Noisy decision thresholds can account for suboptimal detection of low coherence motion
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Noise in sensory signals can vary over both space and time. Moving random dot stimuli are commonly used to quantify how the visual system accounts for spatial noise. In these
stimuli, a fixed proportion of “signal” dots move in the same direction and the remaining “noise” dots are randomly replotted. The _spatial_ coherence, or proportion of signal versus noise
dots, is fixed across time; however, this means that little is known about how temporally-noisy signals are integrated. Here we use a stimulus with low _temporal_ coherence; the signal
direction is only presented on a fraction of frames. Human observers are able to reliably detect and discriminate the direction of a 200 ms motion pulse, even when just 25% of frames within
the pulse move in the signal direction. Using psychophysical reverse-correlation analyses, we show that observers are strongly influenced by the number of near-target directions spread
throughout the pulse and that consecutive signal frames have only a small additional influence on perception. Finally, we develop a model inspired by the leaky integration of the responses
of direction-selective neurons, which reliably represents motion direction and which can account for observers’ sub-optimal detection of motion pulses by incorporating a noisy decision
threshold. SIMILAR CONTENT BEING VIEWED BY OTHERS MOTION DIRECTION IS REPRESENTED AS A BIMODAL PROBABILITY DISTRIBUTION IN THE HUMAN VISUAL CORTEX Article Open access 22 November 2023 BETTER
THAN EXPECTED PERFORMANCE EFFECT DEPENDS ON THE SPATIAL LOCATION OF VISUAL STIMULUS Article Open access 02 January 2025 PERCEPTUAL DECISIONS AND OCULOMOTOR RESPONSES RELY ON TEMPORALLY
DISTINCT STREAMS OF EVIDENCE Article Open access 01 March 2022 INTRODUCTION Sensory systems must continually detect weak signals against a background of noise and discriminate or identify
the properties of those signals. In the domain of visual motion processing, perceptual and neuronal sensitivity has been characterised in detail by manipulating the coherence of random dot
stimuli1,2. In these stimuli, a set of signal dots moves in a single direction, while the remaining noise dots move randomly. As coherence - the proportion of signal dots - is increased,
neuronal responses scale near-linearly3 and tuning bandwidths are slightly reduced4, while perceptual choices become more accurate and more rapid1,5. In most studies, although the specific
dots belonging to the coherent signal population may change over time, the overall signal coherence remains constant6. However, sensory systems must also overcome noisy signals that vary
over time. For example, a school of fish has strong local motion cues associated with individual animals, but to herd the population, a predator may need to determine its average velocity
over time, despite continual fluctuations in the velocity from moment to moment. How does the visual system incorporate temporal variability and over what timescales are noisy sensory
signals integrated? Reverse correlation studies, which rapidly change a single stimulus feature such as position, orientation or direction, have shown that small deviations in stimulus
probability over time influence both neuronal responses and perception7,8. A direction-selective, motion-sensitive neuron in the middle temporal cortical area (MT) is more likely to fire
action potentials after a stimulus moves in its preferred direction9. This is true even if the individual period of motion lasts just 10–20 ms and is embedded in a sequence of non-preferred
directions. Similarly, when viewing a random sequence of motion that changes every 20 ms, human observers are not consciously aware of the direction at all points in time, but are more
likely to report that upwards motion occurred immediately after near-upwards motion is presented10,11. Critically, in this scenario, most individual upwards periods go undetected. Using
reverse correlation techniques, it is thus possible to map out neuronal and perceptual tuning curves, which quantify how strongly each direction influences a neuron’s or human’s response.
Notably, a strong signal may be rendered undetectable because of its brief presentation, or because it is embedded in a sequence of temporally-incoherent noise. While it may be intuitive
that a signal becomes easier to detect as its temporal coherence is increased, the timescales and range of directions over which motion information is integrated remain unclear and little is
known about how temporal coherence affects perception. In order to examine the perceptual integration of direction information over time, we designed a sequential detection-discrimination
task requiring judgments about the presence and direction of brief, noisy motion pulses embedded in a sequence of continually changing motion directions. We show that human observers can
reliably extract direction information from stimuli with low temporal coherence, even when as few as 5 frames, or 12.5% of individual dots within a 20 frame, 200 ms motion pulse, move in a
target direction. Observers are strongly influenced by uninformative, near-target motion directions and weakly influenced by consecutive near-target stimuli. These results are captured by a
model in which a “signal-present” response is generated whenever the leaky integration of the filtered responses of a population of direction-selective neurons exceeds a fixed threshold.
Although sensory noise that differs between observers can account for variability in each observer’s detection performance, it cannot account for biased responses. Critically, we show that
random noise in each observer’s perceptual threshold leads to biases and reduced precision that can account for their suboptimal detection performance. METHODS PARTICIPANTS Experiments were
approved by the Monash Human Research Ethics Committee and carried out in accordance with the National Statement on Ethical Conduct in Human Research and the Australian Code for the
Responsible Conduct of Research. Four participants (ages 20–33; 2 female, 2 male) took part in the study after giving informed consent. Participants wore their standard optical correction,
if required. Two participants were authors on the study (NP, JV) and the other participants were volunteers, naïve with respect to the aims of the study (P1, P2). STIMULUS AND PARADIGM
Stimuli were generated using Matlab (The Mathworks, Natick, MA) and the Psychophysics Toolbox extensions12 and were viewed binocularly on a Sony Multiscan G500 monitor (1280 × 960 pixels;
viewable area 400 × 300 mm; refresh rate 100 Hz; viewing distance 670 mm). To minimise eye movements, participants used a chin-rest and optional forehead-rest and were asked to fixate a
central white cross subtending 0.4°, surrounded by a black circle of 0.8° diameter. We have previously found that the fixation cross minimizes eye movements with similar stimuli13. Stimuli
comprised 50 anti-aliased white dots of diameter 0.1° presented on a black background, limited to a 5° diameter circular aperture centered on the fixation cross. Individual dots were visible
for 2 frames; on each screen refresh, 50% of the dots moved 0.12° (12 °/s) in the same _signal_ direction and the remaining _noise_ dots were replotted randomly maintaining uniform dot
density. The dots defined as _signal_ and _noise_ alternated on every frame. In addition to fixing the spatial coherence of our stimulus at 50%6,14,15, we also precisely controlled its
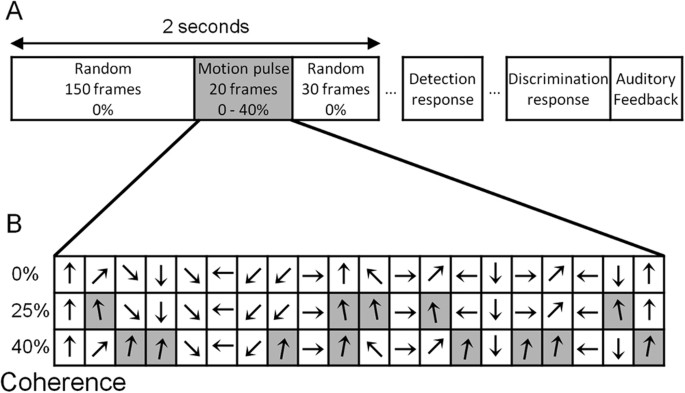
temporal structure. Each trial lasted 2 seconds (200 frames) and contained 3 periods: a 150 frame period of random motion (0% temporal coherence); a 20 frame motion _pulse_ with variable
temporal coherence (0, 25 or 40%); and a 30 frame period of 0% coherence motion (Fig. 1). We define temporal coherence as the percentage of frames in which the signal dots move in the same,
pre-defined _target_ direction. The target direction for a trial was always chosen from 85 or 95°, i.e. 5° left or right relative to vertical. On non-target frames, the stimulus direction
was chosen randomly from a quantized, uniformly distributed set of 24 directions spanning 0 to 345° in 15° steps. During the motion pulse, frames with the target direction were randomly
distributed throughout the 20 frame pulse; e.g. 25% temporal coherence means 5 randomly chosen 20 frames have their signal direction set to the trial’s target direction, while the remaining
frames have a signal direction randomly chosen from the quantized distribution (Fig. 1). Due to the 2 frame dot lifetime and low temporal coherence of the motion pulse, only 12.5–20% of
individual dots in the motion pulse moved in the target direction, thus motion pulses were extremely difficult to identify for untrained observers. Our previous studies have demonstrated
that stimuli with low temporal coherence can strongly influence direction perception16, however, to overcome learning effects, prior to recording data, participants trained for up to 2 hours
until their psychophysical performance reached a plateau. The initial training used longer dot lifetimes and higher temporal coherence, ensuring that all participants knew when to expect
the motion pulse and that they were specifically attending to pulses with directions slightly left or right of vertical. In our pilot studies, we varied spatial coherence, dot displacement,
trial duration and pulse duration. Ultimately, we are interested in how non-target directions influence perceptual decisions, thus we chose stimulus parameters that make the task extremely
difficult. As the task becomes easier, perceptual tuning curves become less informative as they approach a uniform distribution, with no non-target directions affecting pulse detection. Each
participant completed 25–51 blocks of 64 trials (JV – 28; NP – 31; P1 – 51; P2 – 25). Within each block, 32 trials had no pulse (i.e. 0% temporal coherence) and 16 trials had motion pulses
with 25 and 40% coherence. Participants made a sequential detection-discrimination response on each trial (Fig. 1). First, they pressed a button to indicate the presence or absence of a
coherent motion pulse. Subsequently, regardless of their detection response, they pressed a button to indicate whether the pulse direction was left or right of the vertical category
boundary. After both responses had been given, auditory feedback was provided for both detection and discrimination responses, in the form of a beep for correct responses. BEHAVIOURAL
PERFORMANCE For all pulse coherences, we determined the proportion of trials in which the subject indicated that the pulse was present or absent. In analyzing detection responses, we ignored
the accuracy of the subsequent discrimination response. Detection performance was quantified using the _d_′ sensitivity measure, based on the difference between the z-transformed Hit and
False-Detection rates: _d_′ = _z_(_Hit_) − _z_(_FD_). On pulse-present trials (i.e. 25 and 40% coherence), discrimination performance was determined separately for hits (correct detection)
and misses (failed detection). PERCEPTUAL TUNING CURVES Tasks with rapidly changing stimulus parameters are amenable to a probabilistic analysis, in which stimulus probabilities at different
points in time preceding a behavioural or neuronal response can be determined10,11. To maximize the power of this analysis, we chose temporal coherences that ensured that overall
performance for the 4 participants was low. Trials were grouped according to the perceptual response (e.g. false detections, misses, hits, hits with correct discrimination) and for each
trial type we determined the probability that each non-target direction or orientation was presented at each time point. Importantly, target directions are not in the quantized distribution
of directions shown on all non-target frames, allowing us to analyse how each non-target direction influences perception of the target directions. Significance was assessed using a 99%
confidence interval derived from the inverse of the binomial cumulative distribution function with parameters and N. , where c is the temporal coherence and n is the number of non-target
motion directions. For false detections, c = 0, thus . For 25 and 40% coherence trials, and 2.5%, respectively. N is the number of non-signal motion frames across all completed trials.
Full-width half maximum (FWHM) perceptual tuning bandwidths were obtained from perceptual tuning curves after cubic spline interpolation with 5° resolution. SUPERIMPOSITION To increase
statistical power in the analyses described above, motion directions were combined and superimposed in three ways. First, frames with opposite directions but the same axis of motion were
combined (e.g. 15 and 195°) because these directions had indistinguishable effects on perception. Second, for trials ending in Hits and Misses, in order to analyse data as if there was a
single target direction, for all trials with a target direction of 85° we inverted the directions about the vertical discrimination boundary (i.e. θsuperimpose, 85 = 180 − θ85; θsuperimpose,
95 = θ95). Third, for false detection trials, in order to analyse data as if all perceptual reports were aligned, for all frames associated with trials with a choice of “right”, we inverted
the directions relative to vertical (i.e. θsuperimpose, right = 180 − θright; θsuperimpose, left = θleft). These superimposition methods allow us to examine the interaction between the
discrimination and detection tasks and to determine the perceptual bandwidth, or range of directions that influence a perceptual response. RESULTS BEHAVIOURAL PERFORMANCE All observers were
able to reliably identify the presence of a motion pulse with low spatial and temporal coherence that lasted 20 frames. Some observers could identify the direction of motion even when the
pulse contained as few as five, non-consecutive frames moving in the target direction. Importantly, correct motion detection did not guarantee correct direction discrimination. Across
observers, detection performance ranged from 61–73% correct, but based only on trials with correct detection, discrimination performance was only 54–65% correct (Fig. 2). We did not
extensively analyse discrimination performance on Miss trials as some subjects reported that they non-randomly gave a discrimination response when they did not detect a pulse (e.g. they
chose “right” for every trial in a block, or alternated “left” and “right” choices). For all subjects, detection performance was significantly better with 40% than 25% coherence (p <
0.01, normal approximation to the chi-squared test) and discrimination performance was significantly better with the high coherence trials in 2 of 4 subjects (p < 0.05). The d′ values for
each observer, calculated separately for detection performance on 25% and 40% coherence trials were: JV – 0.91, 1.78; NP – 0.38, 0.83; P1 – 0.40, 0.81; P2 – 0.79, 1.37. Although performance
improved with temporal coherence, we wondered how observers’ responses might be influenced by the frequency and relative timing of non-target motion directions. To explore this, the
remainder of the paper focuses on trials grouped by perceptual response (false detections, misses, hits) and analyses the probability distribution of motion directions that precede these
responses. PERCEPTUAL TUNING FOR PULSE-ABSENT TRIALS (FALSE DETECTIONS) The probability distribution of motion directions over time from 154 false detection trials is shown for a single
observer in Fig. 3. The heat-map indicates the probability that each direction, at each time in the 2 second trial, is associated with a false detection. If false detections occur purely
randomly and are not influenced by the stimulus content (e.g. due to guessing) then the heat-maps should have uniform colour corresponding to a probability of 1/24 = 4.17%. However, there
are clear hot-spots around 1.5–1.7 seconds and directions 90° and 270°, indicating that these directions at this specific time cause a false detection. Although absent on false detection
trials, the motion pulse would normally occur from frames 151–170, 1.5–1.7 s after trial onset (vertical lines). Figure 3B shows the perceptual tuning curve, which is the direction
probability density function, integrated across frames 151–170. The peaks at 90–110° and 270–290° suggest that false detections are biased by weak _orientation_-based cues in the noise,
rather than _direction_-specific signals. The reason that the probability peaks are shifted towards directions slightly greater than 90° and 270° is that we treated all trials as if the
reported direction was “left” of vertical (see Superimposition, Methods). We were surprised that for all participants, motion with both upwards and downwards components had high
probabilities on false detection trials, as we expected only near-target upward directions to influence detection. In training, participants were shown the actual motion directions using
pulses with high temporal coherence and long dot lifetimes and were thus aware that the target directions had a close to vertical upwards direction. Moreover, when queried, they reported
qualitatively that the pulses appeared to move upwards. Due to the symmetry of the direction probability distributions in all participants, we collapsed stimulus probabilities across
opposing directions to create an orientation-based PDF (Fig. 3C,D). This more clearly demonstrates that false detections are associated with an increased probability of orientations similar
to those contained in the expected pulses, which occur with timing similar to when pulses are normally expected to occur. As observers may have been influenced by stimulus times other than
the precise frames when a pulse normally occurred, we determined the range of times for which the orientation-based PDF was significantly different from a uniform distribution. To do this,
we calculated PDFs across 10-frame groupings, sampled with 5-frame resolution. Time-points associated with PDFs that were significantly different from uniform (p < 0.05, chi-squared test)
were included in subsequent false detection analysis. Note that we included all significant time-points in this analysis, regardless of the direction that had the highest probability. The
resulting time windows were: JV – frames 146 to 200; NP – frames 146–190; P1 – frames 121–200; P2 – frames 136–185. Similar, but weaker, trends were observed if we restricted our analysis
window to frames 151–170, in which the pulse was expected. Figure 4 shows the perceptual tuning curve for false detection trials averaged across four observers. In Fig. 4A, trial directions
are represented as they were presented to the subject; in Fig. 4B, false detection trial directions have been superimposed as if the subject always responded “left” during the discrimination
task (i.e. trials in which the subject responded “right” are inverted about 90°). False detections are associated with a higher proportion of frames moving in directions 75–105°. For 3
participants (JV, P1, P2), when tuning curves were superimposed according to the discrimination response, the probability of viewing orientations of 90 or 105° on false detection trials was
significantly greater than chance levels (p < 0.05, binomial, see Supplementary Figure 1). The remaining participant (NP) had the highest rate of false detections, suggesting that
non-stimulus factors were most likely the cause of his false detections. PERCEPTUAL TUNING FOR PULSE-PRESENT TRIALS (HITS AND MISSES) In low coherence trials, just 5 frames in the 20 frame
pulse period move in the target direction (85 or 95°). On these trials, observers were more likely to correctly detect a motion pulse if there was a high proportion of vertical motion
directions and were more likely to miss the motion pulse if there was a low proportion of vertical directions (Fig. 4C). However, on high coherence trials, when 40% of frames (8/20) moved in
the target direction, Misses were more influenced by non-target directions than Hits (Fig. 4E). Supplementary Figures 2 and 3 show tuning curves for individual observers with each
coherence. To highlight how each direction contributes to overall detection performance, we calculated a perceptual tuning curve based on the difference between Hits and Misses (Fig. 4D,F).
The Difference tuning curves highlight that the directions most likely to lead to a Miss are 45–60° from the target, not those orthogonal to, or furthest from the target direction. This
“Mexican-hat” shaped tuning curve is reminiscent of similar perceptual tuning curves in an orientation detection task8, but differs from tuning curves previously reported for a direction
detection task11. PULSE DETECTION DEPENDS ON TEMPORAL STRUCTURE For pulse-present trials, the primary factor influencing detection is the mean number of near-target directions, which is
higher on Hit than Miss trials. We wondered whether Hit and Miss trials also differed in their temporal structure and whether this temporal structure could explain additional aspects of
perception. For example, trials during which near-target directions were consecutive or closely spaced in time might be more easily detected. To assess this, for each trial, we examined the
sequence of directions for frames 151–170, defining Near-Target frames as those in which the axis of the signal direction was within 10° of the Target. The choice of a 10° range here is
based on the perceptual tuning curves derived above. We compared the distribution of intervals between Near-Target frames for Hits and Misses; however, as the _number_ of frames influences
both the interval distributions and detection performance (Fig. 5A), the subsequent analyses were all applied to subsets of the data with matched numbers of Near-Target frames. A given
subset was only considered if it included over 10 Hit and 10 Miss trials for each observer. In total, this gave 5 separate conditions, corresponding to 5–9 Near-Target frames within the 20
frame pulse period. Clusters of 3 or 4 near-target frames were more likely to be detected than missed, however, this difference was not significant (Fig. 5B). Note that some numbers of
near-target frames were not associated with any 3- or 4-in-a-row clusters. Thus, while consecutive frames may weakly influence perception, the most important factor is the total number of
near-target frames within the pulse. PERCEPTUAL TUNING FOR DISCRIMINATION For correct-detection trials, we examined how discrimination responses were influenced by the non-target
orientations. For simplicity, we inverted trials with rightward target directions and superimposed them, so that all target directions were equivalent to “left” (95°). Trials were then
grouped according to whether the discrimination judgment was correct or incorrect. Figure 6A shows each individual’s perceptual tuning curves for discrimination performance, based on trials
with 25% coherence. Mean tuning curves for 25% and 40% coherence trials were similar (Fig. 6B). These tuning curves are the difference between the orientation probability density functions
for correct and incorrect judgments; by subtracting the two PDFs, we remove the directions that are only associated with the detection judgment. Therefore, orientations, aligned with the
category boundary (90°) have no influence on the discrimination choice, nor do directions further than 45° from the category boundary. PERCEPTUAL FILTER The prior analyses demonstrate that
detection performance is influenced by the number of near-target directions and to a small degree, the number of consecutive near-target directions within the motion pulse period. Detection
decisions are also determined by how reliably observers apply their decision criterion. To explore how the temporal structure of the stimulus interacts with noise in the observer’s decision
boundary, we developed a model in which the stimulus sequence on each trial ‘_S’_ passes through a perceptual filter with orientation tuning described by a von Mises function and an
exponential decay, producing a time-varying response _R_ (Equation 1). Formally, this is equivalent to a model in which the responses of a population of sluggish, orientation-tuned neurons
are weighted and summed: _R_(_t_) is the filtered response, defined by von Mises concentration parameter κ, exponential decay τ and stimulus sequence _S_(_i_). Note that as our perceptual
responses appeared bidirectional (e.g. 45 and 225° are equivalent), the stimulus directions in the model are all doubled. The decision of the model is generated by comparing the maximum
filtered response within a given time window to a decision threshold T, which has Gaussian-distributed noise T ~ N(μT, σT): if maxt[R(t)] > T, decide “signal present”; otherwise decide
“signal absent”. Model decisions based on the _mean_ filtered response produced similar results (not shown). However, calculating an appropriate mean requires _a priori_ knowledge of when to
average the stimulus properties and the range of stimulus frames that influenced false detections indicate that observers poorly estimated when the motion pulse was likely to occur. The
model contains 4 parameters of interest: the time constant τ; von Mises concentration κ; and threshold mean μT and standard deviation σT. Here, we use the stimulus sequences seen by our
human observers as inputs to the model and assess what parameters maximize the model’s ability to predict the stimulus, i.e. correctly identify pulse-present and pulse-absent trials.
Subsequently, we determine the parameters that maximize the model’s ability to predict each observer’s decisions. Note that additive sensory noise (e.g. in transduction, transmission or
encoding) will impair perceptual detection, but this noise is not incorporated into our model. Moving stimuli that are presented briefly or that rapidly change direction are reliably
represented by direction-selective neurons in area MT with excellent temporal fidelity17,18,19, thus we are concerned here with what factors might affect the read-out of sensory information
that is reliably represented in the brain. Additional sensory noise should impair all detection decisions and will be indistinguishable from increasing the inter-trial variability in
applying the decision threshold (σT), but should not cause changes equivalent to decision biases (μT) to emerge. Figure 7A shows the distribution of filtered responses for the 8583 trials
seen by all observers, with perceptual filter parameters τ = 19 frames and κ = 8 (corresponding to a full-width half-maximum bandwidth of 48°). The vertical black line indicates the
threshold μT that maximizes overall detection performance, correctly classifying 88% of trials if σT = 0 (i.e. there is no noise in the decision threshold). Filtered responses from
pulse-absent trials below this threshold are Correct Rejects, whereas responses above this threshold are false detections. Note that with this threshold, it is possible to correctly detect
76% of trials with 25% coherence (dotted line) and almost all trials with 40% coherence (dashed line), with only a small proportion of false positives at 0% coherence (Fig. 7B). Assuming no
noise in applying the decision threshold, the overall theoretical performance for a range of perceptual filter parameters is shown in Fig. 7C. Detection performance peaks with the filter
parameters shown in Fig. 7A,B, clearly exceeding the observers’ performance on the task, which ranged from 54–73%. This suggests that all observers perform sub-optimally on the detection
task. Having demonstrated that the model can correctly classify the _stimulus_, next we asked how well we could predict an _observer’s response_, given a single trial stimulus sequence.
Assuming zero noise (σT = 0), we found the decision threshold (μT) that maximized the ability to predict each observer’s Yes-No responses across a range of filter parameters (κ,τ) (Fig. 8A).
Prediction performance was well above chance, with the best performing noiseless model having parameters and performance of: JV(κ = 2, τ = 15) − 84%; NP(4, 23) − 65%; P1(4, 13) − 66%; P2(4,
27) − 75%. Across observers, these parameters correspond to direction tuning with a full-width half-maximum bandwidth of 69–96° and integration time constant of 130–270 ms. When compared to
the 200 ms time window within which target frames could occur, these relatively long time constants are consistent with the small influence of clustering of near-target frames that we
observed (Fig. 5). Note that the model’s behaviour is quite different to that of the observers. For example, observer NP has a very high false alarm rate, so the model optimizes its overall
predictive power by having a low threshold – it almost always responds that a pulse was present. Thus, False alarms and Hits with 25 and 40% are always correctly predicted, but Misses are
never correctly predicted. For all 4 observers, misses with 40% coherence were rarely predicted. To examine a more realistic decision scenario, we added Gaussian noise to the decision
threshold (σT > 0) and used exhaustive search to determine the set of model parameters (τ, κ, μT, σT) that best matched overall observer performance. Figure 8B shows the distribution of
max[R(t)] for the best filter parameters τ and κ for each observer. The threshold mean and standard deviation (μT, σT) are indicated by the vertical and horizontal black lines, respectively.
While all observers have a considerable amount of threshold noise, often exceeding the distribution of filtered responses associated with the pulse-absent trials, the size of σT reflects
the pattern of each observer’s performance shown in Fig. 2. JV and P2 have small standard deviations and the best performance; NP and P1 have large standard deviations and perform poorly.
The distribution of decision thresholds required by the model to match each observer’s performance also reflects the biases and sensitivity of each observer. For example, observer JV has a
threshold that is higher than optimal, reflecting their bias to reporting No-pulse. However, their high overall performance reflects the low variability in applying this threshold. The
average performance of the noisy model closely matches the average perceptual performance of each observer (Fig. 8C). In Fig. 7C, the horizontal red line indicates the number of single
trials that are correctly predicted; if the model was performing perfectly, the red lines would be at the top of each bar. However, the model still makes errors on single trials. For
example, “Yes” responses from observer NP are almost always correctly predicted, but “No” responses are rarely predicted. On the other hand, observer JV, who performs more reliably, has the
majority of single trials decisions correctly predicted by the model. DISCUSSION Human observers can reliably detect and subsequently discriminate the direction of a brief motion pulse with
low temporal coherence embedded in an ongoing, noisy stimulus. We show that observers’ detection performance is captured by a model in which the responses of direction-selective neurons are
temporally integrated and compared to a decision threshold; noise in this threshold can account for each observer’s sub-optimal performance. Below we examine why observers were strongly
influenced by motion in directions opposite to those being detected and discriminated, what could account for the narrow perceptual tuning curves and the temporal aspects of our stimulus
that most strongly influence motion detection. BIMODAL PERCEPTUAL TUNING CURVES All observers were similarly influenced by motion in opposite directions along a given axis. This is
surprising as motion 180° from a target direction typically causes the greatest impairment in detection20. As there was no penalty for responding to directions of θ° and (θ + 180)° in the
same manner in our task, might observers have used orientation rather than direction cues? Individual dots in our stimulus had 2-frame lifetimes, so observers were forced to monitor the
average direction of the entire stimulus over time, rather than track the trajectory of individual dots. If observers were insensitive to the relative timing of the dots, they may have
simply extracted the orientation of the dot pair, similar to viewing a Glass pattern21. We think this is unlikely, as observers were trained to expect target directions ±5° relative to
vertically upwards and subjectively reported perceiving upwards motion. Further, in “coarse” discrimination tasks using 2-frame dot lifetimes observers correctly discriminate opposite
directions of motion at lower spatial coherences than those used here1,6. Finally, previous studies of orientation detection using reverse correlation found a Mexican-hat profile in
perceptual tuning curves, with the presence of orientations ~40° from the target significantly impairing detection8; in our study, this profile was also weakly evident, but tuning bandwidths
were broader. While we cannot interpret perceptual tuning curves as proxies for the tuning curves of underlying neurons, they must represent the outcome of weighting a population of neurons
with a range of preferred directions. The bimodal perceptual tuning curves may therefore represent underlying neurons with bimodal direction tuning, the combination of neurons with
opposite, but unimodal tuning, or a non-linear interaction between motion in opposite directions. Neurons with bimodal direction tuning have been found in V1, V2, V3, DM and V6, however,
these areas have not been linked to motion perception22,23,24. The middle temporal area (MT) is closely associated with motion perception in primates25,26 and single MT neurons have unimodal
direction tuning with average bandwidths of 80–90° 18. As it seems unhelpful to combine the responses of neurons with opposing preferred directions, we suggest that the near-symmetric
influence of upwards and downwards motions in our experiment may originate in the temporal properties of motion-sensitive neurons. Critically, many MT and PMLS neurons have biphasic temporal
response profiles, evident as nonlinear interactions between consecutive opposing motion directions9,17,27. For these biphasic neurons, the stimulus sequence most likely to generate an
action potential is anti-preferred followed by preferred direction motion. Therefore, the downwards motion may influence judgments in our task only because it is routinely followed by
upwards motion. One way to resolve whether this biphasic profile could account for our behavioural data is a second-order analysis, looking at the probabilities of all pairs of possible
motion directions associated with each type of detection judgment10,11, however, we had insufficient trials to obtain meaningful second-order kernels. NARROW PERCEPTUAL BANDWIDTHS FOR
DETECTION AND DISCRIMINATION The range of directions that influenced detection judgments in our task (30–50° bandwidth at low coherence) was narrower than those reported previously in most
motion reverse correlation studies −135° in a detection task11 and 60–180° in coarse discrimination tasks28,29. The narrow bandwidth of 35° reported by Busse _et al._ (2008) is attributable
to the high signal-to-noise level of their stimulus, which had 100% temporal and spatial coherence. Given the low coherence of our stimulus, what could account for the narrow bandwidth
reported here? Perceptual bandwidths decrease with increasing viewing duration10 and any stimulus manipulation that increases motion energy (e.g. increased density or number of dots)
facilitates narrower bandwidths. Our stimulus contained 100 dots that moved every 10 ms for 200 ms, whereas earlier studies had fewer dots moving less often, e.g. 32 dots moving every 30 ms
for 300 ms29, or 30 dots moving every 14–28 ms11. This suggests that despite the low temporal coherence, the large motion energy averaged over the long duration of our motion pulse may
promote narrow detection bandwidths. Despite the narrow detection bandwidth, discrimination performance was surprisingly low, ranging from 54–65% across participants, even when only
correct-detection trials were considered. Direction discrimination thresholds of 2° are typical for high signal-to-noise stimuli lasting 200 ms30 and also for noisy stimuli containing a
Gaussian distribution of directions31. As our target directions differed by 10°, the poor discrimination performance in our task is likely due to the low temporal coherence, or the fact that
subjects were primarily attending to the detection task. TEMPORAL FACTORS AFFECTING DETECTION Detection performance was strongly influenced by the number of near-target direction frames
during the expected pulse period; however, for trials with matched numbers of near-target frames, detection was better when those frames occurred consecutively. In motion-sensitive MT
neurons, non-linear interactions in the responses to pairs of frames with the same or opposite directions are strongest when frames are separated by 10–60 ms17,27,32. Our data suggests that
these non-linear neuronal responses also strongly influence perception. Even in the absence of non-linear interactions between the responses to closely-spaced stimuli, it is possible that
bursts of near-target frames strongly influence perception because the time-window of perceptual integration is just a few frames long. However, our model, which does not incorporate
non-linear interactions, suggests that a short integration time-window is unlikely as the model parameters that best predict the stimulus and the overall pattern of observers’ responses
across all coherences have time constants of 10 frames or more (130–270 ms). Thus the ‘leak’ that occurs when integrating the stimulus is quite small due to the 200 ms time window within
which near-target frames were presented. Future studies that vary the duration of the motion pulse could give insight into whether the integration time of the perceptual filter can be
flexibly matched to the expected stimulus duration. NOISY THRESHOLDS CAN ACCOUNT FOR SUB-OPTIMAL DETECTION Despite the presence of noisy stimuli with low temporal coherence, our model could
correctly detect up to 88% of motion pulses if there was no noise in the internal decision threshold. Whereas our observers made frequent false detections on pulse-absent trials and frequent
misses on high-coherence trials, the noiseless model rarely produced errors on these trials. This demonstrates that optimising the model output to match perceptual performance in the
absence of noise gives very unrealistic results. Some of this noise must also arise in the sensory representation of signals and our model is limited in that it cannot distinguish
variability introduced at the level of sensory encoding from variability in applying the decision threshold. However, the temporal fidelity of motion-sensitive neurons is known to be highly
reliable, direction tuning is not expected to vary between trials and therefore sensory noise cannot account for observers’ perceptual biases17,18,19. When decision thresholds were allowed
to vary between trials, our model was able to closely match the general behaviour of all observers on a single trial basis. In matching models independently to each observer, the best models
had similar neural tuning properties (κ of 2–4 and τ of 15–27 frames). Note that for all observers, these time constants are close to the 20 frame duration of the signal pulse. Given the
similar model tuning parameters, differences between observers in both bias (e.g. predisposition to responding pulse present versus absent) and overall performance are well captured by the
mean and standard deviation in the model’s decision threshold. Thus, if human observers can minimize variability in their decision threshold, they should have sufficient sensitivity to
accurately detect noisy motion pulses in our task. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Price, N. S. C. and VanCuylenberg, J. B. Noisy decision thresholds can account for
suboptimal detection of low coherence motion. _Sci. Rep._ 6, 18700; doi: 10.1038/srep18700 (2016). REFERENCES * Britten, K. H., Shadlen, M. N., Newsome, W. T. & Movshon, J. A. The
analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci 12, 4745–4765 (1992). Article CAS Google Scholar * Morgan, M. J. & Ward, R. Conditions
for motion flow in dynamic visual noise. Vision Research 20, 431–435 (1980). Article CAS Google Scholar * Britten, K. H., Shadlen, M. N., Newsome, W. T. & Movshon, J. A. Responses of
neurons in macaque MT to stochastic motion signals. Visual neuroscience 10, 1157–1169 (1993). Article CAS Google Scholar * Britten, K. H. & Newsome, W. T. Tuning bandwidths for
near-threshold stimuli in area MT. J Neurophysiol 80, 762–770 (1998). Article CAS Google Scholar * Cohen, M. R. & Newsome, W. T. Estimates of the contribution of single neurons to
perception depend on timescale and noise correlation. J Neurosci 29, 6635–6648 (2009). Article CAS Google Scholar * Pilly, P. K. & Seitz, A. R. What a difference a parameter makes: a
psychophysical comparison of random dot motion algorithms. Vision Res 49, 1599–1612 (2009). Article Google Scholar * Neri, P. & Levi, D. M. Receptive versus perceptive fields from the
reverse-correlation viewpoint. Vision Res 46, 2465–2474 (2006). Article Google Scholar * Ringach, D. L. Tuning of orientation detectors in human vision. Vision Res 38, 963–972 (1998).
Article CAS Google Scholar * Perge, J. A., Borghuis, B. G., Bours, R. J., Lankheet, M. J. & van Wezel, R. J. Temporal dynamics of direction tuning in motion-sensitive macaque area MT.
J Neurophysiol 93, 2104–2116 (2005). Article Google Scholar * Busse, L., Katzner, S., Tillmann, C. & Treue, S. Effects of attention on perceptual direction tuning curves in the human
visual system. Journal of vision 8, 2 1–13 (2008). Article Google Scholar * Iyer, P. B., Freeman, A. W., McDonald, J. S. & Clifford, C. W. Rapid serial visual presentation of motion:
short-term facilitation and long-term suppression. Journal of vision 11 (2011). * Brainard, D. H. The Psychophysics Toolbox. Spatial vision 10, 433–436 (1997). Article CAS Google Scholar
* Blum, J. & Price, N. S. Reflexive tracking eye movements and motion perception: one or two neural populations? Journal of vision 14, 23 (2014). Article Google Scholar * Newsome, W.
T. & Pare, E. B. A selective impairment of motion perception following lesions of the middle temporal visual area (MT). J Neurosci 8, 2201–2211 (1988). Article CAS Google Scholar *
Schutz, A. C., Braun, D. I., Movshon, J. A. & Gegenfurtner, K. R. Does the noise matter? Effects of different kinematogram types on smooth pursuit eye movements and perception. Journal
of vision 10, 26 (2010). Article Google Scholar * Price, N. S. & Prescott, D. L. Adaptation to direction statistics modulates perceptual discrimination. Journal of vision 12 (2012). *
Bair, W. & Movshon, J. A. Adaptive temporal integration of motion in direction-selective neurons in macaque visual cortex. J Neurosci 24, 7305–7323 (2004). Article CAS Google Scholar
* Price, N. S., Ibbotson, M. R., Ono, S. & Mustari, M. J. Rapid processing of retinal slip during saccades in macaque area MT. J Neurophysiol 94, 235–246 (2005). Article CAS Google
Scholar * Borghuis, B. G. et al. The motion reverse correlation (MRC) method: a linear systems approach in the motion domain. Journal of neuroscience methods 123, 153–166 (2003). Article
Google Scholar * Hol, K. & Treue, S. Different populations of neurons contribute to the detection and discrimination of visual motion. Vision Res 41, 685–689 (2001). Article CAS
Google Scholar * Glass, L. & Perez, R. Perception of random dot interference patterns. Nature 246, 360–362 (1973). Article CAS ADS Google Scholar * Felleman, D. J. & Van Essen,
D. C. Receptive field properties of neurons in area V3 of macaque monkey extrastriate cortex. J Neurophysiol 57, 889–920 (1987). Article CAS Google Scholar * Lui, L. L., Bourne, J. A.
& Rosa, M. G. Functional response properties of neurons in the dorsomedial visual area of New World monkeys (Callithrix jacchus). Cereb Cortex 16, 162–177 (2006). Article Google Scholar
* Galletti, C., Battaglini, P. P. & Fattori, P. Functional Properties of Neurons in the Anterior Bank of the Parieto-occipital Sulcus of the Macaque Monkey. The European journal of
neuroscience 3, 452–461 (1991). Article Google Scholar * Born, R. T. & Bradley, D. C. Structure and function of visual area MT. Annual review of neuroscience 28, 157–189 (2005).
Article CAS Google Scholar * Price, N. S. & Born, R. T. Timescales of sensory- and decision-related activity in the middle temporal and medial superior temporal areas. J Neurosci 30,
14036–14045 (2010). Article CAS Google Scholar * Vajda, I., Borghuis, B. G., van de Grind, W. A. & Lankheet, M. J. Temporal interactions in direction-selective complex cells of area
18 and the posteromedial lateral suprasylvian cortex (PMLS) of the cat. Visual neuroscience 23, 233–246 (2006). Article Google Scholar * Murray, R. F., Sekuler, A. B. & Bennett, P. J.
A linear cue combination framework for understanding selective attention. Journal of vision 3, 116–145 (2003). Article Google Scholar * Neri, P. & Levi, D. Temporal dynamics of
directional selectivity in human vision. Journal of vision 8, 22 21–11 (2008). Article Google Scholar * De Bruyn, B. & Orban, G. A. Human velocity and direction discrimination measured
with random dot patterns. Vision Res 28, 1323–1335 (1988). Article CAS Google Scholar * Watamaniuk, S. N., Sekuler, R. & Williams, D. W. Direction perception in complex dynamic
displays: the integration of direction information. Vision Res 29, 47–59 (1989). Article CAS Google Scholar * Vajda, I., Lankheet, M. J., Borghuis, B. G. & van de Grind, W. A.
Dynamics of directional selectivity in area 18 and PMLS of the cat. Cereb Cortex 14, 759–767 (2004). Article Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by
NHMRC Project Grants 1008287, 1066588 and a Human Frontier Science Program Career Development Award. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Physiology, Monash
University, Clayton, 3800, VIC, Australia Nicholas S. C. Price & John B. VanCuylenberg Authors * Nicholas S. C. Price View author publications You can also search for this author
inPubMed Google Scholar * John B. VanCuylenberg View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.V. and N.P. collected and analysed data.
N.P. wrote the manuscript. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS
AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s
Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the
license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Price, N., VanCuylenberg, J. Noisy decision thresholds can account for suboptimal detection of low coherence motion. _Sci Rep_ 6, 18700 (2016). https://doi.org/10.1038/srep18700 Download
citation * Received: 26 June 2015 * Accepted: 23 November 2015 * Published: 04 January 2016 * DOI: https://doi.org/10.1038/srep18700 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
