Viral-mediated fusion of mesenchymal stem cells with cells of the infarcted heart hinders healing via decreased vascularization and immune modulation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Cell fusion can occur between mesenchymal stem cells (MSCs) transplanted to improve cardiac function and cells of the recipient. The therapeutic benefit or detriment of resultant
cell hybrids is unknown. Here we augment fusion of transplanted MSCs with recipient cardiac cell types via viral fusogens to determine how cardiac function is impacted. Using a
Cre/LoxP-based luciferase reporter system coupled to biophotonic imaging and echocardiography, we found that augmenting fusion with the vesicular stomatitis virus glycoprotein (VSVG)
increased the amount of fusion in the recipient mouse heart, but led to diminished cardiac function. Specifically, MSCs transfected with VSVG (MSC-VSVG) had the lowest mean fold increase in
fractional area change (FAC) and cardiac output (CO). Although the amount of fusion detected had a strong positive correlation (Pearson) with fractional area change and cardiac output at day
7, this effect was lost by day 28. The decrease in cardiac function seen with MSC-VSVG treatment versus MSC alone or sham treatment was associated with decreased MSC retention, altered
immune cell responsiveness and reduced vascularization in the heart. This outcome garners consideration in the context of cellular transplantation to damaged tissues, those with viral
infection or other microenvironmental conditions that might promote fusion. SIMILAR CONTENT BEING VIEWED BY OTHERS CRISPR-CAS9 EDITING OF TLR4 TO IMPROVE THE OUTCOME OF CARDIAC CELL THERAPY
Article Open access 18 March 2023 ENHANCING MYOCARDIAL REPAIR WITH CARDIOCLUSTERS Article Open access 07 August 2020 LIMITED POTENTIAL OF AAV-MEDIATED GENE THERAPY IN TRANSDUCING HUMAN
MESENCHYMAL STEM CELLS FOR BONE REPAIR APPLICATIONS Article 17 August 2020 INTRODUCTION One of the most prevalent health issues in first world countries continues to be myocardial
infarction1. Mesenchymal/multipotent stem/stromal cell (MSC) therapy has been viewed as a promising treatment to solve this issue2,3,4,5,6,7,8. MSCs have the ability to home to injured
tissues9,10, secrete paracrine factors that allow for immune evasion11,12,13 and/or increase angiogenesis10,14,15,16,17,18,19. In the course of these studies, many have observed fusion
between MSCs and cardiac cells20,21,22,23,24,25,26,27,28,29,30. However, the impact of cell fusion in this scenario and subsequent reprogramming on cardiac function at the cellular and
tissue scale is not well understood. Fusion of MSCs with cardiac cell types may improve cardiac function if the fusion products adopt the phenotype and associated function of cardiac cell
types including cardiomyocytes, smooth muscle cells and endothelial cells. Evidence from the literature suggests stem cells and somatic cells can give rise to fusion products with
characteristics of the somatic cell, thereby effectively programming the stem cells. For example, Blau _et al._ fused differentiated mouse muscle cells and human amniocytes and found that
the mature cell phenotype dominated such that the amniocytes expressed human muscle proteins via exchange of cytomplasmic components31. Recent studies have shown that fusion of bone
marrow-derived cells with hepatocytes has a therapeutic effect on the liver because the bone marrow-derived cells repopulate damaged liver tissue and adopt the biochemical functions of
hepatocytes, including maintaining correct levels of serum transaminases, bilirubin and amino acids32,33,34,35. Fusion of MSCs with cardiac cell types could also improve cardiac function if
the fusion products adopt the phenotype and associated function of mesenchymal stem cells, such as self-renewal, pro-angiogenic propensity and anti-inflammatory effects. Evidence from the
literature suggests fusion products of stem cells and somatic cells can serve to effectively reprogram the somatic cell to a less mature state. For example, Cowan _et al._ reverted human
fibroblasts to a pluripotent-like state after fusion with embryonic stem cells36. Tada _et al._ observed a similar pluripotent hybrid cell after fusing embryonic germ cells and
lymphocytes37. Alternatively, fusion of MSCs with cardiac cell types may hinder cardiac function if the fusion products adopt a phenotype and associated function distinct from either cardiac
cell types or mesenchymal stem cells. Blau _et al._ found heterokaryons formed from muscle cells and keratinocytes, expressed a combination of both gene profiles38. A similar result was
seen after fusing intestinal epithelial cells and macrophages in a murine model of intestinal cancer in that cell fusion hybrids retained the transcriptome identity characteristic of both
parental cells, but also expressed genes not activated in either parent cell type39. The activation of previously unexpressed genes is postulated to be responsible for the creation of cancer
stem cells through fusion between tumor cells and bone marrow-derived cells40,41,42. In the present study, we use a Cre/_LoxP_-based molecular approach to detect fusion of transplanted MSCs
to cells of living mice and we utilize echocardiography to determine how MSC fusion affects cardiac function. Using this approach, we found that human mesenchymal stem cells delivered to
the murine heart via a collagen-based patch fuse after delivery and that augmented fusion of MSCs via viral fusogen appears to have a detrimental affect on cardiac function. This negative
outcome was associated with decreased MSC retention, reduced vascularization in the healthy heart and altered immune modulation 56 days after transplantation. RESULTS DETECTION AND
AUGMENTATION OF MSC CELL FUSION _IN VIVO_ Previously, we showed that fusion of MSCs occurs both spontaneously and with the aid of exogenously supplied viral fusogens after transplantation to
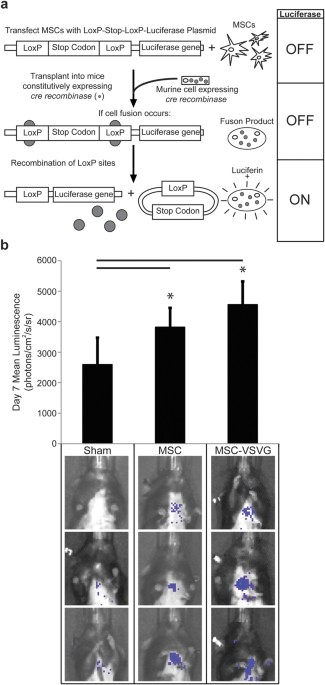
the murine heart20,43,44. In this study, we again utilized a Cre/_LoxP_-based luciferase inducible reporter system coupled to biophotonic imaging to detect and quantify fusion between
transplanted MSCs and cells of mice constitutively expressing Cre recombinase (Fig. 1a,b). The transplanted MSCs (both MSC and MSC-VSVG) were transfected with a _LoxP_-stop
codon-_LoxP_–luciferase construct such that luciferase expression is limited to hybrids between donor and recipient cells (Fig. 1a). Importantly, this imaging method is non-invasive and
fusion can be quantified without sacrificing the mice. Seven days following myocardial infarction and MSC cell transplantation via collagen patch (TissueMend) to the heart, bioluminescence
was measured for mice with no treatment (Sham, n = 5), mice receiving MSCs with no fusogen (MSC, n = 5) and mice receiving MSCs transfected with the VSVG fusogen (MSC-VSVG, n = 5). The sham
mice were used as a negative control to determine background bioluminescence (2591 + 884.9 photons per second per cm2 per steradian (photons/cm2/s/sr)). Fusion was increased in both the MSC
(3818 + 762.6 photons/cm2/s/sr) and MSC-VSVG (4557 + 1317 photons/cm2/s/sr) groups compared to sham (*_P_ < 0.05, compared to sham) (Fig. 1b). The highest amount of bioluminescent signal
and thus fusion, was observed in the MSC-VSVG (not significant compared to MSC group). These data demonstrate a trend towards enhanced fusion between MSCs and cardiac cell types via
expression of viral fusogens in MSCs. AUGMENTED MSC CELL FUSION AFFECTS FUNCTION OF INFARCTED MYOCARDIUM To determine the impact of MSC cell fusion on cardiac function, the mice also
underwent echocardiography at day 3, 7, 14 and 28 after infarction and cell delivery. Fractional area change (%) and cardiac output (mL/min) were measured at each time point for all mice of
the study. The FAC and CO measurements were normalized to the day 3 time point to discern the improvement of each group (sham, MSC, MSC-VSVG) relative to the initial injury. The sham group
showed the largest improvement at day 28 (2.23 + 2.06 fold increase in FAC and 3.02 + 2.80 fold increase in CO), but there was high variability from mouse to mouse (Fig. 2a,b).
Interestingly, at day 28 the MSC (1.31 + 0.48 fold increase in FAC and 1.99 + 1.29 fold increase in CO) and MSC-VSVG (0.86 + 0.48 fold change in FAC and 1.81 + 1.41 fold increase in CO)
groups had a lower average fold change for both FAC and CO compared to the sham group, with the augmented fusion group MSC-VSVG having the lowest relative improvement (Fig. 2a,b). These
results coupled with the observed increase in fusion from the bioluminescent data of the MSC and MSC-VSVG groups (Fig. 1b) suggests that MSC fusion with cells of the mouse heart could be
detrimental to the healing process in the mouse heart following myocardial infarction. However, the variability was also substantial in the MSC and MSC-VSVG groups and there were no
significant differences between groups at any time point. To better probe these outcomes, a focused analysis was performed where the bioluminescent signal at day 7 for each mouse was plotted
against the FAC (Fig. 2c) and CO (Fig. 2d) for each mouse at each time point. At day 7, a strong positive correlation (Pearson’s correlation coefficient) was observed between bioluminescent
signal (fusion) and cardiac function (FAC and CO) for both MSC (ρ = 0.718, FAC; ρ = 0.648, CO) and MSC-VSVG (ρ = 0.793, FAC; ρ = 0.726, CO) mice. However, this trend was mostly lost over
time as the positive Pearson’s correlation coefficient decreased at later time points (day 14 and day 28). The MSC group with no fusogen did exhibit a weak positive correlation through day
14, but the strength of the correlation was decreased for FAC (ρ = 0.718 at day 7 to ρ = 0.393 at day 14) and CO (ρ = 0.648 at day 7 to ρ = 0.336 at day 14). Due to the high level of
variability observed between mice within the same treatment group in this study as well as the loss of correlation between fusion and function over time, the number of mice was capped at
five per group so that an in depth cellular analysis could be performed to probe the mechanism behind differences in cardiac functionality. HUMAN MSC RETENTION IN INFARCTED MURINE HEART To
determine whether retention of the transplanted MSCs over time played a part in the observed functional differences between MSC and MSC-VSVG groups, the hearts of the experimental mice were
explanted, fixed, sectioned at day 56 and probed for human leukocyte antigen (HLA) (Fig. 3a–c) via immunofluorescence. The analysis of the heart tissues was separated into four regions
(TissueMend patch, borderzone between the patch and heart, infarcted heart tissue and healthy heart tissue) to more accurately portray the spatial location in the tissue. The area of HLA
positive signal was normalized to the area of DAPI signal to account for the difference in cell density in a given region. The MSC group showed the highest retention of human cells in the
borderzone (0.526 + 1.06 HLA area/DAPI area), but human cells were also observed in the borderzone of the MSC-VSVG group (0.252 + 0.449 HLA area/DAPI area) albeit to a lesser extent than the
MSC group (not significant) (Fig. 3a). The MSC group also showed significantly increased number of HLA positive cells in the infarcted heart (0.103 + 0.186 HLA area/DAPI area) relative to
both the sham (0.028 + 0.040 HLA area/DAPI area) and MSC-VSVG group (0.025 + 0.046 HLA area/DAPI area) (*_P_ < 0.05), even though the average HLA area/DAPI area was much lower in the
infarct then the borderzone. Also of note, the MSC group showed a strong negative correlation between bioluminescence signal and HLA area/DAPI area (ρ = −0.625) (Fig. 3b). This suggests that
fusion, occurring by means other than exogenously supplied VSV-G, could hinder cell retention. VASCULAR AND IMMUNE RESPONSE In the face of similar or nearly similar retention rates of MSCs
with and without augmented cell fusion, we sought to determine whether altered MSC function at the cellular level could account for differences in tissue-level function. Specifically, we
probed for angiogenic stimulation and immune modulation. Vessel density in the four different heart regions was probed via CD31 expression (fluorescence area) normalized to cell number via
DAPI (fluorescence area)(Fig. 3d–f). Vessel density was similar in the TissueMend, borderzone and infarct regions between the different treatments. However in the healthy heart, the sham and
MSC groups showed significantly higher CD31 area/DAPI area (1.20 + 1.15 and 1.14 + 0.744, respectively) compared to the CD31 area/DAPI area in the MSC-VSVG group (0.493 + 0.455) (***_P_
< 0.001) (Fig. 3d). In addition, the MSC group exhibited a very strong negative correlation between bioluminescent signal and CD31 area/DAPI area (Fig. 3e) (ρ = −0.918). This trend could
imply that fused MSCs lose some ability to promote angiogenesis in the infarcted heart. The healthy region of the infarcted heart typically has to compensate for the loss of contractile
tissue (which would require more energy) and the increase in vessel density observed in the sham and MSC groups might represent an attempt to supply the healthy tissue with increased
metabolites to match the demand due to increased workload. However, if fused MSCs lose the ability to promote angiogenesis or were reprogrammed to prevent angiongenesis, the healthy heart
may have difficulty compensating for increased metabolic demand. Thus the loss of vessel density in the healthy heart region might explain, at least in part, the low-level FAC and CO
improvement of the MSC-VSVG group. Adaptive immune response was probed in the ventricles of treated and untreated mice via staining for T cells (pixel area corresponding to CD3 expression)
relative to area of DAPI signal (Fig. 4a–c). Adaptive immunity was probed since the time point was far later than typical innate immune activation and since MSCs have been shown to modulate
T cell function _in vitro_ and _in vivo._ CD3 positive cells were rare in the sham group in all ventricle regions, as were they rare for the MSC and MSC-VSVG groups in the TissueMend,
infarcted heart and healthy heart. However in the borderzone, the MSC group showed significantly more CD3 area/DAPI area (0.540 + 0.704) compared to the MSC-VSVG (0.185 + 0.244) (**_P_ <
0.005) (Fig. 4a), with only a weak negative correlation with amount of fusion per mouse (Fig. 4b). We actually anticipated the reverse (i.e., higher T cell numbers in MSC-VSVG vs. MSC) since
tissue-level function might be limited by ongoing immune activation. One potential explanation for this observation could be that all or a portion of the T cells detected in the MSC group
are regulatory T cells, which suppress or downregulate induction and proliferation of effector T cells. Multiple studies have shown that human MSCs have the ability to expand regulatory T
cell populations while inhibiting allostimulated T cell proliferation45,46. This could also be the reason for the higher concentration of HLA positive cells remaining the MSC group compared
to the MSC-VSVG group, since the regulatory T cells might prevent clearance of the foreign human MSCs. To determine if regulatory T cells could account for all or a portion of the T cell
population in the MSC group, we stained for CD25, the alpha chain of the IL-2 receptor and a known marker of regulatory T cells. CD25 positive cells were observed in the borderzone in
regions that also stained positive for CD3 in MSC mice (Fig. 4d) representing approximately 33% of the CD3 positive cells. Thus decreased blood vessel density and low levels of regulatory T
cells of the MSC-VSVG group with augmented fusion, suggests a potential loss of paracrine and/or immunomodulatory function of MSCs after fusing. DISCUSSION The aim of this study was to
discern the affect of MSC fusion after transplantation on cardiac recovery following myocardial infarction. Three different treatment groups were utilized to help test this aim: a sham
treatment, a traditional MSC cellular transplant and a treatment wherein fusion was augmented in MSCs by the fusogen VSVG. Fusion was observed in both the MSC and MSC-VSVG group with the
MSC-VSVG group showing the highest level of fusion. Unexpectedly, the sham group demonstrated the highest average fold increase in cardiac function (FAC and CO) with the augmented fusion
group performing the worst for cardiac recovery after infarction. As noted earlier, fusion of MSC with parenchymal cells has been shown to aid in recovery of function in other tissues
especially in the case of the liver32,33,34,35, but up to this point it was still unknown how MSC fusion in the heart might affect cardiac function. Our group and others have observed fusion
of MSCs to cells of recipient heart, although most report a low level of fusion (<1% of transplanted cells) and thus the affect of the fusion on overall cardiac function was assumed to
be minimal25,30. Here we report a decrease in function in the treatment group in which fusion was directly augmented. To our knowledge this is the first study to directly study the
relationship between MSC fusion and cardiac function. High variability in functional outcomes between groups, especially with fusion, led to a shift in our study design from tissue outcomes
and therefore more mice (here we report five per study group) to per mouse correlative analyses and more in depth analysis of cellular-scale outcomes. This led to the separation of each
mouse in the study and direct comparison of the amount of fusion detected in each mouse to the FAC and CO observed during the course of the study. Within one week of infarction and
transplantation, we observed a positive correlation between the amount of fusion and cardiac functional parameters. However, this correlation was lost at later time points in the study. This
suggests that perhaps MSC fusion after transplantation may have a transient positive affect due to increased cell retention at the site of injury as well as increased immune evasion due to
the acquisition of mouse major histocompatibility complexes after fusion. This positive affect appears to be short lived, since the sham treatment is observed to bypass both MSC treatments
at day 28. Especially in the case of the augmented fusion group (MSC-VSVG), it appears that augmenting MSC fusion hinders the ability of MSCs to promote healing. This is in line with very
recent _in vitro_ studies in which human MSCs, when fused with rat neonatal ventricular myocytes, downregulated sarcomeric structures and acquired a non-proliferative and non-contractile
phenotype47. The loss of contractility and proliferation of fusion products between human MSCs and myocytes seen in this _in vitro_ study helps to explain our _in vivo_ observations that MSC
fusion hinders improvement of fractional area change and cardiac output in the infarcted heart. Upon observing a decrease in cardiac function associated with MSC fusion, we probed the
mechanism for decreased function on the cellular level with a focus on MSC retention, vascularization and immune modulation. A cardiac marker (such as cardiac troponin T) was not included in
this study since the frequency of HLA signal in the infarct and healthy heart was extremely low. The MSC group with no fusogen had a higher average level of MSC retention in the borderzone
(though not significant), higher vessel density in the healthy heart as well as a higher concentration of T cells in the borderzone than the MSC-VSVG group with the VSVG fusogen. These
results could explain why the augmented fusion treatment saw reduced tissue-level function compared to the sham group. Increased fusion of MSCs via VSVG, while seeming to promote tissue
function at 7 days after transplantation, appears to prevent MSCs from aiding in recovery at later time points. This could be due to the MSCs undergoing reprogramming after fusion and thus
causing the MSCs to lose their innate abilities such as angiogenesis and immune modulation. The reprogrammed MSCs in the MSC-VSVG group might have lost essential paracrine capabilities,
which rendered them less effective in promoting tissue repair. One might speculate that fusion “forced” via viral fusogen is artificial and therefore is not relevant _in vivo._ However, it
is well documented that viral infection can facilitate fusion _in vivo_20,44,48,49,50,51,52,53,54. In fact, it is possible that viral infection may have been the cause of all or a portion of
the “spontaneous” fusion observed in the MSC group. Indeed, the MSC group saw a negative correlation between amount of fusion and MSC retention as well as vascularization after 56 days.
This spontaneous fusion seen in the MSC group also appears to negatively affect the ability of MSCs to promote healing in the infarcted myocardium. The mice in the MSC group with no fusogen
that exhibited the highest amount of MSC retention and vascularization were the mice with the lowest observed fusion levels. Interestingly, the MSC group with no fusogen and low-level fusion
showed an increase in CD3 positive T cells relative to the MSC-VSVG group and many of these cells were found to colocalize with CD25 positive regulatory T cells in parts the borderzone. The
regulatory T cells may have enabled higher MSC retention and improved cardiac functional response seen in the MSC group. Taken in whole, this study is the first to examine how MSC fusion
after cell transplantation affects cardiac function following myocardial infarction. The negative functional impact observed, even with the small number of mice tested, should be considered
for future clinical trials. MSC transplantation has been shown to be an effective treatment when cell fusion is reported at low levels. However, if cell fusion is somehow increased due to
viral infection or environmental conditions after transplantation, the treatment could result in a loss of function and a negative prognosis (especially in the infarcted heart). METHODS
TRANSGENIC MICE We used transgenic mice that constitutively express Cre recombinase (B6.C-Tg[CMV-cre]1Cgn/J; Jackson Laboratory, Bar Harbor, ME) (15 total mice, 5 per treatment group, 2
months old), such that deletion of _LoxP_-flanked genes occurs in all tissues, including germ cells. The _Cre_ gene is under transcriptional control of the cytomegalovirus (CMV) minimal
promoter and is X-linked. The Cre sequence was introduced to BALB/cJ derived BALB/c-I embryonic stem cells (ESCs). The resulting mice were backcrossed to the BALB/c background for 8
generations and then backcrossed to the C57BL/6J background for 10 generations55. Only male Cre mice were used in the study owing to a false-positive signal detected when imaging the female
transgenic mice (data not shown). CELL CULTURE Human MSCs derived from human embryonic stem cells (MSCs from WA-01, a gift from Dr. Peiman Hematti, University of Wisconsin-Madison, Madison,
WI. WA-01 cells were obtained via a protocol approved by the University of Wisconsin-Madison, Institutional Review Board) were expanded and cultured, as previously described56. In brief,
MSCs were cultured on a 0.1% gelatin (Sigma-Aldrich, St. Louis, MO) pretreated flask containing α-minimum essential medium (MEM)-complete. Complete α-MEM consisted of α-MEM (Invitrogen,
Carlsbad, CA), 10% fetal bovine serum (HyClone Laboratories, Logan, UT), 0.1 mM nonessential amino acids (Invitrogen) and 2 mM l-glutamine (Invitrogen). hMSC cultures were allowed to grow to
60%–70% confluence and were replated at a concentration of 1,500 cells per cm2. These human ESC-derived MSCs have cell surface markers, differentiation potential and immunologic properties
_in vitro_ that are similar to those of adult BM-derived MSCs56. GENE TRANSFER MSCs were transiently transfected with viral fusogen VSV-G20,57 to promote cell-cell fusion (MSC-VSVG) or no
fusogen (MSC). In addition, MSCs in both the MSC and MSC-VSVG groups were simultaneously transfected with the luciferase gene adjacent to a floxed stop codon (p231 pCMVe-betaAc-STOP-luc;
Addgene, Cambridge, MA)23. Transfection was accomplished using the Neon Transfection System (Invitrogen), as previously described58 (Supplementary Figure S1). All recombinant DNA research
was conducted according to NIH guidelines and in accordance with the University of Wisconsin-Madison and University of Minnesota-Twin Cities institutional biosafety committees. MYOCARDIAL
INFARCTION AND CELL DELIVERY Mice underwent an infarction procedure by left coronary artery ligation, such as is routinely performed at the University of Wisconsin Cardiovascular Physiology
Core Facility23,44,59. Transfected MSCs (MSC or MSC-VSVG) were delivered to the myocardium of mice immediately after infarction via a collagen patch (TissueMend; TEI Biosciences, Boston,
MA), as previously described20,43,44. A control (sham) was performed with only the infarction and no patch delivery. TissueMend matrices (2 × 2 × 0.8 mm) were placed in a 24-well plate
(Falcon; Thermo Fisher Scientific, Pittsburgh, PA) and hydrated with α-MEM-complete culture medium. After electroporation, MSCs were seeded on the TissueMend sections at a concentration of 1
× 106 cells per milliliter. The medium was changed at 24 and 48 hours, at which point the TissueMend matrix, containing ∼1 × 105 transfected MSCs, was attached to the myocardium with a
single suture (7-0 Prolene; Ethicon, Johnson & Johnson, New Brunswick, NJ) at each corner of the matrix. A matrix was placed such that it was in contact with both the infarct and the
peri-infarct regions of the myocardium20,43,44. All animal procedures were performed in accordance with the guidelines of the American Association for Laboratory Animal Science and were
approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee and the University of Minnesota-Twin Cities Institutional Animal Care and Use Committee.
BIOLUMINESCENT IMAGING Recipient mice constitutively expressed Cre recombinase; therefore, when transplanted human MSCs fused with cells of the recipient, the _LoxP_ sites were cleaved and
the stop signal was excised, allowing expression of luciferase. Luciferase expression was detected 7 days after cell transplantation in living mice using an _in vivo_ imaging system (IVIS)
(IVIS Spectrum; Caliper Life Sciences, Hopkinton, MA), as previously described23,43. The average radiance was determined by measuring the emitted photons per second per cm2 per steradian of
the heart region using the Living Image _In Vivo_ Imaging Software (PerkinElmer, Life and Analytical Sciences, Waltham, MA). ECHOCARDIOGRAPHY Mice underwent echocardiography 3 days
post-infarction/cell delivery to obtain a baseline measurement of each mouse’s cardiac function. Further echocardiography was repeated at 7, 14 and 28 days after infarction/cell delivery to
track mouse cardiac health over time. Transthoracic echocardiography was performed by using a Visual Sonics 770 ultrasonograph with a 30-MHz transducer (RMV 707B) (Visual Sonics, Toronto).
Mice were lightly anesthestized with isoflurane (1%) and maintained on a heated platform. Two-dimensionally guided M-mode images of the long axis of the LV were acquired with the probe in
different 3 planes, 1) sagittal plane, 2) 45% to the sagittal plane and 3) frontal plane. Images were recorded and the LV endocardial area traced at end-diastole and systole. Volumes were
calculated from these areas and function expressed as fractional area change (FAC, %) and cardiac output (CO, mL/min). All parameters were measured over at least three consecutive cycles.
FLUORESCENCE MICROSCOPY Murine hearts were harvested 8 weeks after cell delivery to determine the amount of MSC retention, vascularization and immune response at the cellular level. After
excision, the hearts were bisected longitudinally through the matrix. The hearts were immediately placed into 10% buffered formalin (pH 7.2; Thermo Fisher Scientific) for 24 hours, followed
by 24 hours of fresh 10% buffered formalin and a final 24-hour incubation in 70% ethanol. The samples were further processed for paraffin embedding and sectioning, as previously described60.
For immunohistochemistry (IHC) analysis, heart sections were deparaffinized by incubating at 60 °C for 1 hour and then washed for 6 minutes in Xylene twice. The sections were rehydrated by
dipping the sections 15 times each in 100% ethanol, 100% ethanol, 95% ethanol, and, finally, ultrapure water. Antigen retrieval was accomplished either by incubating the sections for 20
minutes at 37 °C in 0.5% pepsin (Thermo Fischer Scientific) in 5 mM HCl for human leukocyte antigen (HLA) (monoclonal mouse anti-HLA-A,B,C; EMR8-5; MBL International Corp., Woburn, MA) or by
incubating the sections for 25 minutes at 95 °C in citrate buffer (10 mM sodium citrate (Fisher Scientific), pH 6, 0.01% Triton-X 100 (Sigma-Aldrich)) for CD31 (polyclonal rabbit
anti-PECAM-1(CD31); M-185 sc-28188; Santa Cruz Biotechnology, Santa Cruz, CA), CD3 (monoclonal mouse anti-CD3; F7.2.38; Dako, Carpinteria, CA) and CD25 (monoclonal rat anti-mouse CD25; 7D4
(RUO); BD Pharmingen, San Jose, CA). The sections were removed and allowed to cool for 10 minutes at room temperature. The sections were rinsed in 1x phosphate-buffered saline (PBS) twice
for 3 minutes. A 1:25 dilution of the anti-HLA-A,B,C, -CD3 or -CD25 antibodies or a 1:50 dilution of anti-CD31 was made with dilution buffer containing 5% bovine serum albumin (HyClone), 2%
goat serum (MP Biomedical, Solon, OH), 1% glycine (Sigma-Aldrich) and 0.1% triton-X (MP Biomedical). Next, 40 μl of this antibody solution was placed on each tissue section overnight at 4
°C. The sections were washed with 1x PBS and incubated for 45 minutes at 4 °C with 40 μl of a 1:200 dilution of the secondary antibody (AF647 goat anti-mouse for HLA and CD3 or AF647 goat
anti-rabbit for CD31; Invitrogen) in dilution buffer. The sections were washed with 1x PBS and mounted using 1,4-diazabicyclo[2.2.2]octane (Dabco)/DAPI solution composed of 5% Dabco
(Sigma-Aldrich) and 0.01% DAPI (Sigma-Aldrich) in a mixture of 50% glycerol (Thermo Fischer Scientific) and 50% 2 × PBS on a microscope coverslip sealed with nail polish. Fluorescence
emission was detected using an IX71 inverted deconvolution fluorescence microscope (Olympus, Center Valley, PA). The images were acquired with a 10× or 20× UPlanFluor objective (NA = 0.5),
using Metamorph software (Molecular Devices, Sunnyvale, CA) and analyzed using ImageJ (Fiji; open source software, http://pacific.mpi-cbg.de/wiki/index.php/Fiji). All hearts were stained for
each antibody and 5–20 images per region were quantified for positive expression with the number depending on the size of the region in each heart. Background fluorescence was determined
using a secondary antibody-only control to set a threshold for antibody detection. STATISTICAL ANALYSIS Statistical analyses were performed using analysis of variance with Tukey’s honest
significant difference post hoc test for multiple comparisons or Student’s t test for 2 independent samples; _P_ < 0.05 was considered significant. Correlations that measure the linear
correlation between the luminescent signal (fusion) and a second parameter were calculated using Pearson’s correlation coefficient (ρ). Data were analyzed with SigmaPlot (Systat Software
Inc, San Jose, CA, http://www.systat.com). ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Freeman, B. T. and Ogle, B. M. Viral-mediated fusion of mesenchymal stem cells with cells of the
infarcted heart hinders healing via decreased vascularization and immune modulation. _Sci. Rep._ 6, 20283; doi: 10.1038/srep20283 (2016). REFERENCES * Mozaffarian, D. et al. Heart disease
and stroke statistics–2015 update: a report from the American Heart Association. Circulation 131, e29–322 (2015). Article PubMed Google Scholar * Abdel-Latif, A. et al. Adult bone
marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Archives of internal medicine 167, 989–997 (2007). Article PubMed Google Scholar * Amado, L. C. et al.
Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proceedings of the National Academy of Sciences of the United States of
America 102, 11474–11479 (2005). Article ADS CAS PubMed PubMed Central Google Scholar * Chen, S. et al. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells
for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. The Journal of invasive cardiology 18, 552–556 (2006). PubMed Google Scholar * Chen, S. L. et
al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. The American journal of
cardiology 94, 92–95 (2004). Article PubMed Google Scholar * Ma, J. et al. Time course of myocardial stromal cell-derived factor 1 expression and beneficial effects of intravenously
administered bone marrow stem cells in rats with experimental myocardial infarction. Basic research in cardiology 100, 217–223 (2005). Article CAS PubMed Google Scholar * Shake, J. G. et
al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. The Annals of thoracic surgery 73, 1919–1925; discussion 1926 (2002). Article
ADS PubMed Google Scholar * Tomita, S. et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 100, II247-256 (1999). Article CAS PubMed
Google Scholar * Mouiseddine, M. et al. Human mesenchymal stem cells home specifically to radiation-injured tissues in a non-obese diabetes/severe combined immunodeficiency mouse model. The
British journal of radiology 80 Spec No 1, S49–55 (2007). Article CAS PubMed Google Scholar * Nagaya, N. et al. Intravenous administration of mesenchymal stem cells improves cardiac
function in rats with acute myocardial infarction through angiogenesis and myogenesis. American journal of physiology. Heart and circulatory physiology 287, H2670–2676 (2004). Article CAS
PubMed Google Scholar * Ankrum, J. A., Ong, J. F. & Karp, J. M. Mesenchymal stem cells: immune evasive, not immune privileged. Nature biotechnology 32, 252–260 (2014). Article CAS
PubMed PubMed Central Google Scholar * Chen, G. et al. Marrow stromal cells for cell-based therapy: the role of antiinflammatory cytokines in cellular cardiomyoplasty. The Annals of
thoracic surgery 90, 190–197 (2010). Article PubMed Google Scholar * Caplan, A. I. Why are MSCs therapeutic? New data: new insight. The Journal of pathology 217, 318–324 (2009). Article
CAS PubMed Google Scholar * Caplan, A. I. & Dennis, J. E. Mesenchymal stem cells as trophic mediators. Journal of cellular biochemistry 98, 1076–1084 (2006). Article CAS PubMed
Google Scholar * Gnecchi, M. et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB journal :
official publication of the Federation of American Societies for Experimental Biology 20, 661–669 (2006). Article CAS Google Scholar * Kamihata, H. et al. Implantation of bone marrow
mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands and cytokines. Circulation 104, 1046–1052
(2001). Article CAS PubMed Google Scholar * Kinnaird, T. et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote _in vitro_ and
_in vivo_ arteriogenesis through paracrine mechanisms. Circulation research 94, 678–685 (2004). Article CAS PubMed Google Scholar * Linke, A. et al. Stem cells in the dog heart are
self-renewing, clonogenic and multipotent and regenerate infarcted myocardium, improving cardiac function. Proceedings of the National Academy of Sciences of the United States of America
102, 8966–8971 (2005). Article ADS CAS PubMed PubMed Central Google Scholar * Nygren, J. M. et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency
through cell fusion, but not transdifferentiation. Nature medicine 10, 494–501 (2004). Article CAS PubMed Google Scholar * Kouris, N. A. et al. Directed Fusion of Mesenchymal Stem Cells
with Cardiomyocytes via VSV-G Facilitates Stem Cell Programming. Stem cells international 2012, 414038 (2012). Article PubMed PubMed Central CAS Google Scholar * Matsuura, K. et al.
Cardiomyocytes fuse with surrounding noncardiomyocytes and reenter the cell cycle. The Journal of cell biology 167, 351–363 (2004). Article CAS PubMed PubMed Central Google Scholar *
Oh, H. et al. Cardiac progenitor cells from adult myocardium: homing, differentiation and fusion after infarction. Proceedings of the National Academy of Sciences of the United States of
America 100, 12313–12318 (2003). Article ADS CAS PubMed PubMed Central Google Scholar * Sprangers, A. J., Freeman, B. T., Kouris, N. A. & Ogle, B. M. A Cre-Lox P recombination
approach for the detection of cell fusion _in vivo_. Journal of visualized experiments : JoVE e3581 (2012). * Zhang, S. et al. Both cell fusion and transdifferentiation account for the
transformation of human peripheral blood CD34-positive cells into cardiomyocytes _in vivo_. Circulation 110, 3803–3807 (2004). Article PubMed Google Scholar * Alvarez-Dolado, M. et al.
Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425, 968–973 (2003). Article ADS CAS PubMed Google Scholar * Deb, A. et al. Bone
marrow-derived cardiomyocytes are present in adult human heart: A study of gender-mismatched bone marrow transplantation patients. Circulation 107, 1247–1249 (2003). Article PubMed Google
Scholar * He, X. Q. et al. Co-culture with cardiomyocytes enhanced the myogenic conversion of mesenchymal stromal cells in a dose-dependent manner. Molecular and cellular biochemistry 339,
89–98 (2010). Article CAS PubMed Google Scholar * Kawada, H. et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial
infarction. Blood 104, 3581–3587 (2004). Article CAS PubMed Google Scholar * Terada, N. et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature
416, 542–545 (2002). Article ADS CAS PubMed Google Scholar * van Berlo, J. H. et al. c-kit+cells minimally contribute cardiomyocytes to the heart. Nature 509, 337–341 (2014). Article
ADS CAS PubMed PubMed Central Google Scholar * Blau, H. M., Chiu, C. P. & Webster, C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell 32, 1171–1180
(1983). Article CAS PubMed Google Scholar * Wang, X. et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 422, 897–901 (2003). Article ADS CAS PubMed
Google Scholar * Willenbring, H. et al. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nature medicine 10, 744–748 (2004). Article CAS PubMed Google Scholar
* Willenbring, H. & Grompe, M. Delineating the hepatocyte’s hematopoietic fusion partner. Cell cycle 3, 1489–1491 (2004). Article CAS PubMed Google Scholar * Vassilopoulos, G.,
Wang, P. R. & Russell, D. W. Transplanted bone marrow regenerates liver by cell fusion. Nature 422, 901–904 (2003). Article ADS CAS PubMed Google Scholar * Cowan, C. A., Atienza,
J., Melton, D. A. & Eggan, K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science 309, 1369–1373 (2005). Article ADS CAS PubMed Google
Scholar * Tada, M., Tada, T., Lefebvre, L., Barton, S. C. & Surani, M. A. Embryonic germ cells induce epigenetic reprogramming of somatic nucleus in hybrid cells. The EMBO journal 16,
6510–6520 (1997). Article CAS PubMed PubMed Central Google Scholar * Palermo, A. et al. Nuclear reprogramming in heterokaryons is rapid, extensive and bidirectional. FASEB journal :
official publication of the Federation of American Societies for Experimental Biology 23, 1431–1440 (2009). Article CAS Google Scholar * Powell, A. E. et al. Fusion between Intestinal
epithelial cells and macrophages in a cancer context results in nuclear reprogramming. Cancer research 71, 1497–1505 (2011). Article CAS PubMed PubMed Central Google Scholar * Dittmar,
T., Nagler, C., Niggemann, B. & Zanker, K. S. The dark side of stem cells: triggering cancer progression by cell fusion. Current molecular medicine 13, 735–750 (2013). Article CAS
PubMed Google Scholar * Harkness, T., Weaver, B. A., Alexander, C. M. & Ogle, B. M. Cell fusion in tumor development: accelerated genetic evolution. Critical reviews in oncogenesis 18,
19–42 (2013). Article PubMed Google Scholar * Wei, H. J. et al. FOXF1 mediates mesenchymal stem cell fusion-induced reprogramming of lung cancer cells. Oncotarget 5, 9514–9529 (2014).
Article PubMed PubMed Central Google Scholar * Freeman, B. T., Kouris, N. A. & Ogle, B. M. Tracking fusion of human mesenchymal stem cells after transplantation to the heart. Stem
cells translational medicine 4, 685–694 (2015). Article PubMed PubMed Central Google Scholar * Kouris, N. A. et al. A nondenatured, noncrosslinked collagen matrix to deliver stem cells
to the heart. Regenerative medicine 6, 569–582 (2011). Article CAS PubMed Google Scholar * Aggarwal, S. & Pittenger, M. F. Human mesenchymal stem cells modulate allogeneic immune
cell responses. Blood 105, 1815–1822 (2005). Article CAS PubMed Google Scholar * Selmani, Z. et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to
suppress T lymphocyte and natural killer function and to induce CD4 + CD25 high FOXP3 + regulatory T cells. Stem cells 26, 212–222 (2008). Article CAS PubMed Google Scholar * Shadrin, I.
Y., Yoon, W., Li, L., Shepherd, N. & Bursac, N. Rapid fusion between mesenchymal stem cells and cardiomyocytes yields electrically active, non-contractile hybrid cells. Scientific
reports 5, 12043 (2015). Article ADS PubMed PubMed Central Google Scholar * Clavel, F. & Charneau, P. Fusion from without directed by human immunodeficiency virus particles. Journal
of virology 68, 1179–1185 (1994). Article CAS PubMed PubMed Central Google Scholar * Duelli, D. & Lazebnik, Y. Cell-to-cell fusion as a link between viruses and cancer. Nature
reviews. Cancer 7, 968–976 (2007). Article CAS PubMed Google Scholar * Gao, P. & Zheng, J. High-risk HPV E5-induced cell fusion: a critical initiating event in the early stage of
HPV-associated cervical cancer. Virology journal 7, 238 (2010). Article PubMed PubMed Central Google Scholar * Hu, L. et al. Human papillomavirus 16 E5 induces bi-nucleated cell
formation by cell-cell fusion. Virology 384, 125–134 (2009). Article CAS PubMed Google Scholar * Joag, S. V. et al. Chimeric simian/human immunodeficiency virus that causes progressive
loss of CD4+T cells and AIDS in pig-tailed macaques. Journal of virology 70, 3189–3197 (1996). Article CAS PubMed PubMed Central Google Scholar * Karlsson, G. B. et al. The envelope
glycoprotein ectodomains determine the efficiency of CD4+T lymphocyte depletion in simian-human immunodeficiency virus-infected macaques. The Journal of experimental medicine 188, 1159–1171
(1998). Article CAS PubMed PubMed Central Google Scholar * Kondo, N., Marin, M., Kim, J. H., Desai, T. M. & Melikyan, G. B. Distinct requirements for HIV-cell fusion and
HIV-mediated cell-cell fusion. The Journal of biological chemistry 290, 6558–6573 (2015). Article CAS PubMed PubMed Central Google Scholar * Schwenk, F., Baron, U. & Rajewsky, K. A
cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic acids research 23, 5080–5081 (1995). Article CAS PubMed
PubMed Central Google Scholar * Trivedi, P. & Hematti, P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Experimental
hematology 36, 350–359 (2008). CAS PubMed PubMed Central Google Scholar * Takada, A. et al. A system for functional analysis of Ebola virus glycoprotein. Proceedings of the National
Academy of Sciences of the United States of America 94, 14764–14769 (1997). Article ADS CAS PubMed PubMed Central Google Scholar * Sprangers, A. J., Freeman, B. T. & Ogle, B. M.
Electroporation can efficiently transfect hESC-derived mesenchymal stem cells without inducing differentiation. Open Stem Cell Journal 62–66 (2011). * Michael, L. H. et al. Myocardial
ischemia and reperfusion: a murine model. The American journal of physiology 269, H2147–2154 (1995). CAS PubMed Google Scholar * Ogle, B. M. et al. Spontaneous fusion of cells between
species yields transdifferentiation and retroviral transfer _in vivo_. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 18, 548–550
(2004). Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biomedical Engineering, University of Minnesota – Twin Cities,
Minneapolis, 55455, MN, USA Brian T. Freeman & Brenda M. Ogle * Stem Cell Institute, University of Minnesota – Twin Cities, Minneapolis, 55455, MN, USA Brian T. Freeman & Brenda M.
Ogle * Department of Biomedical Engineering, University of Wisconsin – Madison, Madison, 53706, WI, USA Brian T. Freeman & Brenda M. Ogle * Masonic Cancer Center, University of Minnesota
– Twin Cities, Minneapolis, 55455, MN, USA Brenda M. Ogle * Lillehei Heart Institute, University of Minnesota – Twin Cities, Minneapolis, 55455, MN, USA Brenda M. Ogle * Institute for
Engineering in Medicine, University of Minnesota – Twin Cities, Minneapolis, 55455, MN, USA Brenda M. Ogle Authors * Brian T. Freeman View author publications You can also search for this
author inPubMed Google Scholar * Brenda M. Ogle View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS B.T.F. Conception and design, collection
and/or assembly of data, data analysis and interpretation, manuscript writing B.M.O. Conception and design, data analysis and interpretation, financial support, administrative support,
provision of study materials, manuscript writing, final approval of manuscript ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC
SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party
material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons
license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Freeman, B., Ogle, B. Viral-mediated fusion of mesenchymal stem cells with cells of the infarcted heart hinders healing via decreased
vascularization and immune modulation. _Sci Rep_ 6, 20283 (2016). https://doi.org/10.1038/srep20283 Download citation * Received: 25 August 2015 * Accepted: 30 December 2015 * Published: 05
February 2016 * DOI: https://doi.org/10.1038/srep20283 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
