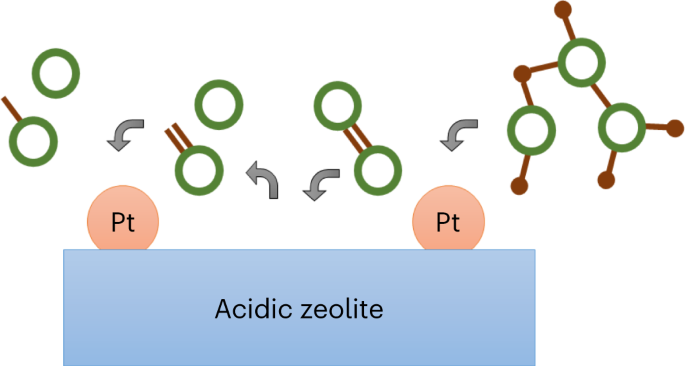
Carbon–carbon bond cleavage for a lignin refinery
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Carbon–carbon bonds, ubiquitous in lignin, limit monomer yields from current depolymerization strategies, which mainly target C–O bonds. Selective cleavage of the inherently inert
σ-type C–C bonds without pre-functionalization remains challenging. Here we report the breaking of C–C bonds in lignin obtained upon initial disruption of labile C–O bonds, achieving
monocyclic hydrocarbon yields up to an order of magnitude higher than previously reported. The use of a Pt (de)hydrogenation function leads to olefinic groups close to recalcitrant C–C
bonds, which can undergo β-scission over zeolitic Brønsted acid sites. After confirming that this approach can selectively cleave common C–C linkages (5–5′, β–1′, β–5′ and β–β′) in lignin
skeletons, we demonstrate its utility in the valorization of various representative lignins. A techno-economic analysis shows the promise of our method for producing gasoline- and jet-range
cycloalkanes and aromatics, while a life-cycle assessment confirms its potential for CO2-neutral fuel production. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per
year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated
during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS LIGNIN
DEOXYGENATION FOR THE PRODUCTION OF SUSTAINABLE AVIATION FUEL BLENDSTOCKS Article 26 November 2024 OXIDATIVE CLEAVAGE OF C–C BONDS IN LIGNIN Article 23 September 2021 ROOM TEMPERATURE
CATALYTIC UPGRADING OF UNPURIFIED LIGNIN DEPOLYMERIZATION OIL INTO BISPHENOLS AND BUTENE-2 Article Open access 13 July 2024 DATA AVAILABILITY All data are available within the manuscript and
Supplementary Information. The atomic coordinates of the optimized computational models are provided in Supplementary Data 1. Source data are provided with this paper. REFERENCES *
Questell-Santiago, Y. M., Galkin, M. V., Barta, K. & Luterbacher, J. S. Stabilization strategies in biomass depolymerization using chemical functionalization. _Nat. Rev. Chem._ 4,
311–330 (2020). Article CAS PubMed Google Scholar * Liao, Y. et al. A sustainable wood biorefinery for low-carbon footprint chemicals production. _Science_ 367, 1385–1390 (2020). Article
CAS PubMed Google Scholar * Li, C., Zhao, X., Wang, A., Huber, G. W. & Zhang, T. Catalytic transformation of lignin for chemicals and fuels. _Chem. Rev._ 115, 11559–11624 (2015).
Article CAS PubMed Google Scholar * Sun, Z. et al. Complete lignocellulose conversion with integrated catalyst recycling yielding valuable aromatics and fuels. _Nat. Catal._ 1, 82–92
(2018). Article CAS Google Scholar * Tuck, C. O., Pérez, E., Horváth, I. T., Sheldon, R. A. & Poliakoff, M. Valorization of biomass: deriving more value from waste. _Science_ 337,
695–699 (2012). Article CAS PubMed Google Scholar * Ragauskas, A. J. et al. Lignin valorization: improving lignin processing in the biorefinery. _Science_ 344, 1246843 (2014). Article
PubMed Google Scholar * Rahimi, A., Ulbrich, A., Coon, J. J. & Stahl, S. S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. _Nature_ 515, 249–252 (2014). Article
CAS PubMed Google Scholar * Li, Y. et al. An ‘ideal lignin’ facilitates full biomass utilization. _Sci. Adv._ 4, eaau2968 (2018). Article PubMed PubMed Central Google Scholar *
Meng, Q. et al. Sustainable production of benzene from lignin. _Nat. Commun._ 12, 4534 (2021). Article CAS PubMed PubMed Central Google Scholar * Katahira, R., Elder, T. J. &
Beckham, G. T. in _A Brief Introduction to Lignin Structure_. (ed Beckham, G. T.) Ch. 1 (Royal Society of Chemistry, 2018). * Zakzeski, J., Bruijnincx, P. C., Jongerius, A. L. &
Weckhuysen, B. M. The catalytic valorization of lignin for the production of renewable chemicals. _Chem. Rev._ 110, 3552–3599 (2010). Article CAS PubMed Google Scholar * Phongpreecha, T.
et al. Predicting lignin depolymerization yields from quantifiable properties using fractionated biorefinery lignins. _Green Chem._ 19, 5131–5143 (2017). Article CAS Google Scholar *
Talebi Amiri, M., Dick, G. R., Questell-Santiago, Y. M. & Luterbacher, J. S. Fractionation of lignocellulosic biomass to produce uncondensed aldehyde-stabilized lignin. _Nat. Protoc._
14, 921–954 (2019). Article CAS PubMed Google Scholar * Biermann, C. J. _Handbook of Pulping and Papermaking_ (Elsevier, 1996). * da Costa Sousa, L. et al. Next-generation ammonia
pretreatment enhances cellulosic biofuel production. _Energy Environ. Sci._ 9, 1215–1223 (2016). Article Google Scholar * Kim, K. H. et al. Integration of renewable deep eutectic solvents
with engineered biomass to achieve a closed-loop biorefinery. _Proc. Natl Acad. Sci. USA_ 116, 13816–13824 (2019). Article CAS PubMed PubMed Central Google Scholar * Luterbacher, J. S.
et al. Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone. _Science_ 343, 277–280 (2014). Article CAS PubMed Google Scholar * Feghali, E., Carrot, G.,
Thuery, P., Genre, C. & Cantat, T. Convergent reductive depolymerization of wood lignin to isolated phenol derivatives by metal-free catalytic hydrosilylation. _Energy Environ. Sci._ 8,
2734–2743 (2015). Article CAS Google Scholar * Deuss, P. J. et al. Phenolic acetals from lignins of varying compositions via iron (III) triflate catalysed depolymerisation. _Green Chem._
19, 2774–2782 (2017). Article CAS Google Scholar * Renders, T. et al. Lignin-first biomass fractionation: the advent of active stabilisation strategies. _Energy Environ. Sci._ 10,
1551–1557 (2017). Article CAS Google Scholar * Wu, X. et al. Solar energy-driven lignin-first approach to full utilization of lignocellulosic biomass under mild conditions. _Nat. Catal._
1, 772–780 (2018). Article CAS Google Scholar * Schutyser, W. et al. Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation and upgrading. _Chem. Soc. Rev._
47, 852–908 (2018). Article CAS PubMed Google Scholar * Rinaldi, R. et al. Paving the way for lignin valorisation: recent advances in bioengineering, biorefining and catalysis. _Angew.
Chem. Int. Ed._ 55, 8164–8215 (2016). Article CAS Google Scholar * Kim, S. et al. Computational study of bond dissociation enthalpies for a large range of native and modified lignins. _J.
Phys. Chem. Lett._ 2, 2846–2852 (2011). Article CAS Google Scholar * Subbotina, E. et al. Oxidative cleavage of C–C bonds in lignin. _Nat. Chem._ 13, 1118–1125 (2021). Article CAS
PubMed Google Scholar * Hemberger, P., Custodis, V. B., Bodi, A., Gerber, T. & van Bokhoven, J. A. Understanding the mechanism of catalytic fast pyrolysis by unveiling reactive
intermediates in heterogeneous catalysis. _Nat. Commun._ 8, 15946 (2017). Article PubMed PubMed Central Google Scholar * Shuai, L. et al. Selective C-C bond cleavage of methylene-linked
lignin models and kraft lignin. _ACS Catal._ 8, 6507–6512 (2018). Article CAS Google Scholar * Zhu, J., Wang, J. & Dong, G. Catalytic activation of unstrained C(aryl)–C(aryl) bonds in
2,2′-biphenols. _Nat. Chem._ 11, 45–51 (2019). Article CAS PubMed Google Scholar * Zhu, J., Xue, Y., Zhang, R., Ratchford, B. & Dong, G. Catalytic activation of unstrained
C(aryl)–C(alkyl) bonds in 2,2′ -methylenediphenols. _J. Am. Chem. Soc._ 144, 3242–3249 (2022). Article CAS PubMed PubMed Central Google Scholar * Wang, W. et al. Microwave-assisted
catalytic cleavage of C–C bond in lignin models by bifunctional Pt/CDC-SiC. _ACS Sustain. Chem. Eng._ 8, 38–43 (2019). Article CAS Google Scholar * Li, X. et al. Scission of C–O and C–C
linkages in lignin over RuRe alloy catalyst. _J. Energy Chem._ 67, 492–499 (2022). Article CAS Google Scholar * Dong, L. et al. Breaking the limit of lignin monomer production via
cleavage of interunit carbon–carbon linkages. _Chem_ 5, 1521–1536 (2019). Article CAS Google Scholar * Luo, Z. et al. Hydrothermally stable Ru/HZSM-5-catalyzed selective hydrogenolysis of
lignin-derived substituted phenols to bio-arenes in water. _Green Chem._ 18, 5845–5858 (2016). Article CAS Google Scholar * Weitkamp, J. Catalytic hydrocracking—mechanisms and
versatility of the process. _ChemCatChem_ 4, 292–306 (2012). Article CAS Google Scholar * Mirena, J. I. et al. Impact of the spatial distribution of active material on bifunctional
hydrocracking. _Ind. Eng. Chem. Res._ 60, 6357–6378 (2021). Article CAS Google Scholar * Chen, G. et al. Interfacial electronic effects control the reaction selectivity of platinum
catalysts. _Nat. Mater._ 15, 564–569 (2016). Article CAS PubMed Google Scholar * Huang, X., Korányi, T. I., Boot, M. D. & Hensen, E. J. Ethanol as capping agent and formaldehyde
scavenger for efficient depolymerization of lignin to aromatics. _Green Chem._ 17, 4941–4950 (2015). Article CAS Google Scholar * Sturgeon, M. R. et al. A mechanistic investigation of
acid-catalyzed cleavage of aryl-ether linkages: implications for lignin depolymerization in acidic environments. _ACS Sustain. Chem. Eng._ 2, 472–485 (2014). Article CAS Google Scholar *
Shuai, L. et al. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. _Science_ 354, 329–333 (2016). Article CAS PubMed Google Scholar *
Anderson, E. M. et al. Differences in S/G ratio in natural poplar variants do not predict catalytic depolymerization monomer yields. _Nat. Commun._ 10, 2033 (2019). Article PubMed PubMed
Central Google Scholar * Humbird, D. et al. _Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic
Hydrolysis of Corn Stover_ (National Renewable Energy Laboratory, 2011); https://www.nrel.gov/docs/fy11osti/47764.pdf * Zhang, C. & Wang, F. Catalytic lignin depolymerization to aromatic
chemicals. _Acc. Chem. Res._ 53, 470–484 (2020). Article CAS PubMed Google Scholar * Moses, C. A. Comparative evaluation of semi-synthetic jet fuels. _Contract_ 33415, 2299 (2008).
Google Scholar * Rahmes, T., Kinder, J. & Crenfeldt, G. Sustainable bio-derived synthetic paraffinic kerosene (Bio-SPK) jet fuel flights and engine tests program results. In _Proc. 9th
AIAA Aviation Technology_, _Integration and Operations Conference_ (_ATIO_) _and Aircraft Noise and Emissions Reduction Symposium_ (_ANERS_) 7002 (AIAA, 2009). * Enright, C. Aviation fuel
standard takes flight. _ASTM Stand. News_ 39, 5 (2011). Google Scholar * _Standard Specification for Aviation Turbine Fuel Containing Synthesized Hydrocarbons_. ASTM Standard D7566-14a
(ASTM international, 2014). * Zijlstra, D. S. et al. Extraction of lignin with high β-O-4 content by mild ethanol extraction and its effect on the depolymerization yield. _J. Vis. Exp_ 143,
58575 (2019). Google Scholar Download references ACKNOWLEDGEMENTS This research was supported financially by the Chemelot Institute for Science and Technology awarded to E.J.M.H. Z.L.
acknowledges support for the RCF experiments, TEA and LCA calculations from the National Natural Science Foundation of China (grant no. 52206236), the Natural Science Foundation of Jiangsu
Province (grant no. BK20220837) and the Fundamental Research Funds for the Central Universities (3203002211A1). J.T.B.d.B. and J.S.L. were supported by the Swiss National Science Foundation
through the National Competence Center Catalysis (grant no. 51NF40_180544). The contribution of A.R. was supported by the European Union’s Horizon 2020 research and innovation programme
under grant agreement no. 883753 (IDEALFUEL). AUTHOR INFORMATION Author notes * These authors contributed equally: Zhicheng Luo, Chong Liu, Alexandra Radu. AUTHORS AND AFFILIATIONS *
Laboratory of Inorganic Materials and Catalysis, Department of Chemical Engineering and Chemistry, Eindhoven University of Technology, Eindhoven, the Netherlands Zhicheng Luo, Alexandra
Radu, Davey F. de Waard, Panos D. Kouris, Michael D. Boot & Emiel J. M. Hensen * MOE Key Laboratory of Energy Thermal Conversion & Control, School of Energy and Environment,
Southeast University, Nanjing, China Zhicheng Luo, Yun Wang, Jun Xiao, Huiyan Zhang & Rui Xiao * State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the
Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian, China Chong Liu * Laboratory of Sustainable and Catalytic Processing, Institute of Chemical Sciences and Engineering, Ecole
Polytechnique Fédérale de Lausanne, Lausanne, Switzerland Jean T. Behaghel de Bueren & Jeremy S. Luterbacher Authors * Zhicheng Luo View author publications You can also search for this
author inPubMed Google Scholar * Chong Liu View author publications You can also search for this author inPubMed Google Scholar * Alexandra Radu View author publications You can also search
for this author inPubMed Google Scholar * Davey F. de Waard View author publications You can also search for this author inPubMed Google Scholar * Yun Wang View author publications You can
also search for this author inPubMed Google Scholar * Jean T. Behaghel de Bueren View author publications You can also search for this author inPubMed Google Scholar * Panos D. Kouris View
author publications You can also search for this author inPubMed Google Scholar * Michael D. Boot View author publications You can also search for this author inPubMed Google Scholar * Jun
Xiao View author publications You can also search for this author inPubMed Google Scholar * Huiyan Zhang View author publications You can also search for this author inPubMed Google Scholar
* Rui Xiao View author publications You can also search for this author inPubMed Google Scholar * Jeremy S. Luterbacher View author publications You can also search for this author inPubMed
Google Scholar * Emiel J. M. Hensen View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Z.L. and E.J.M.H. conceived the idea for lignin
depolymerization. Z.L. and A.R. performed the reactions of lignin and lignin model compounds. C.L. conducted the DFT calculations. Y.W. and J.X. carried out the TEA and LCA calculations with
guidance from H.Z. and R.X. P.D.K., M.D.B. and J.T.B.d.B., supervised by J.S.L., prepared the technical lignins. Z.L. and E.H. wrote the manuscript in close consultation with M.D.B.,
D.F.d.W., C.L., H.Z. and R.X. All authors contributed to the manuscript. CORRESPONDING AUTHORS Correspondence to Zhicheng Luo, Rui Xiao or Emiel J. M. Hensen. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Chemical Engineering_ thanks Changzhi Li, Joseph Samec, Yanqin Wang and the other,
anonymous, reviewer(s) for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. EXTENDED DATA SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Notes 1–5, Figs. 1–29, Tables 1–27 and references 1–40.
SUPPLEMENTARY DATA 1 Atomic coordinates of the optimized computational models. SOURCE DATA SOURCE DATA FIG. 2 Statistical source data. SOURCE DATA FIG. 3 Statistical source data. SOURCE DATA
FIG. 5 Statistical source data. SOURCE DATA FIG. 6 Statistical source data. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights
to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the
terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Luo, Z., Liu, C., Radu, A. _et al._ Carbon–carbon bond cleavage for a
lignin refinery. _Nat Chem Eng_ 1, 61–72 (2024). https://doi.org/10.1038/s44286-023-00006-0 Download citation * Received: 13 April 2023 * Accepted: 22 November 2023 * Published: 11 January
2024 * Issue Date: January 2024 * DOI: https://doi.org/10.1038/s44286-023-00006-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable
link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
