Metal oxyhalide-based heterogeneous catalytic water purification with ultralow H2O2 consumption
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

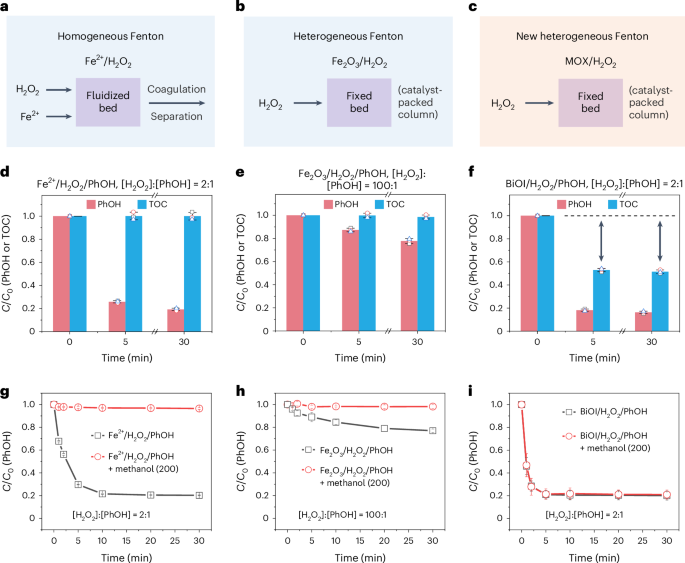
In the quest for advanced water treatment via Fenton and Fenton-like reactions, minimizing the hydrogen peroxide (H2O2) usage by improving its activation efficiency is a critical goal. Here
we report a metal oxyhalide (MOX)-based Fenton reaction system that differs fundamentally from traditional ones in pollutant removal pathway and mechanism. The MOX/H2O2 system enables
efficient coupling and polymerization of organic pollutants via mild surface direct oxidation, bypassing the generation of reactive oxygen species. As a result, pollutants are translocated
and removed from water with ultralow H2O2 consumption, avoiding the formation of toxic by-products. It achieves up to 80% pollutant (50% total organic carbon) removal at a H2O2-to-pollutants
molar ratio of 2:1, outperforming conventional Fenton systems, which are operated at ratios ranging from 20:1 to 1,000:1. The success of these catalytic systems is attributed to the
synergistic actions of O-bridging M and X sites on the catalyst surface, which selectively activate pollutants and H2O2, respectively. The catalyst could be extended to low-cost and
environmentally benign MOX materials such as BiOI, FeOCl and VOCl, and be adopted to construct a dynamic membrane filtration catalytic system for high-performance and energy-saving abatement
of micropollutants in water, providing a promising water purification paradigm.
All data are available in the article and Supplementary Information.
This work was supported by the National Natural Science Foundation of China (51821006, 52192684 and 52027815 to H.-Q.Y.; 51978637 to J.-J.C.; and 52300114 to Y.-J.Z.) and the Instruments
Center for Physical Science at the University of Science and Technology of China (USTC). The numerical calculations in this work were conducted on the supercomputing system in the
Supercomputing Center of the USTC.
These authors contributed equally: Ying-Jie Zhang, Jia-Shu Tao.
CAS Key Laboratory of Urban Pollutant Conversion, Department of Environmental Science and Engineering, University of Science and Technology of China, Hefei, China
Ying-Jie Zhang, Jia-Shu Tao, Yi Hu, Gui-Xiang Huang, Yuan Pan, Wen-Wei Li, Jie-Jie Chen & Han-Qing Yu
Y.-J.Z. came up with the original idea. H.-Q.Y. and J.-J.C. supervised the project. Y.-J.Z., Y.H., W.-W.L., H.-Q.Y. and J.-J.C. designed the experiments. Y.-J.Z., J.-S.T. and Y.H. performed
the experiments and the characterizations. G.-X.H. and Y.P. helped with the data interpretations. Y.-J.Z., W.-W.L., J.-J.C. and H.-Q.Y. wrote and revised the paper. All authors commented on
the paper.
Nature Water thanks Jeonghun Kim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Materials and Methods, Figs. 1–39 and Tables 1–8.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author
self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Anyone you share the following link with will be able to read this content:
