Molecular design of near-ir dyes with different surface energy for selective loading to the heterojunction in blend films
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT We have synthesized three silicon phthalocyanine dyes with different hydrophobic substituents in order to control surface energy in the solid state, aiming at selective loading of
the dyes into blend films of poly(3-hexylthiophene) (P3HT) and polystyrene (PS). These three dyes are differently located at P3HT domains, at P3HT/PS interface and at PS domains,
respectively, which are fully consistent with the locations predicted by the wetting coefficient derived from the surface energy of each material. SIMILAR CONTENT BEING VIEWED BY OTHERS
MOLECULAR ORIENTATION-DEPENDENT ENERGETIC SHIFTS IN SOLUTION-PROCESSED NON-FULLERENE ACCEPTORS AND THEIR IMPACT ON ORGANIC PHOTOVOLTAIC PERFORMANCE Article Open access 04 April 2023
SYNTHESIS OF MODEL HETEROJUNCTION INTERFACES REVEALS MOLECULAR-CONFIGURATION-DEPENDENT PHOTOINDUCED CHARGE TRANSFER Article Open access 20 August 2024 BAND GAP ENGINEERING IN BLENDED ORGANIC
SEMICONDUCTOR FILMS BASED ON DIELECTRIC INTERACTIONS Article 10 June 2021 INTRODUCTION Self-assembling materials play a central role in various functional materials where functional
molecules are precisely arranged on a nanometer scale in a required configuration. For this purpose, various approaches such as the self-assembled monolayer method1,2, Langmuir–Blodgett
technique3,4, layer-by-layer assembly5,6 and microphase separated assembly7,8 have been widely applied. In dye-sensitized solar cells, for example, dye molecules are self-assembled to the
surface of TiO2 nanoparticules, which are covered with iodide/triiodide redox electrolyte solution9,10. This interfacial location of dye molecules is of particular importance for efficient
charge generation because both electron and hole should be transferred from dye to TiO2 and the redox couple, respectively. Owing to the elegant molecular alignment, dye-sensitized solar
cells based on dye-modified TiO2 exhibit highly efficient photovoltaic performance. Similarly, dye sensitization of polymer solar cells can easily extend the light-harvesting wavelength
range11,12,13,14,15,16,17,18,19,20,21,22,23,24. This approach is simple and versatile and therefore can be easily applied to multi-colored sensitization by incorporating different dye
molecules at the same time that have complementary absorption bands in the near-IR region13. As is the case with dye-sensitized solar cells, the key to success in dye sensitization of
polymer solar cells is selective dye loading to the heterojunction of donor/acceptor interface of blend films because both of hole and electron in dye excitons should be transferred to donor
and acceptor materials, respectively, at the same time to generate photocurrent efficiently. Previously, we found that almost all the dye molecules are spontaneously located at the
polymer/fullerene interface by analyzing transient absorption dynamics of ternary blend films14. Furthermore, we have shown that such spontaneous dye segregation into the interface is partly
due to crystallization of polymer, which would expel dye molecules to disordered interface and partly due to intermediate surface energy of dye molecules, which can minimize interfacial
energy when dye molecules are located at the heterojunction15. Inspired by these findings, we have motivated to design new dye molecules with appropriate surface energy so that they are
spontaneously segregated at specified domains in blend films. In this study, we synthesized three silicon phthalocyanine derivatives with different axial ligands in order to study the
relationship between the surface energy of dye molecules and the loading location in polymer blend films. Here, we selected silicon phthalocyanine bis(tri-_n_-hexylsilyl oxide) (SiPc6) as a
standard dye molecule because it serves as an efficient dye sensitizer in polymer/fullerene solar cells as reported previously12,13,14,15,19. To reduce the surface energy, we synthesized
silicon 2,9,16,23-tetra-_tert_-butyl-29_H_,31_H_-phthalocyanine bis(tri-_n_-hexylsilyl oxide) (BuSiPc6), which has four _tert_-butyl groups attached to the phthalocyanine core and two
tri-_n_-hexylsilyl oxide groups in the axial ligand. To increase the surface energy, we synthesized silicon phthalocyanine bis(tribenzylsilyl oxide) (SiPcBz), which has two tribenzylsilyl
oxide groups in the axial ligand. On the other hand, we employed two amorphous polymers to avoid the dye segregation induced by crystallization of polymer matrix as mentioned above. One
polymer is regiorandom poly(3-hexylthiophene) (RRa-P3HT) with a small surface energy25,26,27. The other polymer is polystyrene (PS) with a large surface energy15,27, which is comparable to
that of phenyl-C61-butyric acid methyl ester (PCBM) films15,28. In order to address the location of dye molecules, we measured AFM images of these blend films before and after the films were
immersed in pentane solution, which can extract dye molecules selectively. RESULTS SURFACE ENERGY First, we measured contact angles of ultrapure water dropped on several neat films at room
temperature in order to evaluate the surface energy of each material by using the Neumann's equation29. As summarized in Table 1, the surface energy of matrix materials is evaluated to
be 21 mJ m−2 (RRa-P3HT) and 26 mJ m−2 (PS), which are consistent with previous reports15,25,26,27. On the other hand, the surface energy of SiPc derivatives is evaluated to be 20.5 mJ m−2
(BuSiPc6), 23 mJ m−2 (SiPc6) and 27 mJ m−2 (SiPcBz). In other words, the relationship of the surface energy is as follows: BuSiPc6 < SiPc6 < SiPcBz. The lowest surface energy of
BuSiPc6 is because the phthalocyanine core ring is surrounded by many hydrophobic alkyl chains: four peripheral _tert_-butyl groups and two tri-_n_-hexylsilyl oxide axial ligands. The
highest surface energy of SiPcBz is due to two π-electron rich axial ligands of tribenzylsilyl oxide. As a result, the surface energy of BuSiPc6 is close to that of RRa-P3HT, the surface
energy of SiPc6 is in between RRa-P3HT and PS and the surface energy of SiPcBz is close to that of PS. SURFACE SEGREGATION Next, we measured the dependence of the surface energy of
polymer/dye binary blend films on the dye concentration in order to examine how the surface energy of each material impacts the surface segregation in blend films fabricated by spincoating.
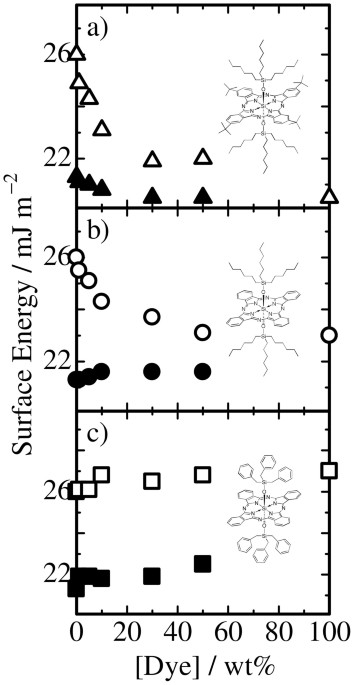
For BuSiPc6 doped in RRa-P3HT or PS films, as shown in Figure 1a, the surface energy of both blend films not linearly but rapidly decreased from that of polymer neat films and approached
that of BuSiPc6 with increasing dye fraction. This is because BuSiPc6 has the lowest surface energy among them and hence can minimize the interface energy at the air/film interface. For
SiPc6 doped in RRa-P3HT or PS films, as shown in Figure 1b, the surface energy of RRa-P3HT/SiPc6 remained the same up to 50 wt% dye fraction while that of PS/SiPc6 rapidly decreased to that
of SiPc6 with increasing dye fraction. This is because SiPc6 has surface energy in between that of RRa-P3HT and PS. For SiPcBz doped in RRa-P3HT or PS films, as shown in Figure 1c, the
surface energy of both blend films remained the same up to 50 wt% dye fraction and finally increased to that of SiPcBz with increasing dye fraction. This is because SiPcBz has the highest
surface energy among them and hence is likely to avoid the air surface. In summary, all these results show that the film surface is likely to be spontaneously covered by a material with the
lowest surface energy to reduce the interfacial energy at the air/film interface. In other words, the surface energy has critical impact on the surface segregation in binary blend
films30,31,32. DYE LOCATION IN BLENDS We now move onto the segregation of dye molecules in RRa-P3HT/PS/dye ternary blend films. In order to directly observe the location of dye molecules, we
measured AFM images of RRa-P3HT/PS/dye ternary blend films before and after immersing the film in pentane solution, which selectively dissolve dye molecules. As shown in Figures 2d and 2e,
RRa-P3HT/PS/BuSiPc6 ternary blend films exhibit a similar sea-island structure before and after the pentane treatment. In contrast, there is a clear difference in the cross-sectional line
profile before and after the treatment. Here, the cross-section profile is a most typical one in which the height of each domain is roughly evaluated as a difference between the average
heights of various sea or island domains and the substrate height. As shown in Figures 2g and 2h, the average height of the island domain is reduced from ~170 to ~120 nm while the height of
the sea domains remains the same after the pentane treatment. Consequently, minor components in the island domains are exposed like a pinholder. A slight dip is observed at the sea/island
interface after the treatment. As shown in Figures 2a and 2b, dye absorption bands at around 350 and 680 nm completely disappear after the pentane treatment, indicating that all the dye
molecules are selectively extracted into pentane solution. After the film is immersed in cyclohexane for 3 min, which dissolves PS selectively as reported previously15,27,31, the sea domains
completely disappear while the island domains still remain as shown in Figures 2f and 2i. We therefore conclude that the majority of BuSiPc6 molecules are located in the RRa-P3HT island
domains in RRa-P3HT/PS/BuSiPc6 ternary blend films. As shown in Figures 3d and 3e, RRa-P3HT/PS/SiPc6 ternary blend films also exhibit a similar sea-island structure before and after the
pentane treatment. Interestingly, a ring structure is additionally observed at the sea/island interface. In the cross-sectional line profile, as shown in Figures 3g and 3h, the height of the
ring portion is higher than that of the other domains before the treatment but sharply depressed after the treatment. No distinct change is found for the other sea/island domains before and
after the treatment. As shown in Figures 3a and 3b, dye absorption bands at around 350 and 680 nm completely disappear after the pentane treatment, again indicating that all the dye
molecules are selectively extracted into pentane solution. We therefore conclude that the majority of SiPc6 molecules are located at the RRa-P3HT/PS interface in RRa-P3HT/PS/SiPc6 ternary
blend films. As shown in Figures 4d and 4e, RRa-P3HT/PS/SiPcBz ternary blend films also exhibit a similar sea-island structure before and after the pentane treatment. In the cross-sectional
line profile, as shown in Figures 4g and 4h, the height of the sea domains is higher than that of the island domains before and after the treatment but slightly reduced from ~270 to ~250 nm
after the treatment while the height of the island domains remains the same before and after the treatment. In addition, several dips and a slight dip are observed in the sea domains and at
the sea/island interface, respectively, after the treatment. As shown in Figures 4a and 4b, dye absorption bands at around 350 and 680 nm decreases by 75% but still observed even after the
pentane treatment, indicating that 75% of dyes are extracted into pentane solution but 25% of dyes still remains in the blend film. After the cyclohexane treatment, as shown in Figures 4c
and 4i, the dye absorption bands completely disappear and no height change is observed for the RRa-P3HT domains. These findings indicate that the remaining dye molecules (25%) are removed
from the PS domains. We therefore conclude that 75% of SiPcBz molecules are located at the surface of the PS sea domains and the other 25% of SiPcBz are located inside the PS sea domains or
the interface in RRa-P3HT/PS/SiPcBz ternary blend films. DISCUSSION For quantitative discussion, we roughly estimate the dye fraction at each location in ternary blend films from the
difference in the AFM images and the absorption spectra before and after the pentane treatment. Details of the estimation are described in the Supplementary Information. In
RRa-P3HT/PS/BuSiPc6 ternary blend films, as summarized in Table 2, 87% of BuSiPc6 are located at RRa-P3HT domains and the rest of them are located at RRa-P3HT/PS interface. In
RRa-P3HT/PS/SiPc6 ternary blend films, 90% of SiPc6 are located at RRa-P3HT/PS interface and the rest of them are located at RRa-P3HT domains. In RRa-P3HT/PS/SiPcBz ternary blend films, 93%
of SiPcBz are located at PS domains and the rest of them are located at RRa-P3HT/PS interface. Although these are rough estimation, it can be safely said that the majority of dye molecules
are spontaneously located at RRa-P3HT domains in RRa-P3HT/PS/BuSiPc6, at RRa-P3HT/PS interface in RRa-P3HT/PS/SiPc6, at PS domains in RRa-P3HT/PS/SiPcBz ternary blend films. These dye
allocations can be predicted in terms of the surface energy of each material as discussed below. As reported previously, the interfacial energy is one of the key parameters for such
spontaneous segregation of the third material in ternary blend films15,33,34,35,36. Sumita et al. introduced a key parameter of the wetting coefficient (ω) evaluated from the surface energy
of each component material to predict allocation of fillers doped as the third material in polymer/polymer binary blends. Here, the surface energy of a matrix polymer A is lower than that of
the other matrix polymer B (γA < γB). The third material filler will be located at polymer A domains when ω > 1, at the A/B interface when −1 < ω < 1 and at polymer B domains
when ω < −1. On the basis of this simple model, we calculate the wetting coefficient of dye (ωdye) to predict the dye location in RRa-P3HT/PS/dye ternary blend films. As a result, dye
molecules are predicated to be located at RRa-P3HT domains (BuSiPc6, ωdye = 1.2), at the RRa-P3HT/PS interface (SiPc6, ωdye = 0.2) and at PS domains (SiPcBz, ωdye = −1.4). These locations
predicted are, as shown in Table 2, fully consistent with the dye location revealed by the AFM measurements. In conclusion, we demonstrated that dye molecules can be selectively loaded into
each domain or interface in polymer/polymer blend films by careful designing the surface energy of dye with appropriate axial ligands. We thus emphasize that our finding can provide a
molecular design perspective for selective loading of small molecules in blend films fabricated even by solution processes such as spin-coating. In particular, this strategy is useful for
developing new dye molecules employed in ternary blend polymer solar cells, in which interfacial dye loading is the key to success for efficient dye sensitization. METHODS DYE SYNTHESIS The
reaction schemes and the chemical structures of SiPc derivatives with various axial groups employed in this study are summarized in the Supporting Information. BuSiPc6: A mixture of silicon
2,9,16,23-tetra-_tert_-butyl-29_H_,31_H_-phthalocyanine dihydroxide (BuSiPc(OH)2) (115 mg), chlorotri-_n_-hexylsilane (250 μL) and dry pyridine (15 mL) was refluxed for 6 h. After the
solution obtained had been allowed to cool, the solvent was evaporated and chloroform was added to the residue. The solution was washed with saturated NaCl solution and then dried over
MgSO4. After evaporation of the solvent, the residue was purified by silica gel column chromatography (toluene/hexane = 1/1 (v/v) as eluent) to afford BuSiPc6 (76.8 mg) as a bluish-green
solid (yield = 38%). UV–visible (toluene): λmax 669 nm (ε = 2.6 × 105 M−1 cm−1); 1H NMR (400 MHz, CDC13):δ = 9.61–9.64 (m, α-Pc, 8H), 8.35–8.37 (m, β-Pc, 4H), 1.82–1.83 (m, tBu, 36H),
0.67–0.71 (m, ε-CH2, 12H), 0.47–0.51 (t, CH3, 18H), 0.35–0.42 (m, δ-CH2, 12H), 0.03–0.04 (m, γ-CH2, 12H), −1.23– −1.29 (m, β-CH2, 12H), −2.40– −2.45 (m, α-CH2, 12H). MALDI-TOF: _m/z_ 1363.9
(M + H). Calcd for C84H126N8O2Si3: _m/z_ 1364.2. SiPc6: A mixture of silicon phthalocyanine dihydroxide (SiPc(OH)2) (85 mg), chlorotrihexylsilane (200 μL) and dry pyridine (10 mL) was
refluxed for 6 h. After the solution obtained had been allowed to cool, the solvent was evaporated and chloroform was added to the residue. The solution was washed with saturated NaCl
solution and then dried over MgSO4. After evaporation of the solvent, the residue was purified by silica gel column chromatography (toluene/hexane = 1/1 (v/v) as eluent) to afford SiPc6 (106
mg) as a blue solid (yield = 62.8%). UV–visible (toluene): λmax 668 nm (ε = 3.0 × 105 M−1 cm−1); 1H NMR (400 MHz, CDC13):δ = 9.63 (m, 3,6-Pc, 8H), 8.31 (m, 4,5-Pc, 8H), 0.81 (m, ε-CH2,
12H), 0.71 (t, CH3, 18H), 0.36 (m, δ-CH2, 12H), 0.02 (m, γ-CH2, 12H), −1.28 (m, β-CH2, 12H), −2.45 (m, α-CH2, 12H). MALDI-TOF: _m/z_ 1139.7 (M + H). Calcd for C68H94N8O2Si3: _m/z_ 1139.8.
SiPcBz: A mixture of silicon phthalocyanine dihydroxide (SiPc(OH)2) (99 mg), chlorotribenzylsilane (270 mg) and dry pyridine (15 mL) was refluxed for 5 h. After the solution obtained had
been allowed to cool, the solvent was evaporated and chloroform was added to the residue. The solution was washed with saturated NaCl solution and then dried over MgSO4. After evaporation of
the solvent, the residue was purified by silica gel column chromatography (dichloromethane/acetone = 30/1 (v/v) as eluent) to afford SiPcBz (126 mg) as a bluish-green solid (yield = 62%).
UV−visible (toluene): λmax 675 nm (ε = 3.0 × 105 M−1 cm−1); 1H NMR (400 MHz, CDC13):δ = 9.58–9.63 (m, 3,6-Pc, 8H), 8.21–8.37 (m, 4,5-Pc, 8H), 6.45–6.53 (m, 4-Ph, 6H), 6.28–6.39 (m, 3,5-Ph,
12H), 4.81–4.98 (m, 2,6-Ph, 12H), −0.96 (m, CH2, 12H). MALDI-TOF: _m/z_ 1175.4 (M + H). Calcd for C74H58N8O2Si3: _m/z_ 1175.6. SAMPLE FABRICATIONS The quartz glass or glass substrates were
cleaned by ultrasonication in toluene, acetone and ethanol each for 15 min, dried with N2 and cleaned with a UV–O3 cleaner for 30 min. For contact angle measurements, neat films (~100 nm) of
RRa-P3HT (Aldrich, head-to-head:head-to-tail = 1:1, _M_w = 90600), PS (Scientific Polymer Products, _M_w = 22000) and SiPc dyes were individually spin-coated on the cleaned quartz glass.
For the dye-concentration dependence measurement, RRa-P3HT and PS films doped with dyes at various concentrations were prepared by spin-coating on the glass substrate from a chlorobenzene
solution to give blend films with a thickness of about 80 nm. For the AFM measurements, RRa-P3HT/PS/dye ternary blend films were spin-coated from a chlorobenzene solution at a weight ratio
of 2:2:1 (10:10:5 mg mL−1). MEASUREMENTS For materials characterization, 1H NMR spectra were recorded on a JEOL EX-400 spectrometer at 400 MHz by using CDCl3 as solvent and MALDI-TOF mass
spectra were obtained on a Bruker Ultraflex MALDI/TOF mass spectrometer with dithranol as matrix. To evaluate the surface energy of each material, contact angle θX was measured on the neat
film of material X using ultrapure water at room temperature. The surface energy γX can be evaluated from θX by the Neumann's equation combined with the Young's equation15,29. From
the surface energy γX of each material, the interface energy γAB between A and B materials was evaluated by the Neumann's equation15,29. The wetting coefficient ωC of material C in a
matrix blend of A and B can be evaluated from the interface energy: ωC = (γBC − γAC)/γAB29,33. Therefore, the dye molecule location can be predicted from the wetting coefficient ωC in a
blend film. Absorption spectra of the blend films were measured with a spectrophotometer (Hitachi, UV-3500). Film morphology and thickness of blend films were measured with AFM (Shimadzu,
SPM-9600) in the contact mode. The AFM images were observed for as-cast films, SiPc dye-removed films and PS-removed films. The SiPc dye-removed films were obtained by immersing the as-cast
films into pentane solution (Wako) for 1 min. The PS-removed films were obtained by immersing the SiPc dye-removed films into cyclohexane (Nacalai Tesque) for 3 min. REFERENCES * Sagiv, J.
Organized monolayers by adsorption. 1. Formation and structure of oleophobic mixed monolayers on solid surfaces. J. Am. Chem. Soc. 102, 92–98 (1980). Article CAS Google Scholar *
Schreiber, F. Structure and growth of self-assembling monolayers. Prog. Surf. Sci. 65, 151–256 (2000). Article CAS ADS Google Scholar * Kuhn, H. & Möbius, D. Systems of monomolecular
layers—assembling and physico-chemical behavior. Angew. Chem., Int. Ed. 10, 620–637 (1971). Article CAS Google Scholar * Fujihira, M. & Yamada, H. Molecular photodiodes consisting of
unidirectionally oriented amphipathic acceptor–sensitizer–donor triads. Thin Solid Films 160, 125–132 (1988). Article CAS ADS Google Scholar * Lvov, Y., Decher, G. & Möhwald, H.
Assembly, structural characterization and thermal behavior of layer-by-layer deposited ultrathin films of poly(vinyl sulphate) and poly(allylamine). Langmuir 9, 481–486 (1993). Article CAS
Google Scholar * Ariga, K. et al. Layer-by-layer nanoarchitectonics: invention, innovation and evolution. Chem. Lett. 43, 36–68 (2014). Article CAS ADS Google Scholar * Fasolka, M. J.
& Mayes, A. M. Block copolymer thin films: physics and applications. Annu. Rev. Mater. Res. 31, 323–355 (2001). Article CAS ADS Google Scholar * Albert, J. N. L. & Epps, T. H.,
III Self-assembly of block copolymer thin films. Mater. Today 13, 24–33 (2010). Article CAS Google Scholar * Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C: Photochem.
Rev. 4, 145–153 (2003). Article Google Scholar * Ahmad, S., Guillén, E., Kavan, L., Grätzel, M. & Nazzeruddin, K. Metal free sensitizer and catalyst for dye sensitized solar cells.
Energy Environ. Sci. 6, 3439–3466 (2013). Article CAS Google Scholar * Peet, J., Tamayo, A. B., Dang, X.-D., Seo, J. H. & Nguyen, T.-Q. Small molecule sensitizers for near-infrared
absorption in polymer bulk heterojunction solar cells. Appl. Phys. Lett. 93, 163306 (2008). Article ADS Google Scholar * Honda, S., Nogami, T., Ohkita, H., Benten, H. & Ito, S.
Improvement of the light-harvesting efficiency in polymer/fullerene bulk heterojunction solar cells by interfacial dye modification. ACS Appl. Mater. Interfaces 1, 804–810 (2009). Article
CAS Google Scholar * Honda, S., Ohkita, H., Benten, H. & Ito, S. Multi-colored dye sensitization of polymer/fullerene bulk heterojunction solar cells. Chem. Commun. 46, 6596–6598
(2010). Article CAS Google Scholar * Honda, S., Yokoya, S., Ohkita, H., Benten, H. & Ito, S. Light-harvesting mechanism in polymer/fullerene/dye ternary blends studied by transient
absorption spectroscopy. J. Phys. Chem. C 115, 11306–11317 (2011). Article CAS Google Scholar * Honda, S., Ohkita, H., Benten, H. & Ito, S. Selective dye loading at the heterojunction
in polymer/fullerene solar cells. Adv. Energy Mater. 1, 588–598 (2011). Article CAS Google Scholar * Kubo, Y. et al. Near-infrared absorbing boron-dibenzopyrromethenes that serves as
light-harvesting sensitizers for polymeric solar cells. Org. Lett. 13, 4574–4577 (2011). Article CAS Google Scholar * Ameri, T. et al. Performance enhancement of the P3HT/PCBM solar cells
through NIR sensitization using a small-bandgap polymer. Adv. Energy Mater. 2, 1198–1202 (2012). Article CAS Google Scholar * Yamamoto, S. & Kimura, M. Extension of light-harvesting
area of bulk-heterojunction solar cells by cosensitization with ring-expanded metallophthalocyanines fused with fluorene skeletons. ACS. Appl. Mater. Interface 5, 4367–4373 (2013). Article
CAS Google Scholar * Xu, H., Wada, T., Ohkita, H., Benten, H. & Ito, S. Dye sensitization of polymer/fullerene solar cells incorporating bulky phthalocyanines. Electrochim. Acta 100,
214–219 (2013). Article CAS Google Scholar * Ito, S. et al. Development of polymer blend solar cells composed of conjugated donor and acceptor polymers. J. Photopolym. Sci. Technol. 26,
175–180 (2013). Article CAS Google Scholar * Huang, J.-S. et al. Polymer bulk heterojunction solar cells employing Förster resonance energy transfer. Nat. Photon. 7, 479–485 (2013).
Article CAS ADS Google Scholar * Chen, Y.-C., Hsu, C.-Y., Lin, R. Y.-Y., Ho, K.-C. & Lin, J. T. Materials for the active layer of organic photovoltaics: ternary solar cell approach.
ChemSusChem 6, 20–35 (2013). Article CAS Google Scholar * Ameri, T., Khoram, P., Min, J. & Brabec, C. J. Organic ternary solar cells: a review. Adv. Mater. 25, 4245–4266 (2013).
Article CAS Google Scholar * Yang, L., Yan, L. & You, W. Organic solar cells beyond one pair of donor–acceptor: ternary blends and more. J. Phys. Chem. Lett. 4, 1802–1810 (2013).
Article CAS Google Scholar * Robinson, L., Isaksson, J., Robinson, N. D. & Berggren, M. Electrochemical control of surface wettability of poly(3-alkylthiophenes). Surf. Sci. Lett.
600, L148–L152 (2006). Article CAS ADS Google Scholar * Jaczewska, J. et al. Swelling of poly(3-alkylthiophene) films exposed to solvent vapors and humidity: evaluation of solubility
parameters. Synth. Met. 157, 726–732 (2007). Article CAS Google Scholar * Jaczewska, J., Budkowski, A., Bernasik, A., Moons, E. & Rysz, J. Polymer vs solvent diagram of film
structures formed in spin-cast poly(3-alkylthiophene) blends. Macromolecules 41, 4802–4810 (2008). Article CAS ADS Google Scholar * Nilsson, S., Bernasik, A., Budkowski, A. & Moons,
E. Morphology and phase segregation of spin-casted films of polyfluorene/PCBM blends. Macromolecules 40, 8291–8301 (2007). Article CAS ADS Google Scholar * Li, D. & Neumann, A. W. A
reformulation of the equation of state for interfacial tensions. J. Colloid Interface Sci. 137, 304–307 (1990). Article CAS ADS Google Scholar * Jones, R. A. L. & Richards, R. W.
Polymers at surfaces and interfaces (Cambridge University Press, Cambridge, 1999). * Walheim, S., Ramstein, M. & Steiner, U. Morphologies in ternary polymer blends after spin-coating.
Langmuir 15, 4828–4836 (1999). Article CAS Google Scholar * Orimo, A. et al. Surface segregation at the aluminum interface of poly(3-hexylthiophene)/fullerene solar cells. Appl. Phys.
Lett. 96, 043305 (2010). Article ADS Google Scholar * Sumita, M., Sakata, K., Asai, S., Miyasaka, K. & Nakagawa, H. Dispersion of fillers and the electrical conductivity of polymer
blends filled with carbon black. Polym. Bull. 25, 265–271 (1991). Article CAS Google Scholar * Wu, M. & Shaw, L. Electrical and mechanical behaviors of carbon nanotube-filled polymer
blends. J. Appl. Polym. Sci. 99, 477–488 (2006). Article CAS Google Scholar * Ma, C. G., Zhang, M. Q. & Rong, M. Z. Morphology prediction of ternary polypropylene composites
containing elastomer and calcium carbonate nanoparticles filler. J. Appl. Polym. Sci. 103, 1578–1584 (2007). Article CAS Google Scholar * Cheng, T. W., Keskkula, H. & Paul, D. R.
Property and morphology relationships for ternary blends of polycarbonate, brittle polymers and an impact modifier. Polymer 33, 1606–1619 (1992). Article CAS Google Scholar Download
references ACKNOWLEDGEMENTS This work was partly supported by the FIRST program (Development of Organic Photovoltaics toward a Low-Carbon Society: Pioneering Next Generation Solar Cell
Technologies and Industries via Multi-manufacturer Cooperation) and the JST PRESTO program (Photoenergy Conversion Systems and Materials for the Next Generation Solar Cells). AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Department of Polymer Chemistry, Graduate School of Engineering, Kyoto University, 615-8510, Katsura, Nishikyo, Kyoto, Japan Huajun Xu, Takaaki Wada,
Hideo Ohkita, Hiroaki Benten & Shinzaburo Ito * Japan Science and Technology Agency (JST), PRESTO, 4-1-8 Honcho Kawaguchi, 332-0012, Saitama, Japan Hideo Ohkita Authors * Huajun Xu View
author publications You can also search for this author inPubMed Google Scholar * Takaaki Wada View author publications You can also search for this author inPubMed Google Scholar * Hideo
Ohkita View author publications You can also search for this author inPubMed Google Scholar * Hiroaki Benten View author publications You can also search for this author inPubMed Google
Scholar * Shinzaburo Ito View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS H.O. and S.I. conceptualized and directed the research project.
H.X. and T.W. synthesized the SiPc dyes and performed measurements. H.X. wrote the original manuscript with the assistance of H.O. All authors discussed the results and commented on the
manuscript. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION SupplementaryInformation
RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the
article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain
permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Xu, H., Wada, T., Ohkita, H. _et al._ Molecular Design of Near-IR Dyes with Different Surface Energy for Selective Loading to the Heterojunction in Blend Films.
_Sci Rep_ 5, 9321 (2015). https://doi.org/10.1038/srep09321 Download citation * Received: 28 October 2014 * Accepted: 02 March 2015 * Published: 20 March 2015 * DOI:
https://doi.org/10.1038/srep09321 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
