Impaired gut-liver-brain axis in patients with cirrhosis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Cirrhosis is associated with brain dysfunction known as hepatic encephalopathy (HE). The mechanisms behind HE are unclear although hyperammonemia and systemic inflammation through
gut dysbiosis have been proposed. We aimed to define the individual contribution of specific gut bacterial taxa towards astrocytic and neuronal changes in brain function using multi-modal
MRI in patients with cirrhosis. 187 subjects (40 controls, 147 cirrhotic; 87 with HE) underwent systemic inflammatory assessment, cognitive testing, stool microbiota analysis and brain MRI
analysis. MR spectroscopy (MRS) changes of increased Glutamate/glutamine, reduced myo-inositol and choline are hyperammonemia-associated astrocytic changes, while diffusion tensor imaging
(DTI) demonstrates changes in neuronal integrity and edema. Linkages between cognition, MRI parameters and gut microbiota were compared between groups. We found that HE patients had a
significantly worse cognitive performance, systemic inflammation, dysbiosis and hyperammonemia compared to controls and cirrhotics without HE. Specific microbial families (autochthonous taxa
negatively and Enterobacteriaceae positively) correlated with MR spectroscopy and hyperammonemia-associated astrocytic changes. On the other hand _Porphyromonadaceae_, were only correlated
with neuronal changes on DTI without linkages with ammonia. We conclude that specific gut microbial taxa are related to neuronal and astrocytic consequences of cirrhosis-associated brain
dysfunction. SIMILAR CONTENT BEING VIEWED BY OTHERS IMPAIRED BRAIN FUNCTION IMPROVED BY L-CARNITINE IN PATIENTS WITH CIRRHOSIS: EVALUATION USING NEAR-INFRARED SPECTROSCOPY Article Open
access 11 August 2020 CEREBRAL SMALL VESSEL DISEASE BURDEN IS ASSOCIATED WITH DECREASED ABUNDANCE OF GUT _BARNESIELLA INTESTINIHOMINIS_ BACTERIUM IN THE FRAMINGHAM HEART STUDY Article Open
access 21 August 2023 THE GUT MICROBIOME IS ASSOCIATED WITH BRAIN STRUCTURE AND FUNCTION IN SCHIZOPHRENIA Article Open access 07 May 2021 INTRODUCTION Hepatic encephalopathy (HE) or brain
dysfunction due to cirrhosis represents a major healthcare burden in subjects with cirrhosis1. Cirrhosis is associated with dysbiosis or an altered gut microbiota that potentiates a systemic
pro-inflammatory milieu2. This inflammatory environment can potentiate neuro-inflammation, brain edema and ultimately neuronal dysfunction in the setting of hyperammonemia3,4. However the
specific contribution of the gut microbiota towards neuro-inflammation, edema and hyperammonemia in cirrhosis is unclear. Brain imaging in patients with cirrhosis and HE reveals differing
impact on neuronal fibers and astrocytes. MR spectroscopy (MRS) findings reveal higher glutamine + glutamate and compensatorily lower myoinositol and choline in astrocytes while diffusion
tensor imaging of long white-matter neuronal tracts shows impaired axonal integrity and edema5. HE treatments, which mostly act on the gut milieu, have varied impacts on MRS and DTI. While
lactulose improves MRS, rifaximin and LOLA do not6,7,8. In contrast rifaximin therapy and lactulose can both favorably impact neuronal integrity as demonstrated by diffusion tensor imaging
(DTI)8,9. While smaller-scale studies have shown that gut bacteria are associated with cognitive performance as a whole, the linkage between microbial taxa and individual components of the
impaired brain response i.e. neuronal and astrocytic impairment is unclear10,11. Given the immense complexity of the gut microbiota and their specific modulation with these therapies, the
relationship between gut taxa and brain MR consequences becomes relevant in defining focused treatment targets. The aim of this study was to define the linkage between gut microbial taxa and
brain MR consequences of neuronal and astrocytic dysfunction in patients with cirrhosis. RESULTS We recruited 187 subjects for this study; 40 healthy controls and 147 cirrhotics.
Eighty-five cirrhotic patients had HE, which was controlled on lactulose and rifaximin. All subjects underwent MRS and 74 subjects underwent DTI. The subjects were age-balanced but controls
had a significantly better cognitive performance and lower systemic inflammation than cirrhotic patients (Table 1), especially those with HE. CIRRHOTIC PATIENTS AND THOSE WITH HE HAD WORSE
COGNITION, INFLAMMATION AND BRAIN MR FINDINGS On MRS, there were significant differences between controls and cirrhotics on creatine ratios of Glx (WM: 1.7 ± 0.3 vs. 2.5 ± 0.8, p <
0.0001; PGM: 1.9 ± 0.2 vs. 2.3 ± 0.7, p = 0.001; ACC: 2.1 ± 0.3 vs. 2.7 ± 0.7, p = 0.004) and mI (WM: 1.0 ± 0.1 vs. 0.5 ± 0.4, p < 0.0001; PGM: 0.8 ± 0.1 vs. 0.5 ± 0.3, p < 0.0001;
ACC: 0.7 ± 0.1 vs. 0.5 ± 0.3, p = 0.001) but not on Choline levels, indicating the impact of hyperammonemia. Cirrhotics with HE were significantly more advanced from the cirrhosis severity
and cognitive impairment perspective compared to cirrhotics without HE (Table 1). Using the PHES and ICT cut-offs separately based on our published healthy control data12, a significantly
higher proportion of prior HE patients had evidence of covert HE by PHES (n = 75, 88% vs. n = 15, 24%,p < 0.001) and by ICT (n = 68, 80% vs n = 20, 32%). Cirrhotics with HE subjects had
higher Glx and lower mI in all three volumes of interest and reduced Cho in all volumes apart from gray matter. In the 74 subjects who underwent DTI, 42 patients had prior HE and had similar
overall demographic and cirrhosis severity characteristics compared to the entire HE group. HE patients had a higher CS and lower FA compared to no-HE patients (Table 2). No changes in MD
between groups were identified. CIRRHOTICS, ESPECIALLY HE PATIENTS, DEMONSTRATED DYSBIOSIS AND ALTERED MICROBIAL FUNCTIONALITY As expected there were significant differences in the
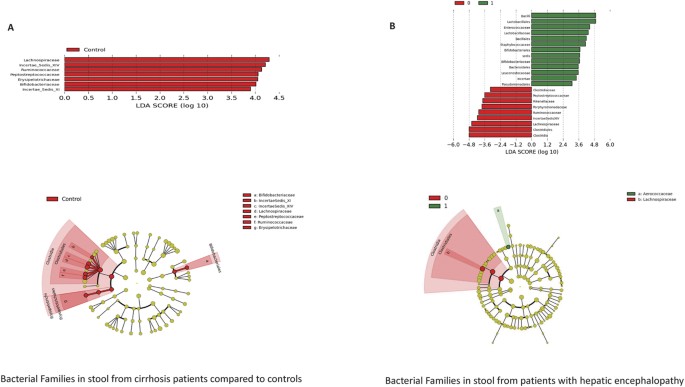
microbiota composition between cirrhotics with and without HE as well as between controls and cirrhotic subjects on UNIFRAC (all p values < = 1.0e − 02). Specifically controls had a
significantly higher proportion of autochthonous bacterial families than cirrhotics (Fig. 1A). Similarly cirrhotic patients with HE had a higher relative abundance of these autochthonous
families and a higher abundance pattern of _Staphylococcaceae, Enterococcaceae, Porphyromonadaceae_ and _Lactobacillaceae_ compared to controls and cirrhotics without HE(Table 3/Fig. 1B). On
PiCRUST, significant differences in predicted stool bacterial functionality was found related to functions of endotoxin and endotoxin-protein synthesis and a shift towards an ammoniagenic
amino acid profile (degradation of branched-chain and metabolism of aromatic amino acids) in controls compared to cirrhotics and in HE patients compared to no-HE cirrhotic patients
(Supplementary Fig. 1A,B). BRAIN MRS AND DTI ARE CORRELATED WITH DIFFERENT GUT MICROBIAL FAMILIES On MRS, there were consistent negative linkages between autochthonous families and positive
ones between potentially pathogenic ones to brain consequences of hyperammonemia i.e. increased Glx and lower mI and Cho, in controls and cirrhotic patients (Figs 2, 3, 4, 5). These brain
changes were also linked with MELD score and serum ammonia levels. These interactions were significantly more complex in patients with HE in whom the non-autochthonous families were
positively linked with poor cognition, brain MRS findings and ammonia. On DTI, a different picture emerged with respect to the families. The family _Porphyromonadaceae_ was linked with all
aspects of DTI. On FA, _Porphyromonadaceae_ relative abundance was correlated negatively with corpus callosum splenium (r = −0.5, p = 0.01), right inferior longitudinal fasciculus (r = −0.4,
p = 0.05), posterior internal capsule (left r = −0.5, p = 0.01, right r = −0.5, p = 0.02), posterior white matter (left r = −0.5, p = 0.01, right p = 0.6, p=0.004). In contrast,
_Prevotellaceae_ relative abundance was positively related with right posterior white matter (r = 0.5, p = 0.04). On spherical isotropy, we found positive correlations between
_Porphyromonadaceae_ and corpus callosum splenium (r = 0.5, p = 0.009), right inferior longitudinal fasciculus (r = 0.4, p = 0.04) and posterior white matter (left r = 0.45, p = 0.03, right
r = 0.6, p = 0.005). There was a significant positive correlation on mean diffusivity between _Porphyromonadaceae_ on corpus callosum genu (r = 0.44, p = 0.03), corpus callosum splenium (r =
0.58, p=0.003), left and right posterior white matter (r = 0.7, p < 0.001 for both), left and right frontal white matter (r = 0.4,p = 0.05 for both)and the right uncinate fasciculus (r =
0.4, p = 0.05). Similarly significant positive correlations were found between _Veillonellaceae_ and mean diffusivity in the bilateral anterior internal capsule (r = 0.4, p = 0.03), corpus
callosum splenium (r = 0.4, p = 0.04), right cingulum (r = 0.5, p = 0.03), external capsule (left r = 0.5, p = 0.03, right r = 0.4, p = 0.05), posterior internal capsule (left r = 0.5, p =
0.02, right r = 0.4, p = 0.05)and the right uncinate fasciculus (r = 0.5, p = 0.004). DISCUSSION The current study is the largest experience of the altered gut-liver-brain axis in humans
with cirrhosis. We found that specific gut microbial changes are linked with systemic inflammation, ammonia and ultimately with neuronal and astrocytic dysfunction in cirrhotic patients,
especially those with HE. Although there is mounting evidence from human and animal studies that gut microbiota modification can impact brain function in cirrhosis, specific linkages between
brain MRI and individual bacterial taxa have not been fully elucidated. While therapies for HE are overwhelmingly gut-based, there is often an additive role for synergistic, systemic
ammonia-scavenging treatments in humans1. There is patho-physiologic evidence supporting both hyperammonemia and inflammation in the causation of HE, with differing effects on neurons and
astrocytes13,14. It has been hypothesized that in humans the dysbiotic gut microbiota is the major source of both ammonia and the systemic pro-inflammatory milieu15. Specifically with the
progression of cirrhosis, the relative reduction in autochthonous commensals and the increase in microbiota such as those belonging to _Enterobacteriaceae_ and _Streptococcaceae_ that can
produce endotoxin and ammonia through their urease activity respectively11. We found indeed that in the human context, the brain MRS, which is largely related to hyperammonemia-associated
astrocytic changes, was related to one set of microbiota while brain DTI, which is related to neuronal integrity and edema, was related to another group of bacteria8. However in patients
with HE there is concurrent hyperammonemia and systemic inflammation, therefore differentiating their individual impact on brain MRI results is not possible. The microbial families that were
negatively linked with brain glial MRS manifestations of ammonia (high Glx and low mI and Cho) were autochthonous families (_Lachospiraceae, Ruminococcaeae_ and Clostridiales XIV). In
contrast families such as _Streptococcacae, Enterobacteriaceae, Lactobacillaceae_ and _Peptostreptococcaceae_ were related positively with ammonia, MELD score and brain MRS manifestations.
These autochthonous taxa are predominant in healthy control studies and mediate several important benefits, such as production of short-chain fatty acids and 7-α de-hydroxylation of bile
acids in hosts16,17. With the progression of cirrhosis in humans, their reduction parallels an increase in potentially harmful taxa such as _Streptococcacae_ and _Enterobacteriaceae_2.
Interestingly, taxa that were associated with DTI were distinct i.e. _Porphyromonadaceae_ relative abundance, from that associated with MRS. Relative abundance of _Porphyromonadaceae_
mirrors a situation of increasing diffuse white matter interstitial edema that is often seen in HE, due to negative associations with FA and positive ones with spherical isotropy and mean
diffusivity6. _Porphyromonadaceae_ have been implicated in cognitive dysfunction and progression of fatty liver disease in prior human and animal studies10,18. They are predominantly oral in
origin but have been implicated in systemic and hepatic inflammation in animal studies that extend beyond the local oral milieu18,19. Our findings now associate this family with neuronal
dysfunction and brain edema. Of note _Porphyromonadaceae_ abundance was not related to ammonia in this dataset. We have reported earlier in a smaller sample size that cognitive dysfunction
in cirrhosis is related with decrease in autochthonous families and increase in _Porphyromonadaceae_10. However our aggregate results indicate that the individual impact of microbial
families may be mediated through impact on different cell types i.e. neurons and astrocytes, in the ultimate pathogenesis of HE-related cognitive dysfunction. Despite prior smaller studies
linking cognition with stool microbiota, this is the largest experience linking gut microbiota with brain MRI results that gives us an understanding of the impaired gut-liver-brain axis in
cirrhosis. This is an association study that helps in hypothesis generation, specifically related to the differences in linkages between bacteria related and unrelated to ammonia in the
pathogenesis of cognitive dysfunction. Given that this was a clinical sample, all of our patients were on treatment with lactulose or rifaximin. However, it would have been unethical to
withdraw this therapy and in a cross-sectional manner, use of these medications did not appreciably reduce systemic inflammation or endotoxemia. We also used the clinically reproducible
definition of prior HE instead of covert HE definitions that are variable based on gold standards1,12. Also as expected, most prior HE patients indeed had evidence of covert HE. It is also
interesting that prior HE treatment studies are varied in their impact on DTI and MRS based on the agent employed, however concurrent microbiota analysis was not performed in most
studies7,8,9. Therefore linking changes in microbiota with changes in brain MRI over time is needed in further studies. Interestingly, _Lactobacillaceae_, which often have probiotic species,
had an increased relative abundance in the stool of HE patients. It was assumed in past human studies that the increased _Lactobacillaceae_ abundance was due to the use of lactulose for HE
therapy20. However, our prior and current human results and prior animal CCL4 studies demonstrate that _Lactobacillaceae_ increase may be a part of an expansion of selected urease-producing
Firmicutes in humans and mouse cirrhosis models2,21,22. An increased cerebral lactate, which has now been hypothesized to be synergistic with glutamine for HE development in some animal
models, could also be precipitated by _Lactobacillaceae_ spp23. We conclude that specific bacterial taxa are associated with astrocytic changes and neuronal changes on brain MRI in humans
with cirrhosis and HE. Specific alterations, which provide potential novel therapeutic targets to restore intestinal and neuronal homeostasis, in the gut microbial milieu could impact
different aspects of brain function in cirrhotic individuals. MATERIALS AND METHODS Outpatients with cirrhosis and age-matched healthy controls were recruited prospectively from Liver
Clinics and the community at Virginia Commonwealth University and McGuire VA Medical Centers. Cirrhosis was diagnosed using biopsy, history of frank decompensation (ascites, HE, variceal
bleeding, jaundice) or varices in a patient with chronic liver disease or those with radiological features of cirrhosis24. We excluded subjects who were unable to provide informed consent,
those with a mini-mental status exam result <25, those who had an uncertain diagnosis of cirrhosis, those with recent (<6 months history) of alcohol or illicit drug misuse and those on
absorbable antibiotics within the last 6 weeks. We only included those with prior type C HE who were controlled on lactulose or rifaximin for at least 3 months with good adherence per
clinic notes and interview before enrollment1. Healthy controls were subjects without chronic diseases who were not receiving any regular medications. All subjects gave written informed
consent and underwent a cognitive assessment consisting of a standard paper-pencil battery of psychometric hepatic encephalopathy score (PHES, number connection tests A and B, digit symbol,
serial dotting and line tracing tests) and the inhibitory control test (lures and targets are outcomes)25. Cirrhotic patients were also divided into covert HE or not using 2 gold standard
tests (PHES and ICT) based on our published control performance12. Blood was drawn for analysis of venous ammonia, endotoxin, inflammatory cytokines (IL-1β, TNF-α, and IL-6) and the MELD
score (validated logarithmic index of serum bilirubin, creatinine and INR)26. Endotoxin and cytokine analysis was performed using validated methods at Assaygate (Ijamsville, MD)27. Subjects
were asked to provide a fresh stool sample for analysis of microbiota relative abundance in a container with RNAlater as described in prior studies28. This protocol was approved by the
Institutional Review Boards at VCU Medical Center and Richmond VA Medical Center and all research activities were carried out in accordance with approved guidelines. SAMPLE ANALYSIS
MICROBIOTA Stool was collected and DNA extracted using published techniques27. We first used Length Heterogeneity PCR (LH-PCR) fingerprinting of the 16S rRNA to rapidly survey our samples
and standardize the community amplification. We then interrogated the microbial taxa associated using Multitag Pyrosequencing (MTPS)29. This technique allows the rapid sequencing of multiple
samples at one time. Microbiome Community Fingerprinting: About 10 ng of extracted DNA was amplified by PCR using a fluorescently labeled forward primer 27F (5′-(6FAM) AGAGTTTGATCCTGGCTCA
G-3′) and unlabeled reverse primer 355R′ (5′-GCTGCCTCCCGTAGGAGT-3′). Both primers are universal primers for bacteria. The LH-PCR products were diluted according to their intensity on agarose
gel electrophoresis and mixed with ILS-600 size standards (Promega) and HiDi Formamide (Applied Biosystems, Foster City, CA). The diluted samples were then separated on a ABI 3130xl
fluorescent capillary sequencer (Applied Biosystems, Foster City, CA) and processed using the Genemapper™ software package (Applied Biosystems, Foster City, CA). Normalized peak areas were
calculated using a custom PERL script and operational taxonomic units (OTUs) constituting less than 1% of the total community from each sample were eliminated from the analysis to remove the
variable low abundance components within the communities. MTPS Specifically, we have generated a set of 96 emulsion PCR fusion primers that contain the 454 emulsion PCR linkers on the 27F
and 355R primers and a different 8 base “barcode” between the A adapter and 27F primer. Thus, each fecal sample was amplified with unique bar-coded forward 16S rRNA primers and then up to 96
samples were pooled and subjected to emulsion PCR and pyrosequenced using a GS-FLX pyrosequencer (Roche). Data from each pooled sample were “deconvoluted” by sorting the sequences into bins
based on the barcodes using custom PERL scripts. Thus, we were able to normalize each sample by the total number of reads from each barcode. We have noted that ligating tagged primers to
PCR amplicons distorts the abundances of the communities and thus it is critical to incorporate the tags during the original amplification step29. MICROBIOME COMMUNITY ANALYSIS We identified
the taxa present in each sample using the Bayesian analysis tool in Version 10 of the Ribosomal Database Project (RDP10). The abundances of the bacterial identifications were then
normalized using a custom PERL script and genera present at >1% of the community were tabulated. We chose this cutoff because of our _a priori_ assumption that genera present in <1% of
the community vary between individuals and have minimal contribution to the functionality of that community and 2,000 reads per sample will only reliably identify community components that
are greater than 1% in abundance. ANALYSIS OF MICROBIOTA QIIME analysis, LEFSe and Kruskal-Wallis tests were used to evaluate changes in overall microbial abundance30. We also performed
Metastats to evaluate changes in relative abundance between groups with correction for the false discovery rate (FDR)31.Predicted bacterial functions were then assessed using PiCRUST
(phylogenetic investigation of communities by reconstruction of unobserved states)32. BRAIN MRI EVALUATION A subset then underwent multi-modal brain MRI assessment on the day of the
cognitive testing and sample collection. All images were acquired on a 3T GE Signa scanner using a quadrature birdcage RF head coil. We tested two strategies to study the impact of cirrhosis
in astrocytes and neurons8,33. For the impact of hyperammonemia-associated astrocytic changes, MR spectroscopy was performed while neuronal white matter integrity was studied using
diffusion tensor imaging (DTI). For MRS, 1H-single voxel spectra were acquired for pre-specified separate volumes of interest (2 × 2 × 2 cm) in the Right Posterior White Matter (RPWM),
anterior cingulate cortex (ACC) and Posterior Gray Matter (PGM) using point-resolved spectroscopy with automated shimming and water suppression (TE/TR/NS/Volume = 35/1500/128/8 cm3). These
specific voxels have been used in several prior studies and have a representation of largely white matter (RPWM), gray matter (PGM) and those with both (ACC)33,34. DTI was performed using a
single shot, spin-echo echoplanar imaging sequence (FOV = 256 mm, slice thickness = 2.0 mm, 70 contiguous axial slices, 128 × 128 matrix, TR = 9000 ms, TE = 80 ms, b-value = 1000 s/mm2, #b0
images = 6, #Diffusion Directions = 64). MRI ANALYSIS MRS The choline (Cho), creatine (Cr), myo-inositol (mI) and glutamate + glutamine (Glx) complex peak areas were computed using LCModel
software and their creatine ratios were used as in prior studies35,36,37. A high Glx and low mI and Cho creatine ratios are associated with cirrhosis and hyperammonemia. DTI: Whole brain
voxel-wise maps of Fractional Anisotropy (FA), mean diffusivity (MD) and spherical isotropy (CS) were constructed from pre-processed DTI images using tools in FSL 5.0.2 (FMRIB’s Software
Library, www.fmrib.ox.ac.uk/fsl)38,39,40,41. These maps were then transformed to standard space using a combination of nonlinear and affine registration tools. The _a priori_ ROIs for major
white matter tracts (Corpus callosum, internal capsule, inferior and superior longitudinal fasciculi, frontal and posterior white matter, uncinate fasciculi, insula and corticospinal tracts)
were created using the DTI-based probabilistic white matter atlases42,43 using a probability threshold of 40%. Mean FA, MD and CS values were extracted from individual maps. A low FA and
high CS and MD indicate interstitial edema. CORRELATION NETWORK ANALYSIS We created correlation networks using tools in the Galaxy Portal at the Microbiome Analysis Center and only included
nodes consisting of microbiota, MELD score, cognitive tests and creatine ratios of mI, Cho and Glx in all three regions of interest which had a p value <0.01 and correlation coefficient
of >0.6 or <−0.6. These networks were created for controls and cirrhotic subjects and then also within cirrhotic subjects into those with and without prior HE44. We then analyzed
correlation differences between cirrhotics with and without HE and these were then visualized in Cytoscape45. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Ahluwalia, V. _et al_. Impaired
Gut-Liver-Brain Axis in Patients with Cirrhosis. _Sci. Rep._ 6, 26800; doi: 10.1038/srep26800 (2016). REFERENCES * Vilstrup, H. et al. Hepatic encephalopathy in chronic liver disease: 2014
Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. _Hepatology_ 60, 715–735, doi: 10.1002/hep.27210
(2014). Article PubMed Google Scholar * Bajaj, J. S. et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. _Journal of hepatology_ 60,
940–947, doi: 10.1016/j.jhep.2013.12.019 (2014). Article CAS PubMed Google Scholar * Shawcross, D. L., Davies, N. A., Williams, R. & Jalan, R. Systemic inflammatory response
exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. _Journal of hepatology_ 40, 247–254 (2004). Article CAS PubMed Google Scholar * Romero-Gomez, M.,
Jover, M., Galan, J. J. & Ruiz, A. Gut ammonia production and its modulation. _Metabolic brain disease_ 24, 147–157, doi: 10.1007/s11011-008-9124-3 (2009). Article CAS PubMed Google
Scholar * Zhang, X. D., Zhang, L. J., Wu, S. Y. & Lu, G. M. Multimodality magnetic resonance imaging in hepatic encephalopathy: an update. _World J Gastroenterol_ 20, 11262–11272, doi:
10.3748/wjg.v20.i32.11262 (2014). Article PubMed PubMed Central Google Scholar * Kale, R. A. et al. Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type
C hepatic encephalopathy. _Hepatology_ 43, 698–706, doi: 10.1002/hep.21114 (2006). Article PubMed Google Scholar * McPhail, M. J. et al. Modulation of neural activation following
treatment of hepatic encephalopathy. _Neurology_ 80, 1041–1047, doi: 10.1212/WNL.0b013e31828726e1 (2013). Article PubMed PubMed Central Google Scholar * Ahluwalia, V. et al. Enhancement
of functional connectivity, working memory and inhibitory control on multi-modal brain MR imaging with Rifaximin in Cirrhosis: implications for the gut-liver-brain axis. _Metabolic brain
disease_ 29, 1017–1025, doi: 10.1007/s11011-014-9507-6 (2014). Article CAS PubMed PubMed Central Google Scholar * Rai, R. et al. Reversal of Low-Grade Cerebral Edema After
Lactulose/Rifaximin Therapy in Patients with Cirrhosis and Minimal Hepatic Encephalopathy. _Clin Transl Gastroenterol_ 6, e111, doi: 10.1038/ctg.2015.38 (2015). Article CAS PubMed PubMed
Central Google Scholar * Bajaj, J. S. et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. _American journal of physiology. Gastrointestinal and liver physiology_
302, G168–175, doi: 10.1152/ajpgi.00190.2011 (2012). Article CAS PubMed Google Scholar * Zhang, Z. et al. Large-scale survey of gut microbiota associated with MHE Via 16S rRNA-based
pyrosequencing. _The American journal of gastroenterology_ 108, 1601–1611, doi: 10.1038/ajg.2013.221 (2013). Article CAS PubMed Google Scholar * Allampati, S. et al. Diagnosis of Minimal
Hepatic Encephalopathy Using Stroop EncephalApp: A Multicenter US-Based, Norm-Based Study. _The American journal of gastroenterology_ 111, 78–86, doi: 10.1038/ajg.2015.377 (2016). Article
PubMed Google Scholar * Butterworth, R. F. Hepatic encephalopathy: a central neuroinflammatory disorder? _Hepatology_ 53, 1372–1376, doi: 10.1002/hep.24228 (2011). Article PubMed Google
Scholar * Bosoi, C. R., Tremblay, M. & Rose, C. F. Induction of systemic oxidative stress leads to brain oedema in portacaval shunted rats. _Liver international : official journal of
the International Association for the Study of the Liver_ 34, 1322–1329, doi: 10.1111/liv.12414 (2014). Article CAS Google Scholar * Shawcross, D. L. Is it time to target gut dysbiosis
and immune dysfunction in the therapy of hepatic encephalopathy? _Expert review of gastroenterology & hepatology_ 9, 539–542, doi: 10.1586/17474124.2015.1035257 (2015). Article CAS
Google Scholar * Chen, Y. et al. Characterization of fecal microbial communities in patients with liver cirrhosis. _Hepatology_ 54, 562–572 (2011). Article PubMed Google Scholar *
Kakiyama, G. et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. _Journal of hepatology_ 58, 949–955 (2013). Article CAS PubMed PubMed Central Google Scholar
* Henao-Mejia, J. et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. _Nature_ 482, 179–185, doi: 10.1038/nature10809 (2012). Article CAS PubMed PubMed
Central ADS Google Scholar * Nakajima, M. et al. Oral Administration of P. gingivalis Induces Dysbiosis of Gut Microbiota and Impaired Barrier Function Leading to Dissemination of
Enterobacteria to the Liver. _Plos one_ 10, e0134234, doi: 10.1371/journal.pone.0134234 (2015). Article CAS PubMed PubMed Central Google Scholar * Riggio, O. et al. Effect of lactitol
and lactulose administration on the fecal flora in cirrhotic patients. _Journal of clinical gastroenterology_ 12, 433–436 (1990). Article CAS PubMed Google Scholar * Fouts, D. E.,
Torralba, M., Nelson, K. E., Brenner, D. A. & Schnabl, B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. _Journal of hepatology_ 56,
1283–1292, doi: 10.1016/j.jhep.2012.01.019 (2012). Article CAS PubMed PubMed Central Google Scholar * Bajaj, J. S. et al. A longitudinal systems biology analysis of lactulose withdrawal
in hepatic encephalopathy. _Metabolic brain disease_ 27, 205–215, doi: 10.1007/s11011-012-9303-0 (2012). Article CAS PubMed Google Scholar * Bosoi, C. R. et al. Increased brain lactate
is central to the development of brain edema in rats with chronic liver disease. _Journal of hepatology_ 60, 554–560, doi: 10.1016/j.jhep.2013.10.011 (2014). Article CAS PubMed Google
Scholar * Schuppan, D. & Afdhal, N. H. Liver cirrhosis. _Lancet_ 371, 838–851, doi: 10.1016/S0140-6736(08)60383-9 (2008). Article CAS PubMed PubMed Central Google Scholar * Bajaj,
J. S., Wade, J. B. & Sanyal, A. J. Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. _Hepatology_ 50, 2014–2021, doi:
10.1002/hep.23216 (2009). Article PubMed Google Scholar * Malinchoc, M. et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts.
_Hepatology_ 31, 864–871, doi: 10.1053/he.2000.5852 (2000). Article CAS PubMed Google Scholar * Bajaj, J. S. et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis
and hepatic encephalopathy and is linked to cognition and inflammation. _American journal of physiology. Gastrointestinal and liver physiology_ 303, G675–685, doi: 10.1152/ajpgi.00152.2012
(2012). Article CAS PubMed PubMed Central Google Scholar * Bajaj, J. S. et al. Gut Microbiota Alterations can predict Hospitalizations in Cirrhosis Independent of Diabetes Mellitus.
_Scientific reports_ 5, 18559, doi: 10.1038/srep18559 (2015). Article CAS PubMed PubMed Central ADS Google Scholar * Gillevet, P., Sikaroodi, M., Keshavarzian, A. & Mutlu, E. A.
Quantitative assessment of the human gut microbiome using multitag pyrosequencing. _Chem Biodivers_ 7, 1065–1075 (2010). Article CAS PubMed PubMed Central Google Scholar * Segata, N. et
al. Metagenomic biomarker discovery and explanation. _Genome Biol_ 12, R60, doi: 10.1186/gb-2011-12-6-r60 (2011). Article PubMed PubMed Central Google Scholar * White, J. R., Nagarajan,
N. & Pop, M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. _Plos Comput Biol_ 5, e1000352 (2009). Article ADS PubMed PubMed
Central Google Scholar * Langille, M. G. et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. _Nat Biotechnol_ 31, 814–821, doi:
10.1038/nbt.2676 (2013). Article CAS PubMed PubMed Central Google Scholar * Ahluwalia, V. et al. Correction of Hyponatremia Improves Cognition, Quality of Life, and Brain Edema in
Cirrhosis. _Journal of hepatology_, doi: 10.1016/j.jhep.2014.07.033 (2014). * Ahluwalia, V. et al. Rifaximin Improves Brain Edema and Working Memory in Minimal Hepatic Encephalopathy: A
Prospective fMRI Study. _Hepatology_ 56, 162A (2012). Google Scholar * Provencher, S. W. Estimation of metabolite concentrations from localized _in vivo_ proton NMR spectra. _Magn Reson
Med_ 30, 672–679 (1993). Article CAS PubMed Google Scholar * Provencher, S. W. Automatic quantitation of localized _in vivo_ 1H spectra with LCModel. _NMR Biomed_ 14, 260–264, doi:
10.1002/nbm.698 (2001). Article CAS PubMed Google Scholar * Sarma, M. K. et al. Multi-dimensional MR spectroscopy: towards a better understanding of hepatic encephalopathy. _Metabolic
brain disease_ 26, 173–184, doi: 10.1007/s11011-011-9250-1 (2011). Article PubMed PubMed Central ADS Google Scholar * Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved
optimization for the robust and accurate linear registration and motion correction of brain images. _NeuroImage_ 17, 825–841 (2002). Article PubMed Google Scholar * Smith, S. M. Fast
robust automated brain extraction. _Human brain mapping_ 17, 143–155, doi: 10.1002/hbm.10062 (2002). Article PubMed PubMed Central Google Scholar * Woolrich, M. W., Ripley, B. D., Brady,
M. & Smith, S. M. Temporal autocorrelation in univariate linear modeling of FMRI data. _NeuroImage_ 14, 1370–1386 (2001). Article CAS PubMed Google Scholar * Westin, C. F. et al.
Processing and visualization for diffusion tensor MRI. _Med Image Anal_ 6, 93–108, doi: S1361841502000531 (2002). Article PubMed Google Scholar * Wakana, S. et al. Reproducibility of
quantitative tractography methods applied to cerebral white matter. _Neuroimage_ 36, 630–644, doi: S1053-8119(07)00138-3 (2007). Article PubMed Google Scholar * Hua, K. et al. Tract
probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. _Neuroimage_ 39, 336–347, doi: S1053-8119(07)00688-X(2008). * Naqvi, A., Rangwala,
H., Keshavarzian, A. & Gillevet, P. Network-based modeling of the human gut microbiome. _Chem Biodivers_ 7, 1040–1050 (2010). Article CAS PubMed PubMed Central Google Scholar *
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. _Genome Res_ 13, 2498–2504 (2003). Article CAS PubMed PubMed Central
Google Scholar Download references ACKNOWLEDGEMENTS Funded partly using grants from NIDDK Grant R01DK089713, VA Merit Review grant CX10076, and the McGuire Research Institute (JSB). The
authors acknowledge Mary Beatty-Brooks from McGuire VAMC for help with figures. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Gastroenterology, Hepatology and Nutrition, Virginia
Commonwealth University and McGuire VA Medical Center, Richmond, Virginia, USA , Vishwadeep Ahluwalia, Melanie B White, Ariel B Unser, Andrew Fagan, Kalyani Daita, Douglas M Heuman &
Jasmohan S Bajaj * Microbiome Analysis Center, George Mason University, Manassas, Virginia, USA Naga S Betrapally, Patrick M Gillevet & Masoumeh Sikaroodi * Microbiology and Immunology,
Virginia Commonwealth University and McGuire VA Medical Center, Richmond, Virginia, USA Phillip B Hylemon & Huiping Zhou Authors * Vishwadeep Ahluwalia View author publications You can
also search for this author inPubMed Google Scholar * Naga S Betrapally View author publications You can also search for this author inPubMed Google Scholar * Phillip B Hylemon View author
publications You can also search for this author inPubMed Google Scholar * Melanie B White View author publications You can also search for this author inPubMed Google Scholar * Patrick M
Gillevet View author publications You can also search for this author inPubMed Google Scholar * Ariel B Unser View author publications You can also search for this author inPubMed Google
Scholar * Andrew Fagan View author publications You can also search for this author inPubMed Google Scholar * Kalyani Daita View author publications You can also search for this author
inPubMed Google Scholar * Douglas M Heuman View author publications You can also search for this author inPubMed Google Scholar * Huiping Zhou View author publications You can also search
for this author inPubMed Google Scholar * Masoumeh Sikaroodi View author publications You can also search for this author inPubMed Google Scholar * Jasmohan S Bajaj View author publications
You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.S.B., P.M.G. and P.B.H. were involved in conceptualization of the study and in all aspects, P.M.G., K.D., H.Z.,
M.S. and N.S.B. were involved in microbiota and systems biology analysis, J.S.B., V.A., M.B.W., A.B.U., A.F. and D.M.H. were involved in recruitment and human study activities, all authors
were involved in critical revision of this manuscript. CORRESPONDING AUTHOR Correspondence to Jasmohan S Bajaj. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (PDF 417 KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International
License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is
not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ahluwalia, V., Betrapally, N., Hylemon, P. _et al._ Impaired Gut-Liver-Brain Axis in
Patients with Cirrhosis. _Sci Rep_ 6, 26800 (2016). https://doi.org/10.1038/srep26800 Download citation * Received: 26 February 2016 * Accepted: 10 May 2016 * Published: 26 May 2016 * DOI:
https://doi.org/10.1038/srep26800 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
