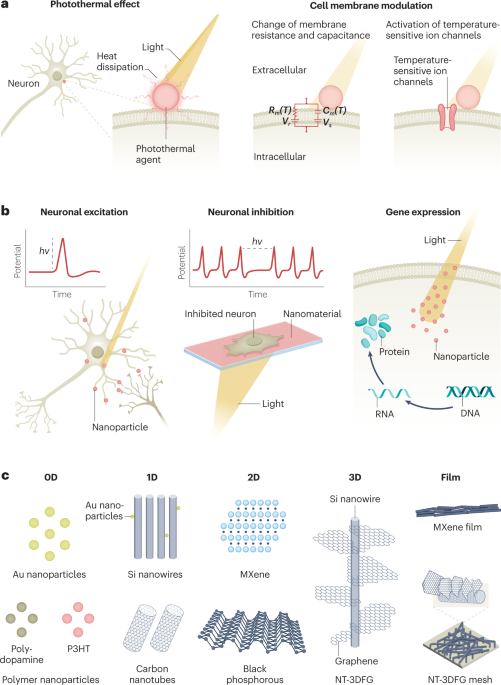
Neural modulation with photothermally active nanomaterials
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Modulating neural electrophysiology with high precision is essential for understanding neural communication and for the diagnosis and treatment of neural disorders. Photothermal
modulation offers a remote and non-genetic method for neural modulation with high spatiotemporal resolution and specificity. This technique induces highly localized and transient temperature
changes at the cell membrane interfaced with photothermally active nanomaterials. This rapid temperature change affects the electrical properties of the cell membrane or
temperature-sensitive ion channels. In this Review, we discuss the fundamental material properties and illumination conditions that are necessary for nanomaterial-assisted photothermal
neural excitation and inhibition. We examine how this versatile technique allows direct investigation of neural electrophysiology and signalling pathways in two-dimensional and
three-dimensional cell cultures and tissues, and highlight the scientific and technological challenges in terms of cellular specificity, light delivery and biointerface stability on the road
to clinical translation. KEY POINTS * Nanomaterial-assisted photothermal modulation is a remote, non-genetic technique for the manipulation of neural activity with high spatiotemporal
resolution and specificity by inducing a rapid temperature increase at the cell–nanomaterial interface. * The fundamental properties of photothermally active nanomaterials (size, dimension,
optical absorbance and photothermal energy conversion) and illumination conditions dictate application-specific material selection. * Altering light illumination conditions (pulse width,
power density and spot size) allows control of neural electrophysiology (excitation and inhibition) and cellular signalling pathways. * Evaluation of the cytotoxicity of nanomaterials,
phototoxicity of light illumination and local temperature increases is necessary for the safe translation of transient and long-term photothermal modulation. Access through your institution
Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 digital issues and
online access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to
local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT
BEING VIEWED BY OTHERS NON-GENETIC PHOTOACOUSTIC STIMULATION OF SINGLE NEURONS BY A TAPERED FIBER OPTOACOUSTIC EMITTER Article Open access 14 July 2021 INFRARED NEURAL STIMULATION AND
INHIBITION USING AN IMPLANTABLE SILICON PHOTONIC MICRODEVICE Article Open access 01 June 2020 TETHER-FREE PHOTOTHERMAL DEEP-BRAIN STIMULATION IN FREELY BEHAVING MICE VIA WIDE-FIELD
ILLUMINATION IN THE NEAR-INFRARED-II WINDOW Article 21 March 2022 REFERENCES * Liang, E., Shi, J. & Tian, B. Freestanding nanomaterials for subcellular neuronal interfaces. _iScience_
25, 103534 (2022). Article Google Scholar * Hong, J.-w, Yoon, C., Jo, K., Won, J. H. & Park, S. Recent advances in recording and modulation technologies for next-generation neural
interfaces. _iScience_ 24, 103550 (2021). Article Google Scholar * Kang, S.-K., Koo, J., Lee, Y. K. & Rogers, J. A. Advanced materials and devices for bioresorbable electronics. _Acc.
Chem. Res._ 51, 988–998 (2018). Article Google Scholar * Rogers, J. A., Someya, T. & Huang, Y. Materials and mechanics for stretchable electronics. _Science_ 327, 1603–1607 (2010).
Article Google Scholar * Song, E., Li, J., Won, S. M., Bai, W. & Rogers, J. A. Materials for flexible bioelectronic systems as chronic neural interfaces. _Nat. Mater._ 19, 590–603
(2020). Article Google Scholar * Hong, G. & Lieber, C. M. Novel electrode technologies for neural recordings. _Nat. Rev. Neurosci._ 20, 330–345 (2019). Article Google Scholar *
Zhang, A. & Lieber, C. M. Nano-bioelectronics. _Chem. Rev._ 116, 215–257 (2016). Article Google Scholar * Balakrishnan, G., Song, J., Mou, C. & Bettinger, C. J. Recent progress in
materials chemistry to advance flexible bioelectronics in medicine. _Adv. Mater._ 34, 2106787 (2022). Article Google Scholar * Garg, R., Roman, D. S., Wang, Y., Cohen-Karni, D. &
Cohen-Karni, T. Graphene nanostructures for input–output bioelectronics. _Biophys. Rev._ 2, 041304 (2021). Article Google Scholar * Bergman, H., Wichmann, T. & DeLong, M. R. Reversal
of experimental parkinsonism by lesions of the subthalamic nucleus. _Science_ 249, 1436–1438 (1990). Article Google Scholar * Spira, M. E. & Hai, A. Multi-electrode array technologies
for neuroscience and cardiology. _Nat. Nanotechnol._ 8, 83–94 (2013). Article Google Scholar * Engel, A. K., Moll, C. K., Fried, I. & Ojemann, G. A. Invasive recordings from the human
brain: clinical insights and beyond. _Nat. Rev. Neurosci._ 6, 35–47 (2005). Article Google Scholar * Polikov, V. S., Tresco, P. A. & Reichert, W. M. Response of brain tissue to
chronically implanted neural electrodes. _J. Neurosci. Methods_ 148, 1–18 (2005). Article Google Scholar * Rivnay, J., Wang, H., Fenno, L., Deisseroth, K. & Malliaras, G. G.
Next-generation probes, particles, and proteins for neural interfacing. _Sci. Adv._ 3, e1601649 (2017). Article Google Scholar * Gulino, M., Kim, D., Pané, S., Santos, S. D. & Pêgo, A.
P. Tissue response to neural implants: the use of model systems toward new design solutions of implantable microelectrodes. _Front. Neurosci._ 13, 689 (2019). Article Google Scholar *
Carnicer-Lombarte, A., Chen, S.-T., Malliaras, G. G. & Barone, D. G. Foreign body reaction to implanted biomaterials and its impact in nerve neuroprosthetics. _Front. Bioeng.
Biotechnol._ 9, 622524 (2021). Article Google Scholar * Salatino, J. W., Ludwig, K. A., Kozai, T. D. & Purcell, E. K. Glial responses to implanted electrodes in the brain. _Nat.
Biomed. Eng._ 1, 862–877 (2017). Article Google Scholar * Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control
of neural activity. _Nat. Neurosci._ 8, 1263–1268 (2005). Article Google Scholar * Sahel, J.-A. et al. Partial recovery of visual function in a blind patient after optogenetic therapy.
_Nat. Med._ 27, 1223–1229 (2021). Article Google Scholar * Chernov, M. & Roe, A. W. Infrared neural stimulation: a new stimulation tool for central nervous system applications.
_Neurophotonics_ 1, 011011 (2014). Article Google Scholar * Packer, A. M., Roska, B. & Häusser, M. Targeting neurons and photons for optogenetics. _Nat. Neurosci._ 16, 805–815 (2013).
Article Google Scholar * Plaksin, M., Shapira, E., Kimmel, E. & Shoham, S. Thermal transients excite neurons through universal intramembrane mechanoelectrical effects. _Phys. Rev. X_
8, 011043 (2018). Google Scholar * Wells, J. et al. Optical stimulation of neural tissue in vivo. _Opt. Lett._ 30, 504–506 (2005). Article Google Scholar * Wells, J. D., Kao, C., Jansen,
E. D., Konrad, P. E. & Mahadevan-Jansen, A. Application of infrared light for in vivo neural stimulation. _J. Biomed. Opt._ 10, 064003 (2005). Article Google Scholar * Jenkins, M. W.
et al. Optical pacing of the embryonic heart. _Nat. Photonics_ 4, 623–626 (2010). Article Google Scholar * Liljemalm, R., Nyberg, T. & von Holst, H. Heating during infrared neural
stimulation. _Lasers Surg. Med._ 45, 469–481 (2013). Article Google Scholar * Wells, J. D. et al. Optically mediated nerve stimulation: Identification of injury thresholds. _Lasers Surg.
Med._ 39, 513–526 (2007). Article Google Scholar * Zimmerman, J. F. & Tian, B. Nongenetic optical methods for measuring and modulating neuronal response. _ACS Nano_ 12, 4086–4095
(2018). Article Google Scholar * Rastogi, S. K. et al. Remote nongenetic optical modulation of neuronal activity using fuzzy graphene. _Proc. Natl Acad. Sci. USA_ 117, 13339–13349 (2020).
THIS ARTICLE REPORTS TEMPLATED GRAPHENE NANOSTRUCTURES THAT ENABLE REMOTE, NON-GENETIC PHOTOTHERMAL STIMULATION WITH LASER ENERGIES AS LOW AS SUB-HUNDRED NANOJOULES WITHOUT GENERATING
CELLULAR STRESS. Article Google Scholar * Chen, R., Romero, G., Christiansen, M. G., Mohr, A. & Anikeeva, P. Wireless magnetothermal deep brain stimulation. _Science_ 347, 1477–1480
(2015). Article Google Scholar * Fang, Y. et al. Texturing silicon nanowires for highly localized optical modulation of cellular dynamics. _Nano Lett._ 18, 4487–4492 (2018). Article
Google Scholar * Sebesta, C. et al. Subsecond multichannel magnetic control of select neural circuits in freely moving flies. _Nat. Mater._ 21, 951–958 (2022). Article Google Scholar *
Duret, G. et al. Magnetic entropy as a proposed gating mechanism for magnetogenetic ion channels. _Biophys. J._ 116, 454–468 (2019). Article Google Scholar * Jiang, Y. & Tian, B.
Inorganic semiconductor biointerfaces. _Nat. Rev. Mater._ 3, 473–490 (2018). Article Google Scholar * Ghezzi, D. et al. A hybrid bioorganic interface for neuronal photoactivation. _Nat.
Commun._ 2, 166 (2011). THIS ARTICLE PRESENTS PHOTOTHERMAL EXCITATION OF PRIMARY NEURONS USING SHORT PULSES OF VISIBLE LIGHT AT THE INTERFACED ORGANIC THIN FILMS. Article Google Scholar *
Bareket-Keren, L. & Hanein, Y. Novel interfaces for light directed neuronal stimulation: advances and challenges. _Int. J. Nanomed._ 9, 65 (2014). Article Google Scholar * Jiang, Y. et
al. Rational design of silicon structures for optically controlled multiscale biointerfaces. _Nat. Biomed. Eng._ 2, 508–521 (2018). Article Google Scholar * Ye, E. & Li, Z.
_Photothermal Nanomaterials_. Vol. 54 (Royal Society of Chemistry, 2022). * Carvalho-de-Souza, J. L. et al. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. _Neuron_
86, 207–217 (2015). Article Google Scholar * Wang, Y. et al. Ti3C2Tx MXene flakes for optical control of neuronal electrical activity. _ACS Nano_ 15, 14662–14671 (2021). THE ARTICLE
REPORTS 2D MXENE FILMS AND FLAKES THAT ENABLE SUBCELLULAR PHOTOTHERMAL STIMULATION WITH ENERGIES AS LOW AS TENS OF MICROJOULES PER PULSE. Article Google Scholar * Lyu, Y., Xie, C.,
Chechetka, S. A., Miyako, E. & Pu, K. Semiconducting polymer nanobioconjugates for targeted photothermal activation of neurons. _J. Am. Chem. Soc._ 138, 9049–9052 (2016). Article Google
Scholar * Carvalho-de-Souza, J. L., Pinto, B. I., Pepperberg, D. R. & Bezanilla, F. Optocapacitive generation of action potentials by microsecond laser pulses of nanojoule energy.
_Biophys. J._ 114, 283–288 (2018). Article Google Scholar * Pinto, B. I., Bassetto, C. A. & Bezanilla, F. Optocapacitance: physical basis and its application. _Biophys. Rev._ 14,
569–577 (2022). THIS ARTICLE EXPLAINS THE THEORETICAL BASIS OF OPTOCAPACITIVE-BASED PHOTOTHERMAL EXCITATION. Article Google Scholar * Jung, S. et al. Photothermal response induced by
nanocage-coated artificial extracellular matrix promotes neural stem cell differentiation. _Nanomaterials_ 11, 1216 (2021). Article Google Scholar * Paviolo, C. et al. Laser exposure of
gold nanorods can increase neuronal cell outgrowth. _Biotechnol. Bioeng._ 110, 2277–2291 (2013). Article Google Scholar * Masini, D. & Kiehn, O. Targeted activation of midbrain neurons
restores locomotor function in mouse models of parkinsonism. _Nat. Commun._ 13, 504 (2022). Article Google Scholar * Zhang, Y. et al. Targeting thalamic circuits rescues motor and mood
deficits in PD mice. _Nature_ 607, 321–329 (2022). Article Google Scholar * Li, M. C. & Cook, M. J. Deep brain stimulation for drug-resistant epilepsy. _Epilepsia_ 59, 273–290 (2018).
Article Google Scholar * Hodgkin, A. L. & Huxley, A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. _J. Physiol._ 117,
500–544 (1952). Article Google Scholar * Manfredi, G. et al. The physics of plasma membrane photostimulation. _APL Mater._ 9, 030901 (2021). THIS ARTICLE DESCRIBES THE PHYSICAL PHENOMENA
RESPONSIBLE FOR PHOTOSTIMULATION USING THE SIMPLE EQUIVALENT CIRCUIT MODEL OF THE CELL MEMBRANE. Article Google Scholar * Eom, K., Byun, K. M., Jun, S. B., Kim, S. J. & Lee, J.
Theoretical study on gold-nanorod-enhanced near-infrared neural stimulation. _Biophys. J._ 115, 1481–1497 (2018). Article Google Scholar * Martino, N. et al. Photothermal cellular
stimulation in functional bio-polymer interfaces. _Sci. Rep._ 5, 8911 (2015). Article Google Scholar * Wu, X. et al. Tether-free photothermal deep-brain stimulation in freely behaving mice
via wide-field illumination in the near-infrared-II window. _Nat. Biomed. Eng._ 6, 754–770 (2022). THIS ARTICLE DEMONSTRATES IN VIVO PHOTOTHERMAL STIMULATION OF TRPV1-EXPRESSING NEURONS
USING NIR-II RESPONSIVE MACROMOLECULAR TRANSDUCTORS. Article Google Scholar * Gribi, S., du Bois de Dunilac, S., Ghezzi, D. & Lacour, S. P. A microfabricated nerve-on-a-chip platform
for rapid assessment of neural conduction in explanted peripheral nerve fibers. _Nat. Commun._ 9, 4403 (2018). Article Google Scholar * Patapoutian, A., Peier, A. M., Story, G. M. &
Viswanath, V. ThermoTRP channels and beyond: mechanisms of temperature sensation. _Nat. Rev. Neurosci._ 4, 529–539 (2003). Article Google Scholar * Ramos, A. P., Cruz, M. A., Tovani, C. B.
& Ciancaglini, P. Biomedical applications of nanotechnology. _Biophys. Rev._ 9, 79–89 (2017). Article Google Scholar * McNamara, K. & Tofail, S. A. Nanoparticles in biomedical
applications. _Adv. Phys. X_ 2, 54–88 (2017). Google Scholar * Vajtai, R. _Springer Handbook of Nanomaterials_ (Springer Science & Business Media, 2013). * Wang, Y. & Guo, L.
Nanomaterial-enabled neural stimulation. _Front. Neurosci._ 10, 69 (2016). Article Google Scholar * Dykman, L. & Khlebtsov, N. Gold nanoparticles in biology and medicine: recent
advances and prospects. _Acta Naturae_ 3, 34–55 (2011). Article Google Scholar * Giljohann, D. A. et al. In _Spherical Nucleic Acids_, 55–90 (Jenny Stanford Publishing, 2020). * Roper, D.
K., Ahn, W. & Hoepfner, M. Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles. _J. Phys. Chem. C._ 111, 3636–3641 (2007). Article Google Scholar * Bera,
D., Qian, L., Tseng, T.-K. & Holloway, P. H. Quantum dots and their multimodal applications: a review. _Materials_ 3, 2260–2345 (2010). Article Google Scholar * Altavilla, C. &
Ciliberto, E. _Inorganic Nanoparticles: Synthesis, Applications, and Perspectives_ (CRC Press, 2017). * Rao, J. P. & Geckeler, K. E. Polymer nanoparticles: preparation techniques and
size-control parameters. _Prog. Polym. Sci._ 36, 887–913 (2011). Article Google Scholar * Zielińska, A. et al. Polymeric nanoparticles: production, characterization, toxicology and
ecotoxicology. _Molecules_ 25, 3731 (2020). Article Google Scholar * Gholami Derami, H. et al. Reversible photothermal modulation of electrical activity of excitable cells using
polydopamine nanoparticles. _Adv. Mater._ 33, 2008809 (2021). Article Google Scholar * Yan, R., Gargas, D. & Yang, P. Nanowire photonics. _Nat. Photonics_ 3, 569–576 (2009). Article
Google Scholar * Shi, J., Sun, C., Liang, E. & Tian, B. Semiconductor nanowire-based cellular and subcellular interfaces. _Adv. Funct. Mater._ 32, 2107997 (2021). Article Google
Scholar * Tian, B. et al. Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. _Science_ 329, 830–834 (2010). Article Google Scholar * Zhou, W., Dai, X.
& Lieber, C. M. Advances in nanowire bioelectronics. _Rep. Prog. Phys._ 80, 016701 (2016). Article Google Scholar * Wagner, A. R. & Ellis, S. W. Vapor-liquid-solid mechanism of
single crystal growth. _Appl. Phys. Lett._ 4, 89–90 (1964). Article Google Scholar * Schmidt, V., Wittemann, J. & Gosele, U. Growth, thermodynamics, and electrical properties of
silicon nanowires. _Chem. Rev._ 110, 361–388 (2010). Article Google Scholar * Tian, B. et al. Coaxial silicon nanowires as solar cells and nanoelectronic power sources. _Nature_ 449,
885–889 (2007). Article Google Scholar * VahidMohammadi, A., Rosen, J. & Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). _Science_ 372, eabf1581 (2021).
Article Google Scholar * Naguib, M. et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. _Adv. Mater._ 23, 4248–4253 (2011). Article Google Scholar * Gogotsi, Y. &
Huang, Q. MXenes: two-dimensional building blocks for future materials and devices. _ACS Nano_ 15, 5775–5780 (2021). Article Google Scholar * Gao, P. et al. Biomedical applications of 2D
monoelemental materials formed by group VA and VIA: a concise review. _J. Nanobiotechnol._ 19, 96 (2021). Article Google Scholar * Driscoll, N. et al. Two-dimensional Ti3C2 MXene for
high-resolution neural interfaces. _ACS Nano_ 12, 10419–10429 (2018). Article Google Scholar * Lin, H., Wang, X., Yu, L., Chen, Y. & Shi, J. Two-dimensional ultrathin MXene ceramic
nanosheets for photothermal conversion. _Nano Lett._ 17, 384–391 (2017). Article Google Scholar * Driscoll, N. et al. MXene-infused bioelectronic interfaces for multiscale
electrophysiology and stimulation. _Sci. Transl. Med._ 13, eabf8629 (2021). Article Google Scholar * Hantanasirisakul, K. & Gogotsi, Y. Electronic and optical properties of 2D
transition metal carbides and nitrides (MXenes). _Adv. Mater._ 30, 1804779 (2018). Article Google Scholar * Yaroslavsky, A. N. et al. Optical properties of selected native and coagulated
human brain tissues in vitro in the visible and near infrared spectral range. _Phys. Med. Biol._ 47, 2059–2073 (2002). Article Google Scholar * Shuck, C. E. et al. Scalable synthesis of
Ti3C2T_x_ MXene. _Adv. Eng. Mater._ 22, 1901241 (2020). Article Google Scholar * San Roman, D., Garg, R. & Cohen-Karni, T. Bioelectronics with graphene nanostructures. _APL Mater._ 8,
100906 (2020). Article Google Scholar * Garg, R. et al. Nanowire-mesh-templated growth of out-of-plane three-dimensional fuzzy graphene. _ACS Nano_ 11, 6301–6311 (2017). Article Google
Scholar * Garg, R. et al. Electron transport in multidimensional fuzzy graphene nanostructures. _Nano Lett._ 19, 5335–5339 (2019). Article Google Scholar * San Roman, D. et al.
Engineering three-dimensional (3D) out-of-plane graphene edge sites for highly selective two-electron oxygen reduction electrocatalysis. _ACS Catal._ 10, 1993–2008 (2020). Article Google
Scholar * Rastogi, S. K. et al. Three-dimensional fuzzy graphene ultra-microelectrodes for subcellular electrical recordings. _Nano Res._ 13, 1444–1452 (2020). Article Google Scholar *
Gong, W. et al. Thermal transport in multidimensional silicon-graphene hybrid nanostructures. _ACS Appl. Mater. Interfaces_ 13, 50206–50212 (2021). Article Google Scholar * Huang, X. &
El-Sayed, M. A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. _J. Adv. Res._ 1, 13–28 (2010). Article Google Scholar * Mie, G.
Contributions to the optics of turbid media, particularly of colloidal metal solutions. _Ann. Phys._ 25, 377–445 (1976). Google Scholar * Papavassiliou, G. C. Optical properties of small
inorganic and organic metal particles. _Prog. Solid. State Chem._ 12, 185–271 (1979). Article Google Scholar * Link, S. & El-Sayed, M. A. Size and temperature dependence of the plasmon
absorption of colloidal gold nanoparticles. _J. Phys. Chem. B_ 103, 4212–4217 (1999). Article Google Scholar * Chen, H. et al. Understanding the photothermal conversion efficiency of gold
nanocrystals. _Small_ 6, 2272–2280 (2010). Article Google Scholar * Lee, K.-S. & El-Sayed, M. A. Dependence of the enhanced optical scattering efficiency relative to that of
absorption for gold metal nanorods on aspect ratio, size, end-cap shape, and medium refractive index. _J. Phys. Chem. B_ 109, 20331–20338 (2005). Article Google Scholar * Anayee, M. et al.
Role of acid mixtures etching on the surface chemistry and sodium ion storage in Ti3C2Tx MXene. _Chem. Commun._ 56, 6090–6093 (2020). Article Google Scholar * Maleski, K., Shuck, C. E.,
Fafarman, A. T. & Gogotsi, Y. The broad chromatic range of two-dimensional transition metal carbides. _Adv. Opt. Mater._ 9, 2001563 (2021). Article Google Scholar * Lin, H. et al. A
90-nm-thick graphene metamaterial for strong and extremely broadband absorption of unpolarized light. _Nat. Photonics_ 13, 270–276 (2019). Article Google Scholar * Campbell, P. &
Green, M. A. Light trapping properties of pyramidally textured surfaces. _J. Appl. Phys._ 62, 243–249 (1987). Article Google Scholar * Yablonovitch, E. Statistical ray optics. _J. Opt.
Soc. Am._ 72, 899–907 (1982). Article Google Scholar * Dipalo, M. et al. Intracellular action potential recordings from cardiomyocytes by ultrafast pulsed laser irradiation of fuzzy
graphene microelectrodes. _Sci. Adv._ 7, eabd5175 (2021). Article Google Scholar * Yong, J. et al. Gold-nanorod-assisted near-infrared stimulation of primary auditory neurons. _Adv.
Healthc. Mater._ 3, 1862–1868 (2014). Article Google Scholar * Jiang, Y. et al. Nongenetic optical neuromodulation with silicon-based materials. _Nat. Protoc._ 14, 1339–1376 (2019). THE
ARTICLE PRESENTS A DETAILED PROTOCOL FOR THE CHARACTERIZATION OF THE LOCAL TEMPERATURE RESPONSE OF NANOMATERIALS USING MICRO-PIPETTE AND NANO-PIPETTE THERMOMETRY. Article Google Scholar *
Tobías, I., Cañizo, C. D. & Alonso, J. In _Handbook of Photovoltaic Science and Engineering_ 255–306 (Wiley, 2003). * Walter, M. G. et al. Solar water splitting cells. _Chem. Rev._ 110,
6446–6473 (2010). Article Google Scholar * Su, Y. et al. Gold nanoparticles-decorated silicon nanowires as highly efficient near-infrared hyperthermia agents for cancer cells destruction.
_Nano Lett._ 12, 1845–1850 (2012). Article Google Scholar * Gao, M., Zhu, L., Peh, C. K. & Ho, G. W. Solar absorber material and system designs for photothermal water vaporization
towards clean water and energy production. _Energy Environ. Sci._ 12, 841–864 (2019). Article Google Scholar * Hao, J. et al. High performance optical absorber based on a plasmonic
metamaterial. _Appl. Phys. Lett._ 96, 251104 (2010). Article Google Scholar * Li, R., Zhang, L., Shi, L. & Wang, P. MXene Ti3C2: an effective 2D light-to-heat conversion material. _ACS
Nano_ 11, 3752–3759 (2017). Article Google Scholar * Lee, U., Yoo, C.-J., Kim, Y.-J. & Yoo, Y.-M. Cytotoxicity of gold nanoparticles in human neural precursor cells and rat cerebral
cortex. _J. Biosci. Bioeng._ 121, 341–344 (2016). Article Google Scholar * Sani, A., Cao, C. & Cui, D. Toxicity of gold nanoparticles (AuNPs): a review. _Biochem. Biophys. Rep._ 26,
100991 (2021). Google Scholar * Eom, K. et al. Enhanced infrared neural stimulation using localized surface plasmon resonance of gold nanorods. _Small_ 10, 3853–3857 (2014). THIS ARTICLE
DEMONSTRATES PHOTOTHERMAL EXCITATION OF THE SCIATIC NERVE USING PULSED INFRARED ILLUMINATION OF INTERFACED PLASMONIC GOLD NANORODS. Article Google Scholar * Lewinski, N., Colvin, V. &
Drezek, R. Cytotoxicity of nanoparticles. _Small_ 4, 26–49 (2008). Article Google Scholar * Tosheva, K. L., Yuan, Y., Pereira, P. M., Culley, S. & Henriques, R. Between life and death:
strategies to reduce phototoxicity in super-resolution microscopy. _J. Phys. D Appl. Phys._ 53, 163001 (2020). Article Google Scholar * Stockley, J. H. et al. Surpassing light-induced
cell damage in vitro with novel cell culture media. _Sci. Rep._ 7, 849 (2017). Article Google Scholar * Kuse, Y., Ogawa, K., Tsuruma, K., Shimazawa, M. & Hara, H. Damage of
photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. _Sci. Rep._ 4, 5223 (2014). Article Google Scholar * Lim, G. P. et al. Cytotoxicity of
MXene-based nanomaterials for biomedical applications: a mini review. _Environ. Res._ 201, 111592 (2021). Article Google Scholar * Matino, L., Rastogi, S. K., Garma, L. D., Cohen-Karni, T.
& Santoro, F. Characterization of the coupling between out-of-plane graphene and electrogenic cells. _Adv. Mater. Interfaces_ 7, 2000699 (2020). Article Google Scholar * Mullick
Chowdhury, S. et al. Cell specific cytotoxicity and uptake of graphene nanoribbons. _Biomaterials_ 34, 283–293 (2013). Article Google Scholar * Li, Z. et al. Cellular level
biocompatibility and biosafety of ZnO nanowires. _J. Phys. Chem. C._ 112, 20114–20117 (2008). Article Google Scholar * Jastrzębska, A. et al. In vitro studies on cytotoxicity of
delaminated Ti3C2 MXene. _J. Hazard. Mater._ 339, 1–8 (2017). Article Google Scholar * Dai, C. et al. Biocompatible 2D titanium carbide (MXenes) composite nanosheets for pH-responsive
MRI-guided tumor hyperthermia. _Chem. Mater._ 29, 8637–8652 (2017). Article Google Scholar * Zhang, L. et al. Mechanisms of reactive oxygen species generated by inorganic nanomaterials for
cancer therapeutics. _Front. Chem._ 9, 630969 (2021). Article Google Scholar * Bergamini, C. M., Gambetti, S., Dondi, A. & Cervellati, C. Oxygen, reactive oxygen species and tissue
damage. _Curr. Pharm. Des._ 10, 1611–1626 (2004). Article Google Scholar * Shields, H. J., Traa, A. & Van Raamsdonk, J. M. Beneficial and detrimental effects of reactive oxygen species
on lifespan: a comprehensive review of comparative and experimental studies. _Front. Cell Dev. Biol._ 9, 628157 (2021). Article Google Scholar * Yarmolenko, P. S. et al. Thresholds for
thermal damage to normal tissues: an update. _Int. J. Hyperth._ 27, 320–343 (2011). Article Google Scholar * Sapareto, S. A. & Dewey, W. C. Thermal dose determination in cancer
therapy. _Int. J. Radiat. Oncol. Biol. Phys._ 10, 787–800 (1984). Article Google Scholar * Dewhirst, M. W., Viglianti, B., Lora-Michiels, M., Hanson, M. & Hoopes, P. Basic principles
of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. _Int. J. Hyperth._ 19, 267–294 (2003). Article Google Scholar * Sharma, H. Hyperthermia influences
excitatory and inhibitory amino acid neurotransmitters in the central nervous system. An experimental study in the rat using behavioural, biochemical, pharmacological, and morphological
approaches. _J. Neural Transm._ 113, 497–519 (2006). Article Google Scholar * Liebregts, M. T., McLachlan, R. S. & Leung, L. S. Hyperthermia induces age‐dependent changes in rat
hippocampal excitability. _Ann. Neurol._ 52, 318–326 (2002). Article Google Scholar * Johannsmeier, S. et al. Gold nanoparticle-mediated laser stimulation induces a complex stress response
in neuronal cells. _Sci. Rep._ 8, 6533 (2018). Article Google Scholar * Battaglini, M. et al. Polydopamine nanoparticles as an organic and biodegradable multitasking tool for
neuroprotection and remote neuronal stimulation. _ACS Appl. Mater. Interfaces_ 12, 35782–35798 (2020). Article Google Scholar * Carvalho-de-Souza, J. L. et al. Cholesterol
functionalization of gold nanoparticles enhances photoactivation of neural activity. _ACS Chem. Neurosci._ 10, 1478–1487 (2018). Article Google Scholar * DiFrancesco, M. L. et al. A hybrid
P3HT-graphene interface for efficient photostimulation of neurons. _Carbon_ 162, 308–317 (2020). Article Google Scholar * Yoo, S., Hong, S., Choi, Y., Park, J. H. & Nam, Y.
Photothermal inhibition of neural activity with near-infrared-sensitive nanotransducers. _ACS Nano_ 8, 8040–8049 (2014). THIS ARTICLE REPORTS THAT PHOTOTHERMAL INHIBITION INDUCED USING
NANOPARTICLES (GOLD NANORODS) CAN BE TUNED BY MODULATING THE ILLUMINATION CONDITIONS. Article Google Scholar * Lee, J. W., Jung, H., Cho, H. H., Lee, J. H. & Nam, Y. Gold
nanostar-mediated neural activity control using plasmonic photothermal effects. _Biomaterials_ 153, 59–69 (2018). Article Google Scholar * Kang, H., Lee, G.-H., Jung, H., Lee, J. W. &
Nam, Y. Inkjet-printed biofunctional thermo-plasmonic interfaces for patterned neuromodulation. _ACS Nano_ 12, 1128–1138 (2018). Article Google Scholar * Feyen, P. et al. Light-evoked
hyperpolarization and silencing of neurons by conjugated polymers. _Sci. Rep._ 6, 22718 (2016). Article Google Scholar * Lavoie-Cardinal, F., Salesse, C., Bergeron, É., Meunier, M. &
De Koninck, P. Gold nanoparticle-assisted all optical localized stimulation and monitoring of Ca2+ signaling in neurons. _Sci. Rep._ 6, 20619 (2016). Article Google Scholar * Eom, K. et
al. Photothermal activation of astrocyte cells using localized surface plasmon resonance of gold nanorods. _J. Biophotonics_ 10, 486–493 (2017). Article Google Scholar * Eom, K. et al.
Synergistic combination of near-infrared irradiation and targeted gold nanoheaters for enhanced photothermal neural stimulation. _Biomed. Opt. Express_ 7, 1614–1625 (2016). Article Google
Scholar * Nakatsuji, H. et al. Thermosensitive ion channel activation in single neuronal cells by using surface-engineered plasmonic nanoparticles. _Angew. Chem. Int. Ed._ 54, 11725–11729
(2015). Article Google Scholar * Paviolo, C., Haycock, J. W., Cadusch, P. J., McArthur, S. L. & Stoddart, P. R. Laser exposure of gold nanorods can induce intracellular calcium
transients. _J. Biophotonics_ 7, 761–765 (2014). Article Google Scholar * Grienberger, C. & Konnerth, A. Imaging calcium in neurons. _Neuron_ 73, 862–885 (2012). Article Google
Scholar * Jiang, Y. et al. Heterogeneous silicon mesostructures for lipid-supported bioelectric interfaces. _Nat. Mater._ 15, 1023–1030 (2016). Article MathSciNet Google Scholar *
Miyako, E. et al. Photofunctional nanomodulators for bioexcitation. _Angew. Chem._ 126, 13337–13341 (2014). Article Google Scholar * Sakmann, B. & Neher, E. Patch clamp techniques for
studying ionic channels in excitable membranes. _Annu. Rev. Physiol._ 46, 455–472 (1984). Article Google Scholar * Tang, M. et al. Injectable black phosphorus nanosheets for wireless
nongenetic neural stimulation. _Small_ https://doi.org/10.1002/smll.202105388 (2021). Article Google Scholar * Jiang, S., Wu, X., Rommelfanger, N. J., Ou, Z. & Hong, G. Shedding light
on neurons: optical approaches for neuromodulation. _Natl Sci. Rev._ 9, nwac007 (2022). Article Google Scholar * Hong, G., Antaris, A. L. & Dai, H. Near-infrared fluorophores for
biomedical imaging. _Nat. Biomed. Eng._ 1, 0010 (2017). Article Google Scholar * Bashkatov, A. N., Genina, E., Kochubey, V. & Tuchin, V. Optical properties of human skin, subcutaneous
and mucous tissues in the wavelength range from 400 to 2000 nm. _J. Phys. D Appl. Phys._ 38, 2543 (2005). Article Google Scholar * Mie, G. Beiträge zur Optik trüber Medien, speziell
kolloidaler Metallösungen. _Ann. Phys._ 330, 377–445 (1908). Article MATH Google Scholar * Rayleigh, J. W. S. B. _On the Scattering of Light by Small Particles_ (Cambridge University
Press, 1871). * Jung, H. & Nam, Y. Optical recording of neural responses to gold-nanorod mediated photothermal neural inhibition. _J. Neurosci. Methods_ 373, 109564 (2022). Article
Google Scholar * An, Y. & Nam, Y. Closed-loop control of neural spike rate of cultured neurons using a thermoplasmonics-based photothermal neural stimulation. _J. Neural Eng._ 18,
066002 (2021). Article Google Scholar * Lee, S. E., Liu, G. L., Kim, F. & Lee, L. P. Remote optical switch for localized and selective control of gene interference. _Nano Lett._ 9,
562–570 (2009). Article Google Scholar * Wijaya, A., Schaffer, S. B., Pallares, I. G. & Hamad-Schifferli, K. Selective release of multiple DNA oligonucleotides from gold nanorods. _ACS
Nano_ 3, 80–86 (2009). Article Google Scholar * Carrow, J. K. et al. Photothermal modulation of human stem cells using light-responsive 2D nanomaterials. _Proc. Natl Acad. Sci. USA_ 117,
13329–13338 (2020). THIS ARTICLE DEMONSTRATES PHOTOTHERMAL MODULATION OF GENE EXPRESSION AND CELLULAR FUNCTIONS IN HUMAN STEM CELLS USING 2D TRANSITION METAL DICHALCOGENIDES SUCH AS MOS2.
Article Google Scholar * Fricker, M., Tolkovsky, A. M., Borutaite, V., Coleman, M. & Brown, G. C. Neuronal cell death. _Physiol. Rev._ 98, 813–880 (2018). Article Google Scholar *
Lodola, F. et al. Conjugated polymers optically regulate the fate of endothelial colony-forming cells. _Sci. Adv._ 5, eaav4620 (2019). Article Google Scholar * Milos, F. et al. High aspect
ratio and light-sensitive micropillars based on a semiconducting polymer optically regulate neuronal growth. _ACS Appl. Mater. Interfaces_ 13, 23438–23451 (2021). Article Google Scholar *
Aziz, I. A. et al. Light-triggered electron transfer between a conjugated polymer and cytochrome C for optical modulation of redox signaling. _iScience_ 23, 101091 (2020). Article Google
Scholar * Huh, D., Hamilton, G. A. & Ingber, D. E. From 3D cell culture to organs-on-chips. _Trends Cell Biol._ 21, 745–754 (2011). Article Google Scholar * Lee, A. et al. 3D
bioprinting of collagen to rebuild components of the human heart. _Science_ 365, 482–487 (2019). Article Google Scholar * Kalmykov, A. et al. Bioelectrical interfaces with cortical
spheroids in three-dimensions. _J. Neural Eng._ 18, 055005 (2021). Article Google Scholar * Andersson, H. A., Kim, Y.-S., O’Neill, B. E., Shi, Z.-Z. & Serda, R. E. HSP70
promoter-driven activation of gene expression for immunotherapy using gold nanorods and near infrared light. _Vaccines_ 2, 216–227 (2014). Article Google Scholar * Chen, X., Chen, Y., Xin,
H., Wan, T. & Ping, Y. Near-infrared optogenetic engineering of photothermal nanoCRISPR for programmable genome editing. _Proc. Natl Acad. Sci. USA_ 117, 2395–2405 (2020). Article
Google Scholar * Fisher, R. S. & Velasco, A. L. Electrical brain stimulation for epilepsy. _Nat. Rev. Neurol._ 10, 261–270 (2014). Article Google Scholar * Ðerek, V., Rand, D.,
Migliaccio, L., Hanein, Y. & Głowacki, E. D. Untangling photofaradaic and photocapacitive effects in organic optoelectronic stimulation devices. _Front. Bioeng. Biotechnol._ 8, 284
(2020). Article Google Scholar * Medagoda, D. I. & Ghezzi, D. Organic semiconductors for light-mediated neuromodulation. _Commun. Mater._ 2, 111 (2021). Article Google Scholar *
Nair, V. et al. Laser writing of nitrogen-doped silicon carbide for biological modulation. _Sci. Adv._ 6, eaaz2743 (2020). Article Google Scholar * Alberts, B. _Molecular Biology of the
Cell_ (Garland Science, 2008). * Nicholls, J. G., Martin, A. R., Wallace, B. G. & Fuchs, P. A. _From Neuron to Brain_. Vol. 271 (Sinauer Associates Sunderland, 2001). * Paulsen, C. E.,
Armache, J.-P., Gao, Y., Cheng, Y. & Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. _Nature_ 520, 511–517 (2015). Article Google Scholar * Crunelli, V.
& Leresche, N. A role for GABAB receptors in excitation and inhibition of thalamocortical cells. _Trends Neurosci._ 14, 16–21 (1991). Article Google Scholar * Mitragotri, S., Burke, P.
A. & Langer, R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. _Nat. Rev. Drug Discov._ 13, 655–672 (2014). Article Google Scholar
* Singh, R. & Lillard, J. W. Jr. Nanoparticle-based targeted drug delivery. _Exp. Mol. Pathol._ 86, 215–223 (2009). Article Google Scholar * Kim, C. Y. et al. Soft subdermal implant
capable of wireless battery charging and programmable controls for applications in optogenetics. _Nat. Commun._ 12, 535 (2021). Article Google Scholar * Ausra, J. et al. Wireless,
battery-free, subdermally implantable platforms for transcranial and long-range optogenetics in freely moving animals. _Proc. Natl Acad. Sci. USA_ 118, e2025775118 (2021). Article Google
Scholar * Reddy, J. W., Lassiter, M. & Chamanzar, M. Parylene photonics: a flexible, broadband optical waveguide platform with integrated micromirrors for biointerfaces. _Microsyst.
Nanoeng._ 6, 85 (2020). Article Google Scholar * Scopelliti, M. G. & Chamanzar, M. Ultrasonically sculpted virtual relay lens for in situ microimaging. _Light Sci. Appl._ 8, 65 (2019).
Article Google Scholar * Kim, D. et al. Ultraflexible organic light-emitting diodes for optogenetic nerve stimulation. _Proc. Natl Acad. Sci. USA_ 117, 21138–21146 (2020). Article Google
Scholar * Nel, A. E. et al. Understanding biophysicochemical interactions at the nano–bio interface. _Nat. Mater._ 8, 543–557 (2009). Article Google Scholar * Petros, R. A. &
DeSimone, J. M. Strategies in the design of nanoparticles for therapeutic applications. _Nat. Rev. Drug Discov._ 9, 615–627 (2010). Article Google Scholar * Benfenati, F. & Lanzani, G.
Clinical translation of nanoparticles for neural stimulation. _Nat. Rev. Mater._ 6, 1–4 (2021). Article Google Scholar * Charbgoo, F. et al. Gold nanoparticle should understand protein
corona for being a clinical nanomaterial. _J. Control. Rel._ 272, 39–53 (2018). Article Google Scholar Download references ACKNOWLEDGEMENTS T.C.-K. acknowledges funding support from the
Defense Advanced Research Projects Agency (Award No. D20AC00002) and the National Institute of Health (Award No. R21EB029164). AUTHOR INFORMATION Author notes * These authors contributed
equally: Yingqiao Wang, Raghav Garg. AUTHORS AND AFFILIATIONS * Department of Materials Science and Engineering, Carnegie Mellon University, Pittsburgh, PA, USA Yingqiao Wang, Raghav Garg
& Tzahi Cohen-Karni * Preclinical education biochemistry, Lake Erie College of Osteopathic Medicine at Seton Hill, Greensburg, PA, USA Devora Cohen-Karni * Department of Biomedical
Engineering, Carnegie Mellon University, Pittsburgh, PA, USA Tzahi Cohen-Karni Authors * Yingqiao Wang View author publications You can also search for this author inPubMed Google Scholar *
Raghav Garg View author publications You can also search for this author inPubMed Google Scholar * Devora Cohen-Karni View author publications You can also search for this author inPubMed
Google Scholar * Tzahi Cohen-Karni View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.W. and R.G. contributed equally to this work.
CORRESPONDING AUTHOR Correspondence to Tzahi Cohen-Karni. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no known competing financial interests or personal
relationships that could have appeared to influence the work reported in this paper. PEER REVIEW PEER REVIEW INFORMATION _Nature Reviews Bioengineering_ thanks Guosong Hong, Diego Ghezzi,
and the other, anonymous, reviewer for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a
society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript
version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wang, Y., Garg, R.,
Cohen-Karni, D. _et al._ Neural modulation with photothermally active nanomaterials. _Nat Rev Bioeng_ 1, 193–207 (2023). https://doi.org/10.1038/s44222-023-00022-y Download citation *
Accepted: 02 January 2023 * Published: 31 January 2023 * Issue Date: March 2023 * DOI: https://doi.org/10.1038/s44222-023-00022-y SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
