High-Throughput Non-Contact Vitrification of Cell-Laden Droplets Based on Cell Printing
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Cryopreservation is the most promising way for long-term storage of biological samples e.g., single cells and cellular structures. Among various cryopreservation methods, vitrification is
advantageous by employing high cooling rate to avoid the formation of harmful ice crystals in cells. Most existing vitrification methods adopt direct contact of cells with liquid nitrogen to
obtain high cooling rates, which however causes the potential contamination and difficult cell collection. To address these limitations, we developed a non-contact vitrification device
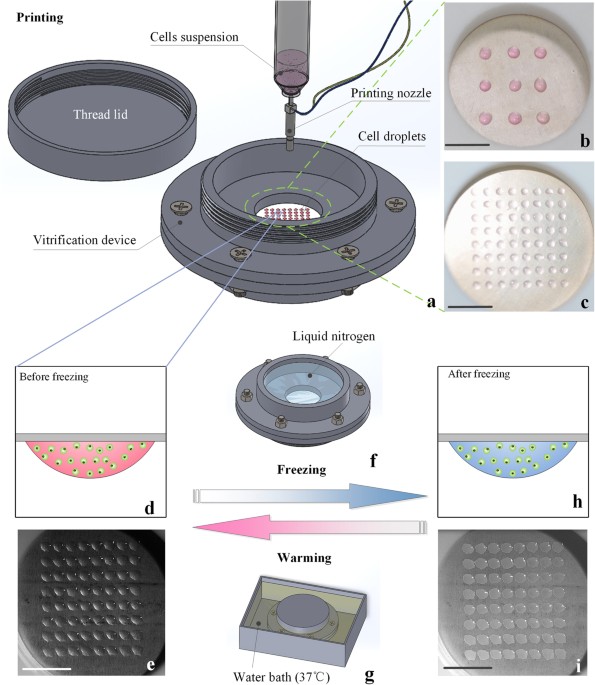
based on an ultra-thin freezing film to achieve high cooling/warming rate and avoid direct contact between cells and liquid nitrogen. A high-throughput cell printer was employed to rapidly
generate uniform cell-laden microdroplets into the device, where the microdroplets were hung on one side of the film and then vitrified by pouring the liquid nitrogen onto the other side via
boiling heat transfer. Through theoretical and experimental studies on vitrification processes, we demonstrated that our device offers a high cooling/warming rate for vitrification of the
NIH 3T3 cells and human adipose-derived stem cells (hASCs) with maintained cell viability and differentiation potential. This non-contact vitrification device provides a novel and effective
way to cryopreserve cells at high throughput and avoid the contamination and collection problems.
Cryopreservation has been widely used for long-term preservation of cells, aggregates, tissues and organs. Careful cryopreservation maintains the functional properties (e.g., proliferation
and differentiation) and genetic characteristics of cellular constructs and protects them from infection, allowing them to be available for off-the-shelf use in both research and clinical
applications1,2. As for now, a variety of cryopreservation methods have been developed including slow freezing and vitrification3,4,5, among which vitrification offers several advantages
over slow freezing in terms of maintaining viability, genetic profiles and cytoskeletal structure of cells6,7. Thus, vitrification holds great promise for applications in biomedical fields
such as reproduction, tissue engineering and organ transplantation to achieve better cryopreservation outcome8,9,10.
The advantages of vitrification attribute to its high cooling rate to transform cell suspensions with cryoprotectant (CPA) from the aqueous phase to a glass state directly11, which can avoid
the cryoinjury induced by intracellular ice formation during freezing process12,13. Besides, the high cooling rate can allow using low CPA concentrations in freezing, thereby reducing the
potential CPA toxicity and osmotic damage of cells5,14. Various methods have been developed to increase cooling rate, such as open pulled straw (OPS)15, quartz micro capillary (QMC)14,
cryotop16 and droplet-based vitrification5,17,18. For instance, in a droplet-based vitrification method, cell-laden droplets with sizes down to nanoliter were ejected (e.g., by cell printer)
directly into liquid nitrogen at high throughput (~0.14 nL per droplet, 1,000 droplets/s5). Due to the small volume of the droplets, a significant high cooling rate was achieved at a
relatively low CPA concentration (e.g., 4.5% ectoine17), which can potentially improve the efficiency of freezing and reduce the risk of toxicity from high CPA concentrations5,18. With this
method, a variety of cells, such as red blood cells17, hepatocytes cells5, oocytes19 and even whole blood20 have been successfully preserved. However, these approaches are associated with
several limitations. Firstly, most methods (i.e., OPS, QMC, cryotop and droplet-based vitrification) have a high contamination risk with pathogenic agents due to a direct contact of the
cells with non-sterile liquid nitrogen21,22. For instance, bone marrow has been found to be contaminated by hepatitis virus in liquid nitrogen during cryopreservation, which caused patient
deaths after transplantation23. Although the vapour phase storage system was developed to avoid the bio-pollutions in liquid nitrogen during storage24,25, it is still challenging to avoid
the potential contamination between bio-specimens and liquid nitrogen during freezing and thawing processes. Although “straw in straw” method has been employed to reduce the risk, its
cooling rate was significantly decreased due to increased thermal barrier (~400 °C/min)26. Secondly, cryotop and droplet-based methods involve the potential issue of sample loss due to an
inefficient cell collection. For example, during droplet-based vitrification, the liquid nitrogen forms nitrogen vapour layer around the droplet that pushes droplets to move around due to
Leidenfrost phenomenon, thus making it difficult for an efficient cell collection27. Additionally, the liquid nitrogen vapour blanket significantly decreases the cooling rate of
droplets27,28. Although precooled substrates have been used to avoid the Leidenfrost phenomenon during freezing process18, the temperature of substrate (~193 K) is not low enough (at least
below glass transition temperature of the droplet, i.e.,
