visits, genotyping and longitudinal follow-up of health outcomes including linkage to medical records. The North West Multi-Centre Ethics Committee granted
ethical approval to the UK Biobank, and all participants provided written informed consent. This study was conducted under project number 13310. Psychiatric diagnoses were assessed using
interviews and a self-report web-based questionnaire (the UK Biobank mental health questionnaire). Interviews were conducted by trained nurses and included questions on past and current
medical conditions and date of diagnosis. The mental health questionnaire was based on the World Health Organization’s Composite International Diagnostic Interview Short Form33 and
integrated elements of other validated tools, such as the Patient Health Questionnaire (9-question version), the Generalized Anxiety Disorder Questionnaire 7-item34 and bespoke additional
questions. Although some participants also have linked medical primary health care records and hospital admission records, these data were not used in the present study as only more recent
records are available electronically; thus, admission dates were not trusted as valid proxies for first onsets of psychiatric disorders. Sample demographic variables include ethnicity. This
was self-reported via touchscreen questionnaire at the initial assessment center visit by the question: ‘What is your ethnic background?’. SELECTION OF INDIVIDUALS FOR ANALYSIS Figure 1
illustrates sample selection. The UK Biobank dataset was restricted to participants of female sex, as reported in the NHS central registry at the time of recruitment; although, in some
instances this was updated by the participant (field identification (ID) 31). Of the 502,357 participants in the UK Biobank, 273,293 (54.4%) were of female sex. The sample was then limited
to the 170,556 participants (62.4%) that had reported menopause occurrence (field ID 2724), which is defined as the cessation of menstrual periods for 12 months29. At each assessment center
visit, participants were asked to self-report if menopause had occurred via touchscreen questionnaire (field ID 2724). Of the 22,971 individuals (13.5%) who had answered this question at
multiple assessment center visits, 42 individuals (0.18%) who answered ‘yes’ but then responded ‘no’ to the same question at a subsequent visit were excluded, leaving 170,514 participants in
the sample. Age at menopause, defined as age at the FMP, was asked via touchscreen questionnaire (field ID 3581) if the participant had responded ‘yes’ when asked if menopause had occurred.
As this question was administered at each assessment center visit, 13,311 participants (7.81%) answered the question more than once. Of these, 442 participants (3.32%) reported different
ages when asked again, and 330 (2.48%) whose reported ages at menopause varied by two or more years were excluded from the study due to potential unreliability. For the 112 individuals
(0.84%) with multiple reported ages at menopause that did not vary by two or more years, the response from the earliest available assessment center visit was taken to reduce the probability
of recall bias. A total of 10,726 participants (6.30%) had no age at menopause reported, having responded, ‘do not know’ or ‘prefer not to answer’ and were subsequently excluded from the
study. A further 6,487 (4.07%) reported an age at menopause (field ID 3581) before 40 years of age and were excluded, as primary ovarian insufficiency is associated with major depression35,
leaving 152,971 participants in the sample. A total of 15,490 participants (10.1%) who reported a hysterectomy (field ID 3591) or a bilateral oophorectomy (field ID 2834) were excluded from
the study as these procedures have also been linked with an increased risk of depression36,37,38. Those who had undergone uterine ablations (8,988) were excluded, as this can cause cessation
of menstrual periods (field ID 41272, OPCS4 codes: Q07, Q08, Q10, Q16). As contraceptive use can also cause cessation of menstrual periods, 199 participants (0.15%) who reported using a
hormonal intrauterine device (IUD) or who reported starting oral contraceptives at the same age they experienced menopause were excluded from analysis, resulting in a sample size of 128,294.
DIAGNOSTIC CRITERIA First onsets of MDD, mania, schizophrenia spectrum disorders and an ‘other diagnoses’ category were investigated in the present study. These disorders were chosen on the
basis of previous literature investigating the effect of childbirth20, as these events both cause changes in sex hormone levels, and thus, new onsets of disorders during these periods may
share hormone-driven etiological pathways2,21. The diagnosis of MDD was based on answers from the online mental health questionnaire and mapped on the Diagnostic and Statistical Manual of
Mental Disorders, Fifth Edition, (DSM-5) criteria. Such approach has been previously validated by Cai and colleagues39, who found this approach to have higher SNP-based heritability than any
other definition of depression available in the UK Biobank, including medical record-based criteria. Details of this diagnostic criteria are available in Supplementary Note: Diagnostic
Criteria. Briefly, a classification of MDD required at least two cardinal symptoms, as well as at least five total symptoms, and excluded individuals with a history of substance abuse and/or
manic/psychotic conditions. Age at first onset was defined as the age at first episode of depression (field ID 20433). Analyses of individuals self-reporting depressive symptoms are
available in Supplementary Note: Diagnostic Criteria (Supplementary Table 4). The diagnoses of mania/bipolar/manic–depression (henceforth referred to as ‘mania’), schizophrenia spectrum
disorders and an ‘other diagnoses’ category were based on the nurse-conducted interviews (field IDs 20002 and 20009). Individuals who reported having schizophrenia at interview were combined
with individuals who reported a diagnosis of schizophrenia or ‘any other type of psychosis or psychotic illness’ in those who completed the mental health questionnaire (field IDs 20544 and
20461) to form a ‘schizophrenia spectrum disorders’ group. The ‘other diagnoses’ group included participants who reported any of the following conditions at a nurse-conducted interview:
‘anxiety/panic attacks’, ‘substance abuse/dependency’, ‘post-traumatic stress disorder’, anorexia/bulimia/other eating disorder’, ‘stress’, ‘obsessive compulsive disorder’ or ‘insomnia’.
Finally, a combined ‘psychiatric disorder’ group was formed of all the above diagnoses combined. In the case of the combined ‘psychiatric disorder’ group, if multiple diagnoses were present
in a single participant, the earliest age at onset was prioritized. For each diagnosis group, individuals were removed from analysis if they met the diagnostic criteria but did not have
onset age data available, as detailed in Supplementary Note: Diagnostic Criteria. STATISTICAL ANALYSIS Age at first onset of any given psychiatric disorder relative to age at FMP was
calculated as self-reported age at menopause (field ID 3581) subtracted from the age at onset. These values were grouped to form the following three life stages: premenopause (6–10 years
before the FMP), perimenopause (2 years before and 2 years following FMP) and postmenopause (6–10 years after the FMP). The values between 2 and 6 years from the FMP were excluded to
increase distinction between the time periods and to minimize the likelihood of misclassification due to inaccuracies in menopausal timing. The premenopause represents the reference life
stage against which the other life stages were compared. The late reproductive stage (6–10 years before the FMP) was used as the refence premenopausal period to reduce the effect of recall
bias and to minimize inclusion of adolescent and postnatal onsets within the reference period. A Kaplan–Meier survival analysis was used for each diagnosis group to determine the likelihood
of disorder onset at each life stage. This was conducted using the ‘survival’ R package in RStudio, version 4.2.1 (refs. 40,41). Incidence rates in person-years for each time period were
calculated as the number of cases divided by four times the total number of cases and controls, as each time period was 4 years long. Incidence RRs were calculated to compare the rate of
first onsets in the perimenopause and postmenopause compared with the premenopausal stage, using the two-sided ‘rateratio.test’ R package42, with a confidence level of 0.95. The false
discovery rate correction was applied to correct for 50 tests (10 tests in Table 2, 30 tests in Supplementary Table 1, 8 tests in Supplementary Table 3 and 2 tests in Supplementary Table
4)43. We provide _P_ values both with and without correction for multiple testing. SENSITIVITY ANALYSES Previous literature has observed that the UK Biobank participants differ from UK
Census data on several socioeconomic, health and lifestyle characteristics30. Participants have been found to be more likely to live in less deprived geographical areas and less likely to be
obese, to currently smoke or to drink alcohol daily. To consider the effect of this sampling bias, we investigated the effects of Townsend Deprivation Index, BMI, smoking status and alcohol
intake frequency on the findings of the present study by stratifying the sample on these characteristics. Further details are available in Supplementary Note: Sensitivity Analysis. ANALYSES
OF MALE PARTICIPANTS It is possible that the observed effects may not be driven by the hormonal fluctuations that characterized the perimenopause. Rather, they could be driven by age or by
unknown ascertainment and assessment bias. The same analyses were, therefore, conducted in male participants enrolled in UK Biobank. First, we matched male participants to female
participants on the basis of their most recent age that they came into the assessment center (field ID 21003). Then, for each male participant, we constructed a timeline centered around the
age at the FMP of their matched female participant. So, for example, if we matched a female and male participant based on the age at the last assessment of 62 and the female participant had
an age at the FMP of 50 years, we then centered the timeline of the male participant around 50. The three time periods considered as a proxy in this case would be 40–44 years for
premenopause, 48–52 years for perimenopause and 56–60 years for postmenopause. This was carried out to account for the variation and distribution of menopausal timing in female participants
to consider the effect of attrition based on the age of most recent follow-up and to create equal sample sizes to homogenize the statistical power. This resulted in 128,294 male participants
being included in analysis. All following statistical analyses mirrored the analyses conducted in the female sample. PATIENT AND PUBLIC INVOLVEMENT People with lived experience were
involved in the design of the study, in the interpretation of the results and in the writing of the manuscript. C.D., a researcher with lived experience and coauthor on the publication,
provided expertise and voiced the issues experienced by people affected by severe mental illness. In her roles within the charities Bipolar UK and Action on Postpartum Psychosis, C.D. has
provided support to women with lived experience of illness during the perimenopause over several years and has worked as patient and public involvement lead. Moreover, the design of the
current study was informed by a Bipolar UK survey designed and conducted by C.D., which received over 1,000 responses, REPORTING SUMMARY Further information on research design is available
in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY All the data used in this study, both raw and derived, are available from the UK Biobank
(https://www.ukbiobank.ac.uk/). This study was conducted under project number 13310. Our access to the data does not allow for data redistribution. CODE AVAILABILITY Code used for analysis
is available online at https://lms-j.github.io/perimeno-first-onsets/. REFERENCES * World Population Prospects 2022. _United Nations, Department of Economic and Social Affairs, Population
Division_ https://population.un.org/wpp/Download/Standard/Population/ (2022). * Brinton, R. D., Yao, J., Yin, F., Mack, W. J. & Cadenas, E. Perimenopause as a neurological transition
state. _Nat. Rev. Endocrinol._ 11, 393–405 (2015). Article PubMed PubMed Central Google Scholar * Tangen, T. & Mykletun, A. Depression and anxiety through the climacteric period: an
epidemiological study (HUNT-II). _J. Psychosom. Obstet. Gynecol._ 29, 125–131 (2008). Article Google Scholar * Lin, H.-L., Hsiao, M.-C., Liu, Y.-T. & Chang, C.-M. Perimenopause and
incidence of depression in midlife women: a population-based study in Taiwan. _Climacteric_ 16, 381–386 (2013). Article PubMed Google Scholar * Timur, S. & Sahin, N. H. The prevalence
of depression symptoms and influencing factors among perimenopausal and postmenopausal women. _Menopause_ 17, 545–551 (2010). Article PubMed Google Scholar * Bromberger, J. T. et al.
Depressive symptoms during the menopausal transition: the Study of Women’s Health Across the Nation (SWAN). _J. Affect. Disord._ 103, 267–272 (2007). Article PubMed PubMed Central Google
Scholar * Maartens, L. W. F., Knottnerus, J. A. & Pop, V. J. Menopausal transition and increased depressive symptomatology: a community based prospective study. _Maturitas_ 42, 195–200
(2002). Article PubMed Google Scholar * Freeman, E. W., Sammel, M. D., Boorman, D. W. & Zhang, R. Longitudinal pattern of depressive symptoms around natural menopause. _JAMA
Psychiatry_ 71, 36–43 (2014). Article PubMed PubMed Central Google Scholar * Schmidt, P. J., Haq, N. & Rubinow, D. R. A longitudinal evaluation of the relationship between
reproductive status and mood in perimenopausal women. _Am. J. Psychiatry_ 161, 2238–2244 (2004). Article PubMed Google Scholar * Bromberger, J. T. & Kravitz, H. M. Mood and menopause:
findings from the Study of Women’s Health Across the Nation (SWAN) over 10 years. _Obstet. Gynecol. Clin._ 38, 609–625 (2011). Article Google Scholar * Perich, T., Ussher, J. & Meade,
T. Menopause and illness course in bipolar disorder: a systematic review. _Bipolar Disord._ 19, 434–443 (2017). Article PubMed Google Scholar * Sommer, I. E. et al. Women with
schizophrenia-spectrum disorders after menopause: a vulnerable group for relapse. _Schizophr. Bull._ 49, 136–143 (2023). Article PubMed Google Scholar * Culbert, K. M., Thakkar, K. N.
& Klump, K. L. Risk for midlife psychosis in women: critical gaps and opportunities in exploring perimenopause and ovarian hormones as mechanisms of risk. _Psychol. Med._ 52, 1612–1620
(2022). Article PubMed PubMed Central Google Scholar * Brinton, R. D. in _Brocklehurst’s Textbook of Geriatric Medicine and Gerontology_ 8th edn, Ch. 13 (eds Fillit H., Rockwood K. and
Young J.) 76–81 (Elsevier, 2017). * Gold, E. B. The timing of the age at which natural menopause occurs. _Obstet. Gynecol. Clin. North Am._ 38, 425–440 (2011). Article PubMed PubMed
Central Google Scholar * Chan, S., Gomes, A. & Singh, R. S. Is menopause still evolving? Evidence from a longitudinal study of multiethnic populations and its relevance to women’s
health. _BMC Womens Health_ 20, 74 (2020). Article PubMed PubMed Central Google Scholar * Sudlow, C. et al. UK Biobank: an open access resource for identifying the causes of a wide range
of complex diseases of middle and old age. _PLoS Med._ 12, e1001779 (2015). Article PubMed PubMed Central Google Scholar * Kennedy, N. et al. Gender differences in incidence and age at
onset of mania and bipolar disorder over a 35-year period in Camberwell, England. _Am. J. Psychiatry_ 162, 257–262 (2005). Article PubMed Google Scholar * Bolton, S., Warner, J., Harriss,
E., Geddes, J. & Saunders, K. E. A. Bipolar disorder: trimodal age-at-onset distribution. _Bipolar Disord._ 23, 341–356 (2021). Article PubMed Google Scholar * Munk-Olsen, T.,
Laursen, T. M., Pedersen, C. B., Mors, O. & Mortensen, P. B. New parents and mental disorders: a population-based register study. _JAMA_ 296, 2582–2589 (2006). Article PubMed Google
Scholar * Gordon, J. L., Sander, B., Eisenlohr-Moul, T. A. & Tottenham, L. S. Mood sensitivity to estradiol predicts depressive symptoms in the menopause transition. _Psychol. Med._ 51,
1733–1741 (2021). Article PubMed Google Scholar * van der Werf, M. et al. Systematic review and collaborative recalculation of 133,693 incident cases of schizophrenia. _Psychol. Med._
44, 9–16 (2014). Article PubMed Google Scholar * Zenebe, Y., Akele, B., W/Selassie, M. & Necho, M. Prevalence and determinants of depression among old age: a systematic review and
meta-analysis. _Ann. Gen. Psychiatry_ 20, 55 (2021). Article PubMed PubMed Central Google Scholar * Schaakxs, R. et al. Associations between age and the course of major depressive
disorder: a 2-year longitudinal cohort study. _Lancet Psychiatry_ 5, 581–590 (2018). Article PubMed Google Scholar * Zisook, S. et al. Effect of age at onset on the course of major
depressive disorder. _Am. J. Psychiatry_ 164, 1539–1546 (2007). Article PubMed Google Scholar * Zhu, T. et al. Admixture analysis of age at onset in major depressive disorder. _Gen. Hosp.
Psychiatry_ 34, 686–691 (2012). Article PubMed Google Scholar * Pedersen, C. B. et al. A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental
disorders. _JAMA Psychiatry_ 71, 573–581 (2014). Article PubMed Google Scholar * Plana-Ripoll, O. et al. Temporal changes in sex- and age-specific incidence profiles of mental disorders—a
nationwide study from 1970 to 2016. _Acta Psychiatr. Scand._ 145, 604–614 (2022). Article PubMed PubMed Central Google Scholar * Harlow, S. D. et al. Executive summary of the stages of
reproductive aging workshop +10: addressing the unfinished agenda of staging reproductive aging. _J. Clin. Endocrinol. Metab._ 97, 1159–1168 (2012). Article PubMed PubMed Central Google
Scholar * Fry, A. et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. _Am. J. Epidemiol._ 186,
1026–1034 (2017). Article PubMed PubMed Central Google Scholar * Keyes, K. M. & Westreich, D. UK Biobank, big data, and the consequences of non-representativeness. _Lancet_ 393, 1297
(2019). Article PubMed PubMed Central Google Scholar * Viktorin, A. et al. The risk of switch to mania in patients with bipolar disorder during treatment with an antidepressant alone
and in combination with a mood stabilizer. _Am. J. Psychiatry_ 171, 1067–1073 (2014). Article PubMed Google Scholar * Kessler, R. C., Andrews, G., Mroczek, D., Ustun, B. & Wittchen,
H.-U. The World Health Organization Composite International Diagnostic Interview short-form (CIDI-SF). _Int. J. Methods Psychiatr. Res._ 7, 171–185 (1998). Article Google Scholar *
Kroenke, K., Spitzer, R. L., Williams, J. B. W. & Löwe, B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. _Gen. Hosp. Psychiatry_
32, 345–359 (2010). Article PubMed Google Scholar * Schmidt, P. J. et al. Depression in women with spontaneous 46, XX primary ovarian insufficiency. _J. Clin. Endocrinol. Metab._ 96,
E278–E287 (2011). Article PubMed Google Scholar * Choi, H. G., Rhim, C. C., Yoon, J. Y. & Lee, S. W. Association between hysterectomy and depression: a longitudinal follow-up study
using a national sample cohort. _Menopause_ 27, 543–549 (2020). Article PubMed Google Scholar * Kim, H. et al. Increased risk of depression before and after unilateral or bilateral
oophorectomy: a self-controlled case series study using a nationwide cohort in South Korea. _J. Affect. Disord._ 285, 47–54 (2021). Article PubMed Google Scholar * Rocca, W. A. et al.
Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. _Menopause_ 25, 1275–1285 (2018). Article PubMed Google Scholar * Cai, N. et al. Minimal phenotyping
yields genome-wide association signals of low specificity for major depression. _Nat. Genet._ 52, 437–447 (2020). Article PubMed PubMed Central Google Scholar * Therneau, T. A Package
for Survival Analysis in R. https://CRAN.R-project.org/package=survival (2022). * R Core Team. R: a language and environment for statistical computing. _R Foundation for Statistical
Computing_ https://www.R-project.org/ (2022). * Fay, M. rateratio.test: exact rate ratio test. https://CRAN.R-project.org/package=rateratio.test (2022). * Benjamini, Y. & Yekutieli, D.
The Control of the False Discovery Rate in Multiple Testing Under Dependency. _Ann. Stat._ https://doi.org/10.1214/aos/1013699998 (2001). Download references ACKNOWLEDGEMENTS We are thankful
for funding from the Medical Research Council (grant MR/W004658/1), awarded to Cardiff University and the National Centre for Mental Health, for supporting this research. A.D.F. is also
funded by the European Research Council (grant 947763). S.E.L. is supported by the National Institute of Mental Health (grant R01MH124873). V.E.-P. is supported by the Medical Research
Council (grants UKDRI-3003 and MR/Y004094/1). We are also grateful to the participants and staff of the UK Biobank for their time in producing the data used in this study. We thank M.
Hamshere for her comments and suggestions, which helped to refine this manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Centre for Neuropsychiatric Genetics and Genomics, Division
of Psychological Medicine and Clinical Neurosciences, School of Medicine, Cardiff University, Cardiff, UK Lisa M. Shitomi-Jones, Ian Jones, George Kirov, Valentina Escott-Price, Sophie E.
Legge & Arianna Di Florio * Bipolar UK, London, UK Clare Dolman * National Centre for Mental Health, School of Medicine, Cardiff University, Cardiff, UK Ian Jones Authors * Lisa M.
Shitomi-Jones View author publications You can also search for this author inPubMed Google Scholar * Clare Dolman View author publications You can also search for this author inPubMed Google
Scholar * Ian Jones View author publications You can also search for this author inPubMed Google Scholar * George Kirov View author publications You can also search for this author inPubMed
Google Scholar * Valentina Escott-Price View author publications You can also search for this author inPubMed Google Scholar * Sophie E. Legge View author publications You can also search
for this author inPubMed Google Scholar * Arianna Di Florio View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.D.F., C.D., G.K. and I.J.
conceptualized the study and acquired funding. Data curation and data analysis were carried out by L.M.S.-J. and supervised by S.E.L., V.E.-P. and A.D.F. L.M.S.-J. and S.E.L. had access to
and verified the data. L.M.S.-J. and A.D.F. prepared the manuscript. Review and editing of the manuscript were conducted by L.M.S.-J., C.D., G.K., I.J., V.E.-P., S.E.L. and A.D.F. The
corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. The study sponsor had no role in study design, data
collection, data analysis, interpretation of data, manuscript preparation or submission for publication. CORRESPONDING AUTHOR Correspondence to Arianna Di Florio. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Mental Health_ thanks Veerle Bergink and the other, anonymous, reviewers for their
contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 INCIDENCE RATE RATIOS OF PSYCHIATRIC DISORDERS FOR MALE PARTICIPANTS DURING MATCHED ‘PERIMENOPAUSE’ AND ‘POSTMENOPAUSE’
PROXIES, RELATIVE TO THE MATCHED ‘PREMENOPAUSE’ PERIOD PROXY. Note that age-based classifications were based on values from matched female participants. Dots indicate the incidence rate
ratio calculated relative to the premenopausal period proxy (6-10 years before the assigned FMP). Whiskers indicate 95% confidence intervals. Total sample sizes analysed for each disorder
are as follows: major depressive disorder (n = 37 719); mania (n = 128 131), schizophrenia spectrum disorders (n = 128 108); other diagnoses (n = 127 449). Perimenopause = Between −2 years
before and 2 years after FMP; Postmenopause = 6-10 years after FMP. EXTENDED DATA FIG. 2 INCIDENCE RATE RATIOS OF PSYCHIATRIC DISORDERS IN FEMALE PARTICIPANTS DURING THE PERIMENOPAUSE (2
YEARS PRIOR - 2 YEARS FOLLOWING FMP) COMPARED TO MALE PARTICIPANTS DURING THE MATCHED ‘PERIMENOPAUSE’ PROXY (2 YEARS PRIOR - 2 YEARS FOLLOWING ASSIGNED FMP). Note that age at FMP in male
participants was based on values from matched female participants. Dots indicate the incidence rate ratio calculated relative to the premenopausal period (6-10 years before FMP). Whiskers
indicate 95% confidence intervals. Sample sizes analysed for each disorder are as follows: major depressive disorder (nfemale = 39 800, nmale = 37 719); mania (nfemale = 128 105, nmale = 128
131), schizophrenia spectrum disorders (nfemale = 128 170, nmale = 128 108); other diagnoses (nfemale = 127 292, nmale = 127 449). Perimenopause = Between −2 years before and 2 years after
FMP; Postmenopause = 6-10 years after FMP. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary methods (diagnostic criteria and sensitivity analysis), Tables 1–4 and Figs. 1
and 2. REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Shitomi-Jones, L.M., Dolman, C., Jones, I. _et al._ Exploration of first onsets of mania, schizophrenia spectrum disorders and major depressive disorder in perimenopause.
_Nat. Mental Health_ 2, 1161–1168 (2024). https://doi.org/10.1038/s44220-024-00292-4 Download citation * Received: 13 November 2023 * Accepted: 24 June 2024 * Published: 15 August 2024 *
Issue Date: October 2024 * DOI: https://doi.org/10.1038/s44220-024-00292-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
