RETRACTED ARTICLE: Acute Toxicity and Gastroprotection Studies of a New Schiff Base Derived Manganese (II) Complex against HCl/Ethanol-Induced Gastric Ulcerations in Rats
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Manganese is a crucial element for health. In this study, the gastroprotective efficacy of Mn (II) complex (MDLA) against acidified ethanol (HCl/Ethanol)-induced gastric ulceration in rats
was evaluated. The animals were distributed into 5 groups. Groups 1 and 2 received carboxymethylcellulose (CMC), group 3 was pretreated with omeprazole, and groups 4 and 5 were given 10 and
20 mg/kg of MDLA, respectively. After one hour, CMC and HCl/Ethanol were given to groups 2–5 whilst the animals in group 1 were ingested with CMC. After sacrifice, gastric lesions were
evaluated by wall mucus, gross appearance, histology, antioxidant enzymes and immunohistochemistry. Group 2 displayed severe gastric damage with a significant reduction in wall mucus.
Conversely, gastric lesions were reduced in groups 3–5 by 85.72%, 56.51% and 65.93%, respectively. The rats in groups 3–5 showed up-regulation of heat shock protein 70 (Hsp70) with
down-regulation of Bcl-2-associated protein x (Bax). Pretreatment with omeprazole or MDLA led to an increase in the uptake of Periodic Acid Schiff (PAS) stain in the glandular part of the
gastric tissue, raised levels of prostaglandin E2 (PGE2) and superoxide dismutase (SOD), and a reduction in malondialdehyde (MDA) concentrations. These results suggested the gastroprotective
action of Mn (II) complex.
Gastric ulcer is the most common gastrointestinal pathology and affects approximately 10–15% of the world’s population; its prevalence rate is associated with age and sex, as well as
lifestyle1. This disease is characterized by mucosal impairment in the gastric accompanied by stomachache, vomiting, loss of appetite and weight, and hemorrhage and perforation. The
progression of gastric ulceration is attributed to infection by Helicobacter pylori, overuse of non-steroidal anti-inflammatory drugs (NSAIDs) and immunosuppressive treatments, as well as
alcohol abuse and smoking2.
Although, the gastrointestinal tract is commonly exposed to countless microbes and harmful substances as well as food antigens, the surface of gastric mucosa is protected by a distinct
barrier mechanism. Certain immune reactions to these antigens enhance the mucosal defense system to sustain homeostasis of the digestive system3. The inability of the defensive factors, such
as bicarbonate secretion, mucus-bicarbonate barrier, surface active phospholipids, prostaglandins (PGs), mucosal microcirculation, cell regeneration, endogenous antioxidants, endogenous
nitric oxide and certain growth factors to adequately suppress the aggressive factors, which comprise gastric acid and pepsin secretion, Helicobacter pylori, refluxed bile, release of
leukotrienes and reactive oxygen species (ROS), result in stomach injury4.
Even though developments have been conducted in terms of a cure of gastric ulcerations, the mortality rates are still high5. The current medications for the treatment of ulcerations suffer a
serious disadvantage as they are accompanied by high decline rates and increasing side effects6.
Authentication of the effectiveness and utilization of synthetic agents for the cure of gastric ulceration disease is a promising way to overcome the shortcomings of orthodox medications7.
In this respect, Schiff bases are deemed to be a substantial category of organic agents in the field of medicinal chemistry8, and the study of new Schiff base complexes accompanied by
therapeutic efficacy is attracting the interest of researchers9. Schiff bases, along with their transition metal complexes, are multifunctional agents resulting from the reaction of an amino
candidate with a carbonyl agent. In addition, they are extensively utilized for industrial objectives and in a wide range of pharmacological applications comprising antifungal,
antibacterial, antimalarial, antiproliferative, anti-inflammatory, antiviral, and antipyretic activities10.
Manganese (Mn), which is a crucial element for human health and acts as a significant part of the antioxidant system, is found in several enzymes, such as mitochondrial superoxide
dismutases, glutamine synthetase, alkaline phosphatase, and arginase11. Although surplus Mn2+ is poisonous and can result in damage, and lead to a Parkinsonian-like syndrome12, lower
concentrations can have a defensive effect by decreasing the radical dotOH to yield Mn(OH)2+13. In addition, manganese-containing superoxide dismutase (Mn-SOD) has attracted specific
interest since it enhances the antioxidant properties and contributes to cancer protection14.
The present study was performed to study the mechanism of the anti-ulcerogenic effect of the new Mn (II) complex with a Schiff base derived from 4-dimethylaminobenzaldehyde with L-asparagine
(MDLA) against acidified ethanol (HCl/Ethanol)-induced gastric ulcers in rats.
All chemicals and reagents were purchased from Sigma (Sigma Aldrich, Germany) and used without further purification. In addition, malondialdehyde (MDA), superoxide dismutase (SOD) and
prostaglandin E2 (PGE2) Kits were obtained from Cayman Chemical Company (Cayman, USA). Omeprazole was utilized as a reference antiulcer medication and was acquired from the University of
Malaya Medical Centre (UMMC) Pharmacy. This medication was prepared as a suspension in 0.5% (w/v) carboxymethylcellulose (CMC) and intragastrically administered to the rats at a dose of 20
mg/kg body weight (5 ml/kg) according to the suggestions of Miranda et al.15.
The Schiff base formed from 4-dimethylaminobenzaldehyde and L-asparagine was prepared by adding 25 ml of 4-dimethylaminobenzaldehyde ethanolic solution (1.49, 0.01 mol) to an equal amount of
ethanolic solution of L-asparagine (0.01 mol). The mixture was refluxed for two hours. The product that formed was collected by filtration, washed several times with ethanol and
recrystallized from hot ethanol16.
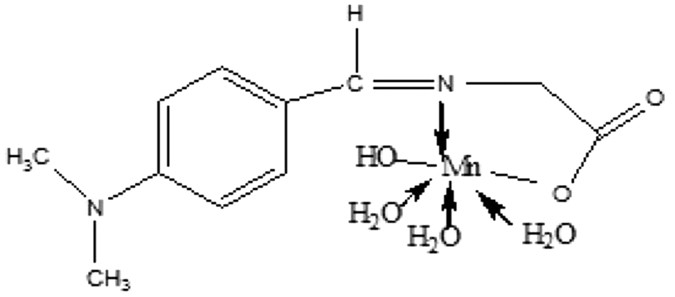
The Mn (II) complex (Fig. 1) was prepared by adding 25 ml of ethanolic solution of metal chloride (0.01 mole) with ethanolic solutions of the prepared Schiff base (0.01 mole) followed by the
drop-wise addition of aqueous ammonia. The resulting mixture was refluxed for two hours and the metal complex compounds that precipitated out were filtered and then washed repeatedly with
hot ethanol until the washing was colorless. The product was air dried over phosphorus penta-oxide16. Elemental analysis and spectral characterization for the ligand and its metal complex
are presented in Table 1.
All the methods were carried out in accordance with the approved guidelines of the Institutional Animal Ethical Committee of the University of Malaya [Ethic certificate no. (PM/27/7/2014/RAB
(R)]. Pathogen-free Sprague-Dawley rats with an average body weight of (200–220 g), were provided by the Animal House, Faculty of Medicine, University of Malaya, Kuala Lumpur. The rats were
fed a standard diet and tap water ad libitum, and were kept separately in cages with wide-mesh wire bottoms to prevent coprophagia throughout the experiment.
The acute toxicity study was carried out to determine a nontoxic dosage for MDLA. Thirty-six rats (18 male and 18 female) were separately and equally allocated into 3 groups labeled as
vehicle (0.5% CMC, 5 ml/kg) or as 500 or 1000 mg/kg of MDLA (5 ml/kg). The animals were deprived of food overnight before treating. Food was withdrawn for an additional 3 to 4 h after
treatment. The rats were monitored for 48 hours after the intragastric administration of the MDLA for toxicological signs. Death cases were recorded over a duration of 14 consecutive days.
All the rats were killed via an overdose of xylazine and ketamine anesthesia on the 15th day and then histological evaluation and serum analysis were implemented following the standard
techniques17,18.
The animals were randomly distributed into 5 groups of 6 rats each in separate cages with wide-mesh wire bottoms to prevent coprophagia during the experiment. Animals were deprived of food
for 24 h but allowed free access to drinking water up to 2 hours before conducting the experimentation. The gastric ulceration model was induced using acidified ethanol solution (150 mM
Hcl/absolute ethanol) 40: 60 v/v, (Hcl/ethanol solution) based upon a published protocol with some modification15. For groups 1 and 2, the vehicle (0.5% CMC) was administered
intragastrically. Meanwhile, group 3 received an oral dosage of 20 mg/kg omeprazole in 0.5% CMC (5 ml/kg), and groups 4–5 were administered MDLA at doses of 10 and 20 mg/kg, respectively.
These dosages were administered as pre-treatment. One-hour after pre-treatment, the vehicle and acidified ethanol (HCl/Ethanol) were intragastrically administered to group 1 and groups 2–5,
respectively. The rats were euthanized (xylazine and ketamine) after 60 min, and their stomach tissues were dissected.
The stomachs were removed, opened along the greater curvature, and their contents were placed in labeled tubes and centrifuged at 2000 rpm for 10 min. The pH of the resultant supernatant was
recorded using a digital pH meter (PA 200, Marconi S.A, Brazil). Quantitative estimation assay of the gastric mucus was implemented according to the methodology previously reported by Corne
et al.19.
The ulcerative injuries (mm2) were investigated using a 10× magnifier lens to assess the formation of ulcerations. The sum of the ulcer area for each animal was calculated and used as the
ulcer index (UI). The Inhibition/Gastroprotection percentage (I %) was calculated according to the following formula:
Inhibition percentage (I %) = [(UI control − UI treated) ÷ UI control] × 100%.
Gastric tissue specimens were rinsed thoroughly and then homogenized using a mortar. The homogenized tissues (10% w/v) were prepared in an ice-cold 50 mM phosphate buffer (pH 7.4) comprising
a mammalian protease inhibitor cocktail. The homogenates were then centrifuged at 4,000 rpm for 10 minutes (4 °C). The resulting supernatant was employed to quantify the enzymatic
activities.
SOD activity was determined using the method described by Sun et al.20. The suppression of the photochemical reduction of nitroblue tetrazolium (NBT) to produce blue colored formazan salt in
existence of phenazine methosulphate (PMS) besides reduced nicotinamide adenine dinucleotide (NADH) was assessed at 560 nm using n-butanol as blank. SOD activity was expressed as units/mg
protein.
Tissue malondialdehyde (MDA) (mmol/L) was measured using the previously reported method of Draper and Hadley21. In brief, the reaction mixture comprising 8.1% sodium dodecyl sulfate, 20%
acetate buffer (pH 3.5), and 0.8% thiobarbituric acid (TBA) was added to 0.2 ml of gastric tissue homogenate for 3 min. Subsequently, the mixture was incubated at 95 °C for 60 min and then
left for cooling. After that, the TBA-reactive substance, MDA, was extracted with 1 ml of H2O and 2.5 ml of an n-butanol:pyridine mixture (15:1, v/v). The surface organic layer comprising
the MDA, which was generated by lipid peroxidation, was read at 532 nm. The absorbance assessed at 532 nm was presented as nM of MDA.
The stomachs mucosa were weighed, crushed by scissors, and then homogenized at 48 °C in a phosphate buffered saline (PBS) buffer. After that, homogenates were centrifuged at 13,400 rpm for
10 min at room temperature. The pure supernatants were served to determine the concentrations of PGE2 through a PGE2 monoclonal enzyme immunoassay kit (Sigma-Aldrich, Malaysia).
Small pieces of the stomach wall were fixed using 10% buffered formalin for 18 h at 4 °C and then immersed in paraffin wax. Subsequently, sections of the stomach were prepared by the
microtome at a thickness of 5 μm, and stained with Hematoxylin and Eosin (H & E) for histological assessment.
Sections of 5 μm thickness from the gastric glandular part were stained with Periodic acid Schiff (PAS) stain to detect the mucus secretion and to assess the variations in both acidic and
basic glycoproteins22.
The immunohistochemical technique was conducted using (Dako cytomation, USA). Briefly, the tissue section slides were placed in a hot-air oven for 25 min at 60 °C (Venticell, MMM,
Einrichtungen, Germany). De-paraffinization of the tissue sections was done using xylene and graded alcohol. After that, slides were boiled in antigen retrieval solution and then incubated
with biotinylated primary antibodies of heat shock protein 70 (Hsp70) (1:500) and Bcl-2-associated protein x (Bax) (1:200) for 15 min. Subsequently, streptavidin conjugated to horseradish
peroxidase was added to the slides and then incubated for 15 min. Further incubation for 5 min was done after adding DAB-substrate-chromagen to the slides. Finally, slides were immersed in
hematoxylin for 5 sec, washed with distilled water and dipped in weak ammonia (0.037 mol/L) 10 times. Positive results of the immunohistochemical staining can be observed as brown areas
under a light microscope.
All the results were presented as mean ± S.E.M. The data were analyzed using one-way ANOVA followed by Tukey’s post hoc test for multiple comparison using SPSS 18 (Statistical Package for
the Social Sciences) software. The probability of p
