The multifaceted risa regulon of bordetella pertussis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The whooping cough agent _Bordetella pertussis_ regulates the production of its virulence factors by the BvgA/S system. Phosphorylated BvgA activates the virulence-activated genes
(_vag_s) and represses the expression of the virulence-repressed genes (_vrg_s) via the activation of the _bvgR_ gene. In modulating conditions, with MgSO4, the BvgA/S system is inactive,
and the _vrg_s are expressed. Here, we show that the expression of almost all _vrg_s depends on RisA, another transcriptional regulator. We also show that some _vag_s are surprisingly no
longer modulated by MgSO4 in the _risA_− background. RisA also regulates the expression of other genes, including chemotaxis and flagellar operons, iron-regulated genes, and genes of unknown
function, which may or may not be controlled by BvgA/S. We identified RisK as the likely cognate RisA kinase and found that it is important for expression of most, but not all
RisA-regulated genes. This was confirmed using the phosphoablative RisAD60N and the phosphomimetic RisAD60E analogues. Thus the RisA regulon adds a new layer of complexity to _B. pertussis_
virulence gene regulation. SIMILAR CONTENT BEING VIEWED BY OTHERS FOUR SINGLE-BASEPAIR MUTATIONS IN THE _PTX_ PROMOTER OF _BORDETELLA BRONCHISEPTICA_ ARE SUFFICIENT TO ACTIVATE THE
EXPRESSION OF PERTUSSIS TOXIN Article Open access 30 April 2021 _BORRELIA BURGDORFERI_ SERINE PROTEASE HTRA IS A PLEIOTROPIC REGULATOR OF STRESS RESPONSE, MOTILITY, FLAGELLAR HEMOSTASIS, AND
INFECTIVITY Article Open access 01 March 2025 STREAMLINED COPPER DEFENSES MAKE _BORDETELLA PERTUSSIS_ RELIANT ON CUSTOM-MADE OPERON Article Open access 08 January 2021 INTRODUCTION Whooping
cough or pertussis is a life-threatening respiratory disease and remains one of the major causes of infant mortality despite a global vaccination coverage of >85%. It represents today
the most prevalent vaccine-preventable disease in infants1, thereby illustrating the shortcomings of current vaccination programs. The development of improved vaccines will certainly benefit
from a more thorough understanding of the biology of _Bordetella pertussis_, the main etiologic agent of whooping cough. _B. pertussis_ produces a large array of _bona fide_ virulence
factors whose production is under the transcriptional control of the two-component system BvgA/S (for review see ref. 2). BvgS is the transmembrane sensor component of the system, which,
after a complex cascade of autophosphorylation finally transfers a phosphate group to the cytoplasmic transcriptional regulator BvgA. The BvgA/S system is functional by default, but can be
turned off by growth at low temperatures or in the presence of sulphate or nicotinic acid, a process referred to as antigenic or phenotypic modulation3,4. During the Bvg+ or virulence phase,
BvgA is phosphorylated and triggers the transcription of the genes coding for adhesins, toxins and other virulence factors. These genes are collectively called virulence-activated genes
(_vag_s). By contrast, in the Bvg− or avirulence phase, BvgA is not phosphorylated, and the _vag_s are not expressed. Instead, another set of genes, collectively called virulence-repressed
genes (_vrg_s), is expressed in the Bvg− phase5. Two genes were demonstrated to be involved in the regulation of _vrg_s: _bvgR_ and _risA_6,7. RisA is a member of the OmpR family of
two-component response regulators and was shown to be required for maximal expression of at least some _vrg_s7,8. The _risA_ gene is adjacent to _risS_ coding for the putative sensor kinase
of the RisA/S two-component system. Whereas for the closely related animal pathogen _Bordetella bronchiseptica, risA_ and _risS_ are required for resistance of this organism to oxidative
stress and for _in vivo_ persistence9, _risS_ is a pseudogene in the genome of all _B. pertussis_ isolates examined so far8. These observations suggest that in _B. pertussis_ RisA may be an
orphan response regulator, that its activity is independent of phosphorylation or that it is phosphorylated by an as yet unidentified kinase distinct from RisS. In this study we
characterized the RisA regulon by whole-genome transcriptomic analysis, identified the likely RisA kinase RisK and examined the role of RisA phosphorylation and of BvgR in RisA activity in
_B. pertussis_. RESULTS TRANSCRIPTOMIC PROFILING OF _B. PERTUSSIS_ BPSM GROWN IN MODULATING AND NON-MODULATING CONDITIONS To identify the full set of _vag_ and _vrg_ genes of _B. pertussis_
BPSM, the organism was grown in the presence or absence of 50 mM MgSO4 and then subjected to microarray analyses covering 3552 open reading frames. The threshold to identify genes that were
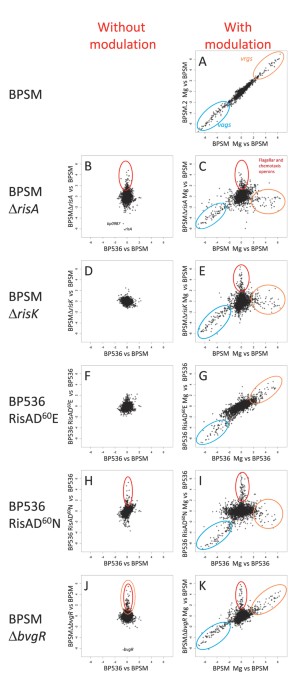
differentially transcribed was set at a 4-fold difference in transcript abundance between bacteria grown under modulating and non-modulating growth conditions (Fig. 1A, blue circle and Table
S1). The genes more abundantly transcribed in non-modulating conditions than in modulating conditions include the well-known _vag_s, such as the _ptx_/_ptl_ operon, _fhaB, bvgA_/_S, bvgR,
prn, tcfA, vag8, sphB1, brkA, fim2, fimABC_ and some genes involved in siderophore production, whereas the genes more abundantly transcribed in modulating conditions than in non-modulating
conditions include the _vrg_s, such as _fim3_, genes encoding the putative capsule, _vrg6_ and many genes with unknown function (Fig. 1A, orange circle, see also Fig. S1). These data are in
agreement with previously reported transcriptomic profiles of _B. pertussis_ grown in modulating versus non-modulating conditions10. THE RISA REGULON IN NON-MODULATING CONDITIONS To
characterize the RisA regulon, we constructed a _risA_-deficient mutant in the _B. pertussis_ BPSM background. The mutant strain, named BPSM∆RisA, carries a 735-bp internal deletion within
the _risA_ gene. A comparison of the transcriptome of BPSM with that of BPSM∆RisA, both grown in non-modulating conditions, identified 53 genes differentially regulated in BPSM∆RisA compared
to BPSM. Among these 53 genes, 22 genes were less abundantly transcribed and 31 genes had higher transcript levels in BPSM∆RisA as compared to BPSM (Figs 1B and 2 and Table S2).
Approximately half of the genes with decreased transcript abundance in BPSM∆RisA are _vrg_s (labelled in red in Fig. 2). They include _tviD_ (capsular operon), _osmB, vrg-6, bfrG_ but also
_bipA_, while the remaining genes were not identified as BvgA/S-regulated genes. With the exception of _bp0987_, the gene with the strongest decrease in transcript abundance in BPSM∆RisA (42
fold), all the other genes showed only a 5-fold decrease in transcript abundance (Fig. 1B). Among the 31 genes presenting more transcripts in BPSMΔRisA than in BPSM, none could be
identified as BvgA/S-regulated genes. However, many of them belong to the flagellar and chemotaxis operons (Fig. 2). These data suggest that under non-modulating conditions RisA does not
regulate _vag_ expression but modestly regulates the expression of many _vrg_s in addition to non-BvgA/S regulated genes in _B. pertussis_. RISA REGULATION IN MODULATING CONDITIONS The
modest regulation of the _vrg_s by RisA under non-modulating conditions may be enhanced when the bacteria are grown in modulating conditions. Therefore, we compared the transcriptional
profiles of BPSMΔRisA and BPSM grown under modulating conditions (in the presence of 50 mM MgSO4) to the transcriptional profile of BPSM grown under non-modulating conditions (Fig. 1C). Six
different gene clusters were identified (Fig. 3 and Table S3). The first cluster is composed of many _vag_s, which were less transcribed in both modulated BPSM and modulated BPSMΔRisA as
compared to non-modulated BPSM, arguing that RisA is not involved in the regulation of most _vag_s, as already proposed by Stenson _et al_.8. However, surprisingly, the second cluster is
also composed of _vag_s, but they were less transcribed in modulated BPSM than in modulated BPSMΔRisA (Fig. 3). These include the gene coding for pertactin (_prn_), whose transcripts are
less abundant in modulated BPSM (fold change of −89.26) than in non-modulated BPSM. In contrast, in modulated BPSMΔRisA, _prn_ shows only a 2.34 fold change as compared to non-modulated BPSM
(Fig. S2). Other genes that fall in cluster 2 include _fhaB_, genes involved in fimbrial biogenesis and the adenylate cyclase toxin gene. Thus, for some _vag_s the repression by modulation
appears to depend on RisA. The third cluster is composed of genes, such as _osmC_ and 7 other genes of unknown function, whose transcripts were less abundant in modulated BPSMΔRisA, but not
in modulated BPSM, as compared to non-modulated BPSM. The fourth cluster contains genes that were more abundantly transcribed in modulated BPSMΔRisA than in modulated BPSM (Fig. 3). They
comprise genes of the chemotaxis and flagellar operons (Fig. 1C red circle; Fig. S3, labelled in yellow), iron-regulated genes (Fig. S3, labelled in blue) and genes coding for a putative
type II secretion system (Fig. S3, labelled in green). Among the genes involved in iron acquisition, some, such as _bfrD_, are regulated by BvgA/S11, whereas others, such as _tonB, exbB_ and
_exbD_, are not. The expression of these genes has been shown to be increased by iron starvation12,13,14. The fifth cluster is composed of genes that were transcribed at higher levels in
both modulated BPSMΔRisA and modulated BPSM as compared to non-modulated BPSM (Fig. 3). Only 6 genes fall in this cluster (_bp0627, bp0628, bp1704, bp2496, bp3501, bp3871_), all of unknown
function. Based on their expression in modulated BPSM, these genes would be classified as _vrg_s. However, their expression does not appear to require RisA. The sixth cluster is composed of
genes that were much more transcribed in modulated BPSM but not in modulated BPSMΔRisA, compared to non-modulated BPSM (Fig. 3) and contains most of the _vrg_s. Thus, with the exception of
the 6 genes in cluster 5, the expression of all the _vrg_s depends on functional RisA. IDENTIFICATION OF RISK (BP3223) AS THE PUTATIVE KINASE OF RISA Since RisA is required for the
expression of most of the _vrg_s, as well as for a set of genes that do not appear to be modulated by MgSO4 (Fig. 3, cluster 4), we further investigated the regulation mechanism of RisA and
examined the requirement for its phosphorylation in its activity. To address this issue, we first set out to identify the main kinase involved in RisA phosphorylation. The _B. pertussis_
genome contains 17 genes coding for putative two-component system kinases. One of them is _risS_, a gene separated from _risA_ by only 4 bp and thus likely co-transcribed with _risA_ within
the same operon. However, the deletion of _risS_ does not perturb the activity of RisA8. In addition, _risS_ is a pseudogene in _B. pertussis_, in contrast to _B. bronchiseptica_8, which
makes it unlikely that RisS is the cognate RisA kinase in _B. pertussis_. To search for an alternative RisA kinase, we made use of the concept of co-evolving residue pairs to predict the
interacting partner of RisA (http://biohealth.snu.ac.kr/cgi-bin/platcom/tcs/intro.cgi). Among all intact putative _B. pertussis_ histidine kinases, RisA was predicted to interact most
strongly with the _bp3223_ gene product that we therefore propose to call RisK. We deleted the _risK_ gene from the BPSM chromosome and analysed the transcriptomic profiles of BPSM∆RisK in
modulating and non-modulating conditions. In non-modulating conditions, BPSMΔRisK presented a transcriptomic profile almost identical to that of BPSM and slightly different from that of
BPSM∆RisA (Figs 1D and 4 and Table S4). Under modulating conditions, BPSM∆RisK presented a transcriptional profile similar to that of BPSMΔRisA (Figs 1E and 4), indicating that RisA requires
the presence of RisK to express its full transcriptional activities. Notable exceptions include some of the _vag_s (Fig. 4, genes in green) and _bipA_, which were more transcribed in
modulated BPSMΔRisA than in modulated BPSM∆RisK. In addition, RisK appears to play a minor role in regulating the expression levels of the RisA-dependent _vrg_s in non-modulating conditions,
whereas in modulating conditions, RisK is absolutely required for the upregulation of the expression of these genes (Fig. 4, genes in red). Some of the non-_vrg_ RisA-repressed genes (Fig.
3, cluster 4 and Fig. 4, genes in blue) were more abundantly transcribed in modulated BPSMΔRisA than in modulated BPSMΔRisK, whereas others (Fig. 4, genes in yellow) were more transcribed in
both modulated BPSMΔRisA and BPSMΔRisK as compared to non-modulated BPSM. Hence, in modulating conditions, some genes require RisK for their RisA-mediated repression, whereas for other
genes the involvement of RisK in regulation appears to be dispensable. To confirm the results obtained by the microarray experiments, quantitative RT-PCR analyses were performed on several
selected genes, covering all 6 identified clusters on Fig. 3 in BPSM, BPSMΔRisA and BPSM∆RisK grown in modulating or non-modulating conditions. In all cases the quantitative RT-PCR results
confirmed the data obtained by DNA microarray (Fig. S4). TRANSCRIPTOMIC PROFILES OF _B. PERTUSSIS_ RISAD60E AND RISAD60N To further deepen our understanding of the role of RisA
phosphorylation in transcriptional activation, we used _B. pertussis_ mutant strains producing phosphomimetic or phosphoablative RisA derivatives. Phosphorylation of two-component response
regulators usually occurs at a conserved aspartate residue. Asp-60 of RisA is the most conserved aspartate residue in the RisA/OmpR family of response regulators and is therefore likely to
be the site of RisA phosphorylation8. RisA was thus genetically replaced by the phosphomimetic RisAD60E or the phosphoablative RisAD60N analogue, and we compared the transcriptional profiles
of the RisAD60E- or RisAD60N-producing strains to those of the parental strain in modulating and non-modulating conditions. The phosphomimetic RisAD60E mutant presented a transcriptomic
profile almost identical to that of its parental strain, both in modulating and in non-modulating conditions, suggesting that the RisAD60E protein is fully functional (Figs 1F,G and 4 and
Table S4). In contrast, the phosphoablative RisAD60N mutant showed many differences with the parental strain, and its transcriptomic profile was similar, but not identical to that of the
RisA-deficient mutant (Figs 1H,I and 4), suggesting that the loss of phosphorylation leads to a strong but not complete loss of RisA functions. These observations suggest that some of the
RisA-dependent genes do not require phosphorylation of RisA. In non-modulating conditions, genes that are less transcribed in the absence of RisA, but not in the case of its replacement with
RisAD60N, include genes coding for a putative glycosyl transferase and for a biotin synthase (labelled in purple on Fig. 4). In modulating conditions, all the _vag_s identified in cluster 2
of Fig. 3 (labelled in green in Fig. 4), produced lower levels of transcripts in the RisAD60N-producing strain than in BPSMΔRisA. These data suggest that phosphorylated RisA is not required
to regulate these genes and confirm that, in addition to modulation, a second level of regulation of these genes is RisA-dependent. Some genes of the chemotaxis, flagellar and iron
acquisition operons (labelled in yellow and blue, respectively, in Fig. 4) appear to be differentially regulated between the RisAD60N-producing strain and BPSMΔRisA. Finally, with the
exception of the 6 genes of cluster 5 (Fig. 3), the expression of the _vrg_s appears to require phosphorylated RisA, as their transcripts were less abundant in the modulated RisAD60N mutant
than in the modulated parental strain (Fig. 4, genes highlighted in red). The transcriptome of the RisAD60N mutant was very similar to that of BPSM∆RisK, suggesting that phosphorylation of
RisA is required for the transcription of most genes belonging to the RisA regulon. However, the transcription of some of them does not require RisA phosphorylation. Furthermore, the effects
of the loss of regulation by RisA through the absence of its phosphorylation were enhanced in modulating conditions. RISA-MEDIATED REGULATION IN RECENT CLINICAL _B. PERTUSSIS_ ISOLATES
Since the above studies were all done with derivatives of the TohamaI laboratory strain, it was important to determine whether a similar role of RisA could be observed in more recent
clinical isolates. We therefore attempted to construct _risA_ mutants in several clinical isolates, including the highly virulent _B. pertussis_ D420 strain15. Although several attempts to
construct _risA_-deletion derivatives of these clinical strains were unsuccessful, we were able to obtain a D420 derivative in which RisA was replaced by the phosphoablative analogue
RisAD60N. Using quantitative RT-PCR we compared the transcription of the set of representative genes for each cluster presented in figure S4 in the D420 RisAD60N mutant with that of the
parent strain, both grown in modulating and in non-modulating conditions. As shown in Fig. 5A, the transcriptional profile of these genes in the D420 background was very similar to the
profile seen in the TohamaI derivatives (Fig. S4). Notable exceptions include the _fim2_ and _fim3_ genes. Since D420 does not produce serotype 2 fimbriae15, _fim2_ was not expressed in
D420, as expected, in contrast to the TohamaI derivatives. The _fim3_ gene was highly transcribed in non-modulated D420, non-modulated D420 producing RisAD60N and in modulated D420 but was
less transcribed in the modulated D420 RisAD60N mutant, indicating that _fim3_ is not a _vrg_ in D420 in contrast to BPSM (see Fig. 3), but has maintained RisA-mediated regulation in
modulating conditions. RISA-MEDIATED REGULATION IN IRON AND IN GLUTAMATE DEPLETED CONDITIONS Since RisA is involved in the regulation of expression of several iron-regulated genes (Fig. S3,
labelled in blue), we investigated the role of iron on the expression of the set of representative genes in Fig. 5A by qRT-PCR. BPSM and BPSM∆RisA were therefore grown in iron depleted
conditions with or without 50 mM MgSO4. As expected, _bp1560_ and _hurI_ showed a higher transcript abundance in iron depleted conditions than in iron replete conditions, with respective
50.20 and 490.29 fold changes (Fig. 5B). However, the transcriptional profile of the remaining genes in iron depleted conditions was very similar to that seen in iron replete conditions in
both BPSM and BPSM∆RisA, arguing that the iron depletion did not modify the regulatory properties of RisA. Furthermore, it has recently been shown that the depletion of glutamate in the
growth medium may also have a major impact on gene regulation in _B. pertussis_16. We therefore analyzed by qRT-PCR the transcription of the set of representative genes described above in
BPSM and BPSM∆RisA, grown in glutamate depleted conditions with and without 50 mM MgSO4. As previously shown17, glutamate starvation led to increased transcript abundance of _hurI_, with a
9.96 fold change (Fig. 5C). However, the transcriptional profile of the remaining subset of genes in glutamate-depleted conditions was generally identical to the profile seen in glutamate
replete conditions in both BPSM and BPSM∆RisA (Fig. 5C), indicating that, while glutamate depletion has an effect on the transcription of some _B. pertussis_ genes, it does not modify the
global pattern of regulation of RisA. THE ROLE OF BVGR IN RISA-REGULATED GENE EXPRESSION Since expression of the _vrg_s depends on RisA but is also regulated by BvgR and can be modulated by
the presence of MgSO4, which itself results in reduced _bvgR_ expression, we investigated the link between modulation, _bvgR_ expression and RisA-dependent transcription. Therefore, the
transcriptomic profile of a _bvgR_-deficient strain was compared to that of its parental strain and of the _risA_-deficient strain, all of which were grown under modulating and
non-modulating conditions. In non-modulating conditions, the transcripts of most _vrg_s were more abundant in BPSM∆BvgR than in BPSM (Fig. 1J,K, labelled in red in Fig. 6 and Table S5),
confirming the role of BvgR in repressing the _vrg_s. As expected, in modulating conditions the _vag_s were less transcribed in both BPSM and BPSM∆BvgR (labelled in green in Fig. 6), whereas
the _vrg_s were more transcribed (labelled in red in Fig. 6) as compared to non-modulated BPSM. Additionally, all the genes related to the flagellar and chemotaxis operons (labelled in
yellow in Fig. 6) were also more transcribed in BPSM∆BvgR compared to BPSM in modulating and non-modulating conditions. The comparison of the transcriptional profiles of modulated versus
non-modulated BPSM∆BvgR with those of BPSM∆RisA grown in the same conditions indicated that the expression level of most of the chemotaxis and flagellar genes is lower in BPSMΔRisA than in
BPSMΔBvgR, especially in modulating conditions. The transcripts of the flagellum-related genes _bp1022_ and _bp1023_ were more abundant in modulated and non-modulated BPSMΔRisA and BPSM∆BvgR
than in non-modulated BPSM. They code for the transcriptional activators FlbB and FlhC, respectively, suggesting that RisA is involved in the repression of these two regulators and
therefore may indirectly affect the expression of the remaining genes of the flagellar and chemotaxis operons. DISCUSSION In this study we used microarray analysis to investigate the role of
the _risA, risK_ and _bvgR_ genes in the global _B. pertussis_ transcriptomic regulation in both modulating and non-modulating conditions. The vast majority of the genes affected by the
_risA, risK_ or _bvgR_ mutations are all members of the RisA regulon. RisA is a member of the two-component system transcriptional regulator family. However, its putative cognate kinase RisS
encoded by the gene located immediately downstream of _risA_ is not functional in _B. pertussis_ because of a frame shift in _risS_. Instead, we found that the RisK kinase is required for
the full regulatory activities of RisA, strongly suggesting that RisK is its cognate kinase. This was confirmed biochemically by some of us using the Phos-Tag technology, showing that the
phosphorylation state of RisA is altered in the _risK_-deficient strain (Chen _et al_., submitted). The transcriptomic profile of BPSM∆RisK, the strain that lacks _risK_, was nearly
identical to that of BPSM∆RisA, suggesting that phosphorylation is important for RisA function. However, the expression of some genes was affected by the deletion of _risA_ but not by the
deletion of _risK_. Since the phosphoablative RisAD60N variant presented the same transcriptional profile as the _risK_-deletion mutant, we conclude that the genes differentially regulated
in the _risA_ and _risK_ mutants are regulated by non-phosphorylated RisA, rather than through phosphorylation via cross-talk with another histidine kinase. The _risK_ gene is co-transcribed
with the immediate upstream _bp3222_ gene, a member of the _ompR_ gene family, located within the same bicistronic operon on the _B. pertussis_ chromosome. This gene arrangement is typical
for operons that code for two-component systems and suggests that RisK may also be a cognate kinase of the OmpR-like protein and that therefore _risK_ deletion may have an impact on the
regulatory function of BP3222. However, since the transcriptional profile of BPSM∆RisK was found to be nearly identical to that of the RisAD60N mutant, it is likely that RisK exclusively
acts on RisA. We found that the transcriptome of a _bp3222_–deficient BPSM derivative was nearly identical to that of BPSM (data not shown). The few genes that were less transcribed in the
_bp3222_–deficient strain compared to BPSM, were not thus affected in the _risK_–deficient strain, suggesting either that their expression did not require BP3222 phosphorylation, or that
BP3222 is phosphorylated by a kinase different from RisK. In either case, these observations indicate that the transcriptomic profile observed for the _risK_ mutant is mediated through the
loss of RisA phosphorylation rather than through an effect on the OmpR-like protein. Many of the genes that are under the control of RisA are _vrg_s. The _risA_ deletion affects even the
basal level of most _vrg_s in non-modulating conditions, indicating that RisA is required for _vrg_ expression even without modulation (Figs 2 and 3). In modulating conditions, the effect of
the _risA_ deletion is stronger than in non-modulating conditions, as expected. However, the expression of some _vrg_s was not affected by the _risA_ deletion in non-modulating conditions.
These include _bp0874 (vir-18_) and _fim3_. Thus, RisA may act differentially on a subset of _vrg_s. Alternatively, the basal level of transcription of these genes in non-modulating
conditions may be too low to detect a difference between BPSM and BPSM∆RisA. Among the 71 overexpressed genes in modulated versus non-modulated BPSM and therefore identified as _vrg_s, as
confirmed by using a ΔBvgA mutant (data not shown), the expression of only 6 was independent of RisA. These 6 RisA-independent _vrg_s may have evolved from the other _vrg_s to become
independent of RisA-mediated transcriptional activation or may have additional regulatory systems that override the lack of RisA. The _vrg_s are also regulated by BvgR, as the presence of
this protein represses _vrg_ expression. However, binding of BvgR to _vrg_ operator sites has not been observed, suggesting that BvgR does not act as a transcriptional repressor. Instead,
BvgR contains a conserved EAL sequence found in diguanylate phosphodiesterases that are involved in the degradation of c-di-GMP. Additionally, RisA was shown recently to interact with
c-di-GMP (Warfel _et al_., in preparation). Thus, BvgR may participate in the control of intracellular levels of c-di-GMP, a secondary messenger that might regulate the activity of RisA, as
has already been shown for other two-component response regulators (for review, see ref. 18). We found that the expression of the _vrg_s is strongly enhanced in the _bvgR_-deficient strain
BPSM∆BvgR, even in non-modulating conditions. This observation suggests that, in order to be fully functional, RisA requires the presence of c-di-GMP, the concentration of which is reduced
by the presence of BvgR. Our results also suggest that the expression of some RisA-dependent genes requires phosphorylated RisA but not c-di-GMP, whereas another subset of genes does not
require phosphorylated RisA but requires the presence of c-di-GMP. Interestingly, we have also detected genes that were more abundantly transcribed in BPSM∆RisA than in BPSM in
non-modulating conditions. They mainly belong to the flagellar and chemotaxis operons. These genes were not identified as _vrg_s in _B. pertussis_, while they have previously been described
as _vrg_s in _B. bronchiseptica_19,20. In addition, they were also shown to be regulated by c-di-GMP21. It remains to be investigated whether they are directly regulated by RisA acting as a
repressor, or indirectly involving a regulation intermediate that has yet to be identified. The deletion of _bvgR_ resulted in a transcriptomic profile for these genes that is similar to
that observed for BPSM∆RisA, with the exception of _flhC_ and _flhD_, encoding putative transcriptional regulators. The flagellar genes were also more strongly transcribed in the ∆_bvgR_
background compared to the ∆_risA_ background in modulating conditions (see Fig. 6), suggesting that c-di-GMP acts at two different levels in the regulation of flagellar gene expression.
Hence, in addition to acting with RisA, c-di-GMP may also act on the FlhC and FlhD regulators. A similar mechanism has been shown for the _B. bronchiseptica_ flagellum operon21, where in
modulating conditions or in the absence of BvgR, the basal activity of the FlbB and FlhC regulators is enhanced, resulting in a higher expression of the remaining genes related to the
flagellar and chemotaxis operons. Several genes related to iron acquisition systems were also more transcribed in modulated BPSM∆RisA than in modulated BPSM. It is not known whether RisA
acts directly on the expression of these genes or whether it modifies the bacterial perception of the environmental iron concentration. However, not all the genes that were demonstrated to
be more transcribed in iron starvation conditions are regulated by RisA. RisA-independent iron-regulated genes include the operon coding for alcaligin biosynthesis (_bp2456-2461_)12,13,14.
We also found that iron depletion does not perturb the general pattern of RisA regulation. Surprisingly, in the RisA-deficient background 18 _vag_s lost their repression mediated by the
addition of MgSO4 (Fig. 4), whereas they were strongly repressed by the addition of MgSO4 in BPSM. In contrast, the expression of all other _vag_s was still repressed in modulated BPSM∆RisA,
similarly to modulated BPSM. Several of the former _vag_s, like _prn_ and _fhaB_, belong to the class of early _vag_s, while others, like _cyaA_, belong to the class of late _vag_s,
suggesting that RisA may act on various kinds of _vag_s involved in the pathogenesis at different times during infection22. RisA phosphorylation was not required to suppress the modulatory
effect of MgSO4 of these _vag_s, while, in contrast, RisA phosphorylation was required for the transcription of all the _vrg_s in modulating conditions. In conclusion, the data presented
here prompted us to propose a model integrating the roles of _bvgA_/_S, bvgR, risA_ and _risK_ in the regulation of _B. pertussis_ virulence genes (Fig. 7). According to this model, in
non-modulating conditions, BvgA is phosphorylated by BvgS and activates the _vag_s, including _bvgR_. Expression of _bvgR_ leads to the degradation of intracellular c-di-GMP. RisA is the
transcriptional activator of most of the _vrg_s, but in the absence of c-di-GMP RisA is not able to induce the expression of these genes, but can nevertheless activate the basal level of
_vrg_ expression and repress the expression of the flagellar and chemotaxis genes and of genes involved in iron acquisition. In modulating conditions, BvgS is not active and does not
phosphorylate BvgA. Non-phosphorylated BvgA does not activate the expression of _bvgR_. Therefore, the concentration of c-di-GMP increases. The cofactor c-di-GMP binds to phosphorylated and
non-phosphorylated RisA participating in the induction or repression of the RisA regulon, including the _vrg_s, the RisA-regulated _vag_s and other genes of unknown function. Although the
role of the RisA regulon in the pathogenesis of pertussis is not yet known, RisA-mediated regulation may perhaps be required to allow the bacteria to adapt to different phases during the
infectious cycle. In addition, fine-tuning of adhesin production may contribute to transmission and/or the colonization of a specific niche in the respiratory tract. Furthermore, we have
analysed the RisA regulon in standard _in vitro_ growth conditions, in iron depleted conditions and in glutamate depleted conditions in the absence or presence of 50 mM MgSO4. It remains to
be investigated whether the RisA-dependent transcriptome may vary in different growth conditions or during infection. We cannot exclude the possibility that under different growth
conditions, additional members of the RisA regulon might be identified. METHODS CONSTRUCTION OF _B. PERTUSSIS_ MUTANT STRAINS The _B. pertussis_ strains used in this study were derived from
Tohama I derivatives BPSM23 or BP53624, or from the clinical isolate D42015. _B. pertussis_ BPSM∆RisA, BPSMΔRisK and BPSMΔBvgR were obtained by homologous recombination using either pSS1129
or pJQ200 mp18 rpsL as allelic exchange vectors25,26,27. The recombinant plasmids were introduced into _B. pertussis_ by conjugation via _Escherichia coli_ SM1028. BPSMΔRisA carries a 735 bp
internal deletion in the _risA_ gene (BP3554). It was obtained as follows. Two 800-bp DNA fragments flanking the region to be deleted were obtained by PCR using the BPSM genomic DNA as
template and the oligonucleotide pairs 5′-GAATTCGCGGCCACGCCGCCGCCATCCCGCCAGGCC-3′ and 5′-TCTAGAGGCCGGAAATGTAACAGTGA-3′ and 5′-TCTAGACCTAATGGCCCGCCCCGGGC-3′ and
5′-AAGCTTCGCCAGCGGCGTGCACAGGTCGTGTGAAATGCC-3′ as primers. The resulting _Eco_RI-_Xba_I and _Xba_I-_Hin_dIII fragments were then introduced into _Eco_RI-_Hin_dIII-digested pJQ200 mp18 rpsL,
yielding pJQ∆RisA. This construct was used for allelic exchange in BPSM, yielding BPSMΔRisA, in which _risS_ is located directly downstream of the _risA_/_S_ promoter. BPSMΔRisK carries a
1392-bp internal deletion in the _risK_ gene (BP3223). It was obtained as follows. Two 400-bp DNA fragments flanking the region to be deleted were obtained by PCR using the BPSM genomic DNA
as template and the oligonucleotide pairs 5′-TATAAAGCTTACGACTACCTCGGCAAGCCCTT-3′ and 5′-TATACTCGAGGCGGAGCAGTTTCATCAGGG-3′ and 5′-TATACTCGAGCCGCTTGCGAAGGCTTGACC-3′ and
5′-TATAGGATCCTGGAGCAATACGGCCCACCT-3′ as primers. The resulting _Hin_dIII-_Xho_I and _Xho_I-_Bam_HI fragments were successively introduced into the _Hin_dIII-_Bam_HI sites of pSS1129,
yielding pSS1129 BP3223. This construct was used for allelic exchange in BPSM, yielding BPSMΔRisK. The internal deletion in the _risK_ gene led to a truncated RisK protein constituted by the
first and last five amino acids of the original RisK protein. BPSMΔBvgR carries a 804-bp internal deletion in the _bvgR_ gene (BP1876). It was obtained as follows. Two 470-bp DNA fragments
flanking the region to be deleted were obtained by PCR using the BPSM genomic DNA as template and the oligonucleotide pairs 5′-TATAAAGCTTCAATCCGCGCCATCCAGGTC-3′ and
5′-TATACTCGAGAGCCTCGAAGCTGCTGCGAG-3′ and 5′-TATACTCGAGCGCCGCGAGATGCCGCCCAA-3′ and 5′-TATAGGATCCCGCGCCGGCCACGGACGACG-3′ as primers. The resulting _Hin_dIII-_Xho_I and _Xho_I-_Bam_HI fragments
were successively introduced into the _Hin_dIII-_Bam_HI sites of pSS1129, yielding pSS1129 BvgR. This construct was used for allelic exchange in BPSM, yielding BPSMΔBvgR. The internal
deletion in the _bvgR_ gene led to a truncated BvgR protein constituted by the first 14 and last 9 amino acids of the original BvgR protein. Strains QC3296 (RisAD60N) and BP1942 (RisAD60E)
were constructed as follows. Plasmid pSS4894 (Chen _et al_., unpublished results) was used as the allelic exchange vector for the construction of pSS5085 (pSS4894::Δ_BP-risA)_, pSS5085.5
(pSS4894::_BP-risA_D60N), and pSS5086 (pSS4894::_BP-risA_D60E). For each, fragments comprising sequences flanking the mutation were synthesized using PCR amplification with BP536 chromosomal
DNA as a template. For pSS5085, the upstream fragment was amplified with primers 5′-TATAGGTCTCCGGCCGCGGTGGTGAAGGCCACCTTGTC-3′ and 5′-TATAGGTCTCCGGGTTTTGCGTGTTCATGGCCGGAAATGTAACAGTG-3′. The
downstream fragment was amplified with primers 5′-TATAGGTCTCAACCCGGATGGCGGCAGTTGACCTAATG-3′ and 5′-TATAGGTCTCGGATCCGATCTGGCCGAGGTCCTCGTCGATG-3′. Both fragments were digested with the type
II-S restriction enzyme _Bsa_I to create cohesive ends compatible with _Not_I, _Bam_HI or unique, compatible cohesive ends in the vicinity of the deletion endpoint. The two digested
fragments were ligated together with pSS4984 digested with _Not_I and _Bam_HI, transformed, and screened to create pSS5085. For pSS5085.5 the upstream fragment was amplified with primers
5′-TATAGGTCTCCGGCCGCGGTGGTGAAGGCCACCTTGTC-3′ and 5′-TATAGGTCTCCATCAGGTTGAGAACCAGCAGGTCAAAGTG-3′. The downstream fragment was amplified with primers
5-TATAGGTCTCGGATCCGATCTGGCCGAGGTCCTCGTCGATG-3′ and 5′-TATAGGTCTCCTGATGCTGCCGGGCGAGGATGGCCTGTCGATC-3′. For pSS5086 synthesis was similar, with the exception that primer
5′-TATAGGTCTCCATCAGTTCGAGAACCAGCAGGTCAAAGTG-3′ was used, such that the D60E, rather than the D60N, mutation was incorporated. Transfer of pSS5085 to _B. pertussis_ BP536 was accomplished by
conjugation following its transformation into the _dap E. coli_ strain RHO3, with selection on LB supplemented with gentamicin and DAP. Prior to mating, the recipient BP536 was grown on BG
agar plus streptomycin and 50 mM MgSO4. Mating was performed by swabbing the _E. coli_ donor strain together with the _B. pertussis_ recipient strain on BG agar plus MgSO4 and DAP. After
incubation at 37 °C for 3 hours, bacteria were recovered by swabbing and re-swabbed onto BG agar plus gentamicin (100 μg/ml), streptomycin (50 μg/ml) and 50 mM MgSO4 to select for transfer
and integration by a single crossover. MgSO4 was included to maintain repression of I-_Sce_I synthesis from the pSS4894 vector and the higher level of gentamicin was used to reduce
background typically observed under modulating conditions. Discrete colonies arising on these selection plates were then restreaked onto BG agar lacking MgSO4 to induce the synthesis of
I-_Sce_I enzyme driven by P_ptx_, the subsequent cleavage of the integrated pSS4894 vector, and the stimulation of homologous recombination to repair the resulting double stranded cleavage,
resulting in loss of the vector and either incorporation of the mutant allele or a return to the wild-type allele. Colonies that arose on these plates were screened by PCR for incorporation
of the deletion allele and also for sensitivity to gentamicin to verify loss of the plasmid vector. In this way strain BP1928 was created. In a similar way, the D60N and D60E mutations were
incorporated into the BP536 genetic background using pSS5085.5 and pSS5086 as allelic exchange constructs. However, in these crosses BP1928 was used as the recipient, in order to be able to
distinguish the resident deletion allele from the incoming D60 substitution alleles by PCR in the screening step. In this way the strains QC3296 (_risA_D60N) and BP1942 (_risA_D60E) were
created. Strain QC4470 (_B. pertussis_ D420, _risA_D60N) was constructed as follows QC4470 was created by allelic exchange with the plasmid pQC2266 in _B. pertussis_ strain D420. This
plasmid was created by cloning a 1 kb gene fragment containing sequences flanking the D60N mutation as well as synonymous changes to introduce a _Sma_I restriction site nearby. The latter
was included to facilitate screening of recombinants. The fragment was synthesized as a gBlock (Integrated DNA Technologies, Inc.) with flanking _Bam_HI and _Not_I sites and was cloned,
following digestion, into the same sites of pSS4894. The sequence of the fragment is given in Table S6. Transfer of pQC2266 to D420 was accomplished by conjugation following its
transformation into the _dap E. coli_ RHO3, with selection on LB supplemented with gentamicin and DAP. To maintain modulation in the first stages of this allelic exchange, The recipient D420
was grown on BG agar containing 20 mM MgSO4 and mating was performed on BG with 20 mM MgSO4 plus DAP. Mating was for 3 hours at 37 °C after which exconjugants were selected after reswabbing
on BG agar containing 20 mM MgSO4, and gentamicin, but without DAP. Discrete colonies arising on these selection plates were then restreaked onto BG agar lacking MgSO4 to allow the cross
out of the plasmid vector. Colonies that arose on these plates were screened by sensitivity to gentamicin and PCR of the _risA_ region followed by SmaI digestion of the resulting product.
The DNA sequence of the _risA_ locus in the final strain QC4470 was verified by sequencing the PCR product. All _B. pertussis_ strains were grown on Bordet Gengou agar (BG), in liquid
modified Stainer-Scholte medium as described by Locht _et al_.29, in liquid iron-depleted Stainer-Scholte medium as described by Alvarez Hayes _et al_.13 or in liquid modified
Stainer-Scholte medium containing 20% of the standard glutamate concentration, as described by Hanawa _et al_.16. The culture media were supplemented with 100 mg/ml streptomycin and 50 mg/ml
magnesium sulphate where appropriate. MICROARRAY PRODUCTION AND ANALYSIS Long oligonucleotide probes were designed on the sequences of the 3552 open reading frames, including all coding
sequences, except those of the transposases in IS elements, of _B. pertussis_ Tohama I genome using OligoArray v2.130. Oligonucleotides were synthesized by Sigma-Aldrich and spotted on
Nexterion AL slides (Schott Nexterion) in 1× SciSpot-AM buffer (Scienion) using a Q-Array II spotter (Genetix). Total RNA was extracted from bacterial pellets harvested from two or three
individual cultures for each mutant strain, using TriReagent (Ambion) following manufacturer’s instructions. For each sample, 5 μg of total RNA was reverse transcribed with 400 units of
SuperScript III (Invitrogen) in the presence of 100 μM Cy3-dCTP or Cy5-dCTP (GE) and 300 mM of random hexanucleotides (Roche). The labelled cDNA was treated with 1 M NaOH to degrade the RNA
and then purified on the Qiaquick PCR purification kit (Qiagen). Hybridization was performed in 40% formamide, 5× Denhardt’s solution, 0.1% SDS, 1 mM Sodium pyrophosphate and 5× SSC during
14–16 h at 52 °C under agitation. Slides were then washed sequentially in 2× SSC/0.2% SDS during 5 min, 0.5× SSC during 10 min, 0.05× SSC during 5 min and 0.01× SSC during 1 min before
drying. Hybridized slides were scanned using an Innoscan 700 (Innopsys) microarray scanner and analysed with Mapix v3.1 (Innopsys). For normalisation and differential expression analyses the
LIMMA package (Linear Models for Microarray Data)31, running under the statistical language R v2.11.1, was used. Statistically significant regulation was identified using moderated
t-statistic with empirical Bayes shrinkage of the standard errors32. Because of multiple testing, obtained P-values were corrected using the Benjamini & Hochberg method to control false
discovery rates33. The expression data shown corresponding to each of the 3552 open reading frames were calculated by a means of 2 to 4 individual cultures that were each analysed in 4
technical replicates. All the microarray data are under GEOarchive GSE77754. GENERATION OF CDNA RNA was extracted from bacterial pellets of mid exponential phase cultures grown in
Stainer-Scholte medium, using TriReagent (Ambion) following the manufacturer’s instructions. For each sample, 5 μg of total RNA was reverse transcribed with 400 units of SuperScript III
(Invitrogen) and 300 mM of random hexanucleotides (Roche). The cDNA products were treated with 1 M NaOH to degrade the RNA and then purified on the Qiaquick PCR purification kit (Qiagen).
QUANTITATIVE REAL-TIME POLYMERASE CHAIN REACTION Polymerase chain reaction (PCR) was performed in an Roche LightCycler® 480 Instrument II using 10 μl of 2× Master Mix SybrGreen (Roche), 1 μl
of cDNA product (30 ng), 1 μl of 5 μM forward+ reverse primer mix and water to reach a final reaction volume of 20 μl. The primers used are presented in Supplementary Table S6. A 15-min
cycle at 95 °C was followed by 40 cycles of 15 s at 95 °C, 8 s at 64 °C and 1 min at 72 °C. At completion of the PCR run, a dissociation curve from 55 °C to 95 °C was run to determine that a
single product was generated. The efficiency for each primer pair was determined by serial dilution. The expression of the housekeeping gene _bp3416_ was used as reference to normalize the
expression of the genes of interest. The experiments were done 3 times with at least 4 technical replicates for each measurement. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Coutte, L.
_et al_. The multifaceted RisA regulon of _Bordetella pertussis._ _Sci. Rep._ 6, 32774; doi: 10.1038/srep32774 (2016). REFERENCES * World Health Organisation. WHO vaccine-preventable
diseases: monitoring system, 2016 global summary. http://apps.who.int/immunization_monitoring/globalsummary/Date of access: 02/08/2016. * Stibitz, S. The _bvg_ regulon in _Bordetella_
Molecular Microbiology. (ed. Locht, C. ) 47–67 (Horizon Bioscience, 2007). * Lacey, B. W. Antigenic modulation of _Bordetella pertussis_. J Hyg 31, 423–434 (1960). Google Scholar * Melton,
A. R. & Weiss, A. A. Environmental regulation of expression of virulence determinants in _Bordetella pertussis_. J. Bacteriol 171, 6206–6212 (1989). Article CAS Google Scholar *
Knapp, S. & Mekalanos, J. J. Two trans-acting regulatory genes (_vir_ and _mod_) control antigenic modulation in _Bordetella pertussis_. J. Bacteriol 170, 5059–5066 (1988). Article CAS
Google Scholar * Merkel, T. J. & Stibitz, S. Identification of a locus required for the regulation of _bvg_-repressed genes in _Bordetella pertussis_. J. Bacteriol 177, 2727–2736
(1995). Article CAS Google Scholar * Croinin, T. O., Grippe, V. K. & Merkel, T. J. Activation of the _vrg6_ promoter of _Bordetella pertussis_ by RisA. J. Bacteriol 187, 1648–1658
(2005). Article CAS Google Scholar * Stenson, T. H., Allen, A. G., Al-Meer, J. A., Maskell, D. & Peppler, M. S. _Bordetella pertussis risA_, but not _risS_, is required for maximal
expression of Bvg-repressed genes. Infect Immun 73, 5995–6004 (2005). Article CAS Google Scholar * Jungnitz, H., West, N. P., Walker, M. J., Chhatwal, G. S. & Guzman, C. A. A second
two-component regulatory system of _Bordetella bronchiseptica_ required for bacterial resistance to oxidative stress, production of acid phosphatase, and _in vivo_ persistence. Infect Immun
66, 4640–4650 (1998). CAS PubMed PubMed Central Google Scholar * Cummings, C. A., Bootsma, H. J., Relman, D. A. & Miller, J. F. Species- and strain-specific control of a complex,
flexible regulon by _Bordetella_ BvgAS. J. Bacteriol 188, 1775–1785 (2006). Article CAS Google Scholar * Antoine, R. et al. New virulence-activated and virulence-repressed genes
identified by systematic gene inactivation and generation of transcriptional fusions in _Bordetella pertussis_. J. Bacteriol 182, 5902–5905 (2000). Article CAS Google Scholar * Brickman,
T. J., Cummings, C. A., Liew, S. Y., Relman, D. A. & Armstrong, S. K. Transcriptional profiling of the iron starvation response in _Bordetella pertussis_ provides new insights into
siderophore utilization and virulence gene expression. J. Bacteriol 193, 4798–4812 (2011). Article CAS Google Scholar * Alvarez Hayes, J. et al. Identification of a new protective antigen
of _Bordetella pertussis_. Vaccine 29, 8731–8739 (2011). Article CAS Google Scholar * Vidakovics, M. L. et al. Profiling the _Bordetella pertussis_ proteome during iron starvation. J.
Proteome Res 6, 2518–2528 (2007). Article Google Scholar * Boinett, C. J. et al. Complete Genome Sequence of Bordetella pertussis D420. Genome Announc 3, e00657–15 (2015). PubMed PubMed
Central Google Scholar * Hanawa et al. Glutamate Limitation, BvgAS Activation, and (p)ppGpp Regulate the Expression of the _Bordetella pertussis_ Type 3 Secretion System. J. Bacteriol 198,
343–351 (2016). Article CAS Google Scholar * Nakamura, M. M. et al. Growth phase- and nutrient limitation-associated transcript abundance regulation in _Bordetella pertussis_. Infect.
Immun. 74, 5537–5548 (2006). Article CAS Google Scholar * Römling, U., Galperin, M. Y. & Gomelsky, M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger.
Microbiol Mol Biol Rev 77, 1–52 (2013). Article Google Scholar * Akerley, B. J. & Miller, J. F. Flagellin gene transcription in _Bordetella brobchispetica_ is regulated by the BvgAS
virulence control system. J. Bacteriol 175, 3468–3479 (1993). Article CAS Google Scholar * Akerley, B. J., Monack, D. M., Falkow, S. & Miller, J. F. The _bvgAS_ locus negatively
controls motility and synthesis of flagella in _Bordetella bronchiseptica_. J Bacteriol 174, 980–990 (1992). Article CAS Google Scholar * Sisti, F., Ha, D. G., O’Toole, G. A., Hozbor, D.
& Fernandez, J. Cyclic-di-GMP signalling regulates motility and biofilm formation in _Bordetella bronchiseptica_. Microbiology 159, 869–879 (2013). Article CAS Google Scholar *
Veal-Carr, W. L. & Stibitz, S. Demonstration of differential virulence gene promoter activation _in vivo_ in _Bordetella pertussis_ using RIVET. Mol Microbiol 55, 788–798 (2005). Article
CAS Google Scholar * Menozzi, F. D. et al. Heparin-inhibitable lectin activity of the filamentous hemagglutinin adhesin of _Bordetella pertussis_. Infect Immun 62, 769–778 (1994). CAS
PubMed PubMed Central Google Scholar * Stibitz, S. & Yang, M. S. Subcellular localization and immunological detection of proteins encoded by the _vir_ locus of _Bordetella pertussis_.
J Bacteriol 173, 4288–4296 (1991). Article CAS Google Scholar * Quandt, J. & Hynes, M. F. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative
bacteria. Gene 127, 15–21 (1993). Article CAS Google Scholar * Stibitz, S., Black, W. & Falkow, S. The construction of a cloning vector designed for gene replacement in _Bordetella
pertussis_. Gene 50, 133–140 (1986). Article CAS Google Scholar * Stibitz, S. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol 235, 458–465
(1994). Article CAS Google Scholar * Simon, R., Priefer, U. & Pühler, A. A broad host range mobilization system for _in vivo_ genetic engineering: transposon mutagenesis in gram
negative bacteria. Biotechnology 1, 784–791 (1983). Article CAS Google Scholar * Locht, C., Geoffroy, M. C. & Renauld, G. Common accessory genes for the _Bordetella pertussis_
filamentous hemagglutinin and fimbriae share sequence similarities with the _papC_ and _papD_ gene families. EMBO J 11, 3175–3183 (1992). Article CAS Google Scholar * Rouillard, J. M.,
Zuker, M. & Gulari, E. OligoArray 2.0: design of oligonucleotide probes for DNA microarrays using a thermodynamic approach. Nucleic Acids Res 31, 3057–3062 (2003). Article CAS Google
Scholar * Smyth, G. K., Yang, Y. H. & Speed, T. Statistical issues in cDNA microarray data analysis. Methods Mol Biol 224, 111–136 (2003). CAS PubMed Google Scholar * Lonnstedt, I.
& Speed, T. Replicated microarray data. Stat Sinica 12, 808 (2002). MathSciNet MATH Google Scholar * Benjamini, Y. & Hochberg, Y. Controlling the False Discovery Rate - a
Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met 811, 289–300 (1995). MathSciNet MATH Google Scholar Download references ACKNOWLEDGEMENTS We thank Dr Raymond
Pierce for carefully reading the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institut Pasteur de Lille, Center for Infection and Immunity of Lille, Lille, France Loïc Coutte,
Ludovic Huot, Rudy Antoine, Stephanie Slupek, David Hot & Camille Locht * Univ. Lille, Lille, France Loïc Coutte, Ludovic Huot, Rudy Antoine, Stephanie Slupek, David Hot & Camille
Locht * Centre National de la Recherche Scientifique (CNRS), Lille, UMR 8204, France Loïc Coutte, Ludovic Huot, Rudy Antoine, Stephanie Slupek, David Hot & Camille Locht * Institut
National de la Santé et de la Recherche Médicale (Inserm), Lille, U1019, France Loïc Coutte, Ludovic Huot, Rudy Antoine, Stephanie Slupek, David Hot & Camille Locht * Divison of
Bacterial, Parasitic and Allergenic Products, Center for Biologics Evaluation and Research, FDA, Silver Spring, MD, USA Tod J. Merkel, Qing Chen & Scott Stibitz Authors * Loïc Coutte
View author publications You can also search for this author inPubMed Google Scholar * Ludovic Huot View author publications You can also search for this author inPubMed Google Scholar *
Rudy Antoine View author publications You can also search for this author inPubMed Google Scholar * Stephanie Slupek View author publications You can also search for this author inPubMed
Google Scholar * Tod J. Merkel View author publications You can also search for this author inPubMed Google Scholar * Qing Chen View author publications You can also search for this author
inPubMed Google Scholar * Scott Stibitz View author publications You can also search for this author inPubMed Google Scholar * David Hot View author publications You can also search for this
author inPubMed Google Scholar * Camille Locht View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.C., D.H. and C.L. designed the conceptual
framework of this study. L.C., L.H. and S. Slupek carried out the experiments. L.C., R.A., D.H. and C.L. analysed the data. T.J.M., Q.C. and S. Stibitz substantially contributed to the
discussion and provided important unpublished information. L.C., Q.C. and S. Stibitz constructed the mutated strains, and L.C. and C.L. wrote the paper. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLES RIGHTS AND PERMISSIONS This work is licensed
under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless
indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the
material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Coutte, L., Huot, L., Antoine, R.
_et al._ The multifaceted RisA regulon of _Bordetella pertussis_. _Sci Rep_ 6, 32774 (2016). https://doi.org/10.1038/srep32774 Download citation * Received: 10 March 2016 * Accepted: 09
August 2016 * Published: 13 September 2016 * DOI: https://doi.org/10.1038/srep32774 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
