Fabrication of si negative electrodes for li-ion batteries (libs) using cross-linked polymer binders
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Currently, Si as an active material for LIBs has been attracting much attention due to its high theoretical specific capacity (3572 mAh g−1). However, a disadvantage when using a Si
negative electrode for LIBs is the abrupt drop of its capabilities during the cycling process. Therefore, there have been a few studies of polymers such as poly(vinylidene fluoride) (PVdF),
carboxymethyl cellulose (CMC), styrene butadiene rubber (SBR) and polyacrylic acid (PAA) given that the robust structure of a polymeric binder to LIBs anodes is a promising means by which
to enhance the performance of high-capacity anodes. These studies essentially focused mainly on modifying of the linear-polymer component or on copolymers dissolved in solvents.
Cross-linking polymers as a binder may be preferred due to their good scratch resistance, excellent chemical resistance and high levels of adhesion and resilience. However, because these
types of polymers (with a rigid structure and cross-linking points) are also insoluble in general organic solvents, applying these types in this capacity is virtually impossible. SIMILAR
CONTENT BEING VIEWED BY OTHERS A CONDUCTIVE SELF HEALING POLYMERIC BINDER USING HYDROGEN BONDING FOR SI ANODES IN LITHIUM ION BATTERIES Article Open access 11 September 2020 DEVELOPMENT OF
DESIGN STRATEGIES FOR CONJUGATED POLYMER BINDERS IN LITHIUM-ION BATTERIES Article 09 November 2022 AN ENTANGLEMENT ASSOCIATION POLYMER ELECTROLYTE FOR LI-METAL BATTERIES Article Open access
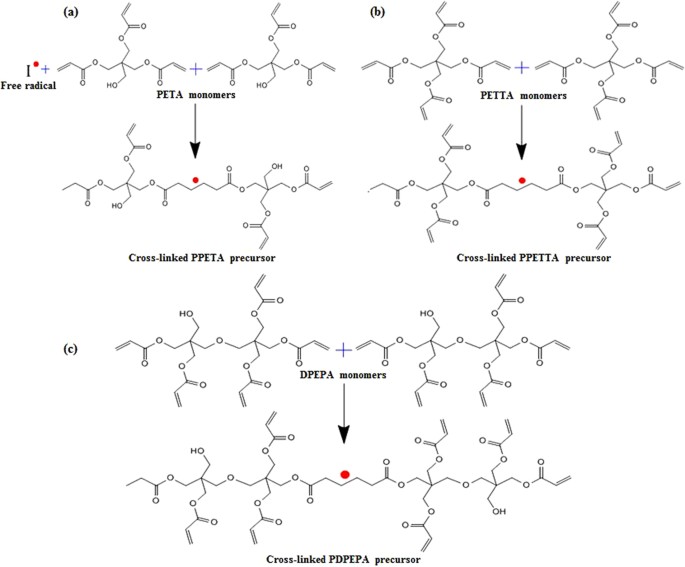
20 March 2024 INTRODUCTION In theory, at least, a multifunctional monomer can easily cross-link by itself1,2,3,4,5, and our laboratory utilized pentaerythritol triacrylate (PETA),
pentaerythritol tetraacrylate (PETTA) and dipentaerythritol pentaacrylate (DPEPA) multifunctional monomers containing three, four and five carbon-carbon double bonds at the backbone. The
Si/carbon black (CB)/(poly)pentaerythritol triacrylate (PPETA) composite (PPETA-composite), Si/CB/(poly)pentaerythritol tetraacrylate (PPETTA) composite (PPETTA-composite) and
Si/CB/(poly)dipentaerythritol pentaacrylate (PDPEPA) composite (PDPEPA-composite) were fabricated via a curing process from a Si/CB/pentaerythritol triacrylate (PETA) composite mixture
(PETA-composite mixture), a Si/CB/pentaerythritol tetraacrylate (PETTA) composite mixture (PETTA-composite mixture) and a Si/CB/dipentaerythritol pentaacrylate (DPEPA) composite mixture
(DPEPA-composite mixture), respectively2,3,4. The resulting charge (delithiation) rates were close to 4, 5 and 3 times higher than a Si/CB/PVdF composite containing the well-known PVdF
binder for 15 cycles. Specifically, the discharge (lithiation) of the Si/CB/PVdF/PPETTA composite (1:2) (PVdF/PPETTA-composite (1:2)) (approximately 3013 mAh g−1) containing the PVdF/PPETTA
(1:2) blended polymer (PVdF/PPETTA (1:2) binder) (blending ratio, 1.0/2.0) as a binder was improved by approximately 654 mAh g−1 compared to the Si/CB/PVdF composite (PVdF-composite) (about
2359 mAh g−1). The purpose of this study was to determine whether a relationship exists between the application of a cross-linking polymer binder and the performance of Si negative
electrodes for LIBs. Another objective was to present a novel process by which to fabricate Si negative electrodes for LIBs with a cross-linked polymer binder system. Figure 1 shows the
cross-linking routes of the PETA, PETTA and DPEPA multifunctional monomers. RESULTS AND DISCUSSION FABRICATION OF COMPOSITE ELECTRODES First, the PETA-composite mixture, the PETTA-composite
mixture, the DPEPA-composite mixture and the PVdF/PETTA-composite mixtures were all fabricated by the direct mixing of 60% Si, 25% CB, 15% of a binder and 2,20-azobisiso-butyronitrile (a
radical initiator, AIBN) in N-methyl-2-pyrrolidone (NMP). Secondly, the composite mixtures were cast on a Cu-foil, and then cured in a silicon-packed mold for polymerization at 85 °C for 2
h3,4. Finally, the resulting composite electrodes were dried in a vacuum oven at 120 °C for 1 h. In order to enhance the inter-particle contact, the composite electrodes were roll-pressed.
The entire fabrication process to create the PVdF/PPETTA-composites is shown in Fig. 2. The materials used for the fabrication of the PVdF-composite, PPETA-composite, PPETTA-composite,
PDPEPA-composite and PVdF/PPETTA-composites are summarized in Table 1. Photographs of the PVdF-composite, PPETA-composite, PPETTA-composite, PDPEPA-composite and PVdF/PPETTA-composites are
shown in Fig. 3. The cell production process used to create the composite electrodes is shown in Fig. 4. Images of the PVdF-composite, PPETA-composite, PPETTA-composite, PDPEPA-composite and
PVdF/PPETTA-composites after 10 cycles are shown in Fig. 5. FABRICATION OF RAW-BINDER SAMPLES The PETA, PETTA, DPEPA, PVdF/PETTA (1:1) mixture, PVdF/PETTA (1:2) mixture and PVdF/PETTA (1:5)
mixture were dissolved in NMP to form the homogeneous solutions described in Table 2. Then, a radical initiator, AIBN, was added to the solutions and directly poured into a silicon-packed
mold (size: 3.0 × 3.0 cm) to carry out cross-linking polymerization for 2 h at 85 °C. After the polymerization step was complete, raw PPETA, PPETTA, PDPEPA, PVdF/PPETTA (1:1), PVdF/PPETTA
(1:2) and PVdF/PPETTA (1:5) binder samples with a thickness of about 0.2 cm were obtained. The resulting samples were dried in a vacuum oven at 120 °C for 1 h. The raw-PVdF binder sample was
prepared by a solution casting method. The materials for the fabrication and the resulting electrolyte uptake (EU) of each raw-binder are summarized in Table 2. FE-SEM IMAGE OF COMPOSITE
ELECTRODES Figure 6 shows a FE-SEM image of the Si used in this study. The morphology of the Si in this case was highly irregular. The average particle size was approximately 10 um. Figure 7
shows FE-SEM images of the PVdF-composite, the PPETTA-composite and the PVdF/PPETTA-composite (1:1). As shown in Fig. 7(a) 1 and 2, the surface of the PVdF-composite cracked frequently. In
addition, the CB particles in this case were highly aggregated (Fig. 7(a) 3). However, there were far fewer, surface cracks in the PPETTA-composite (Fig. 7(b) 1) and the
PVdF/PPETTA-composite (1:1) (Fig. 7(c) 1) compared to the PVdF-composite. The CB particles in these case (Fig. 7(b) 3 and (c) 3) were very well dispersed. Judging from this, the cross-link
networks of the PPETTA as a binder played a role in reinforcing the binding strength between the electrode particles within the composite electrodes5,6. This most likely occurs because the
CB particle inter-distances for the PPETTA-composite and the PVdF/PPETTA-composite (1:1) expanded as the PETTA monomer chains were extended through a curing process3,4,5. ELECTROLYTE UPTAKE
(EU) OF THE BINDERS Figure 8(a) shows the electrolyte uptake of the PVdF, PPETA, PPETTA, PDPEPA and PVdF/PPETTA binders. Interestingly, the electrolyte uptake levels for the PPETA, PPETTA
and PDPEPA samples were approximately 154.4%, 188.5% and 98.2%, close to 8, 10 and 5 fold greater than that of the PVdF sample (approximately 18.9%) despite the fact that they are
cross-linking polymers. The PPETTA sample had the best electrolyte uptake. Because the electrolyte solution is absorbed only into the hydrophobic segments4,5,7, we consider that the
electrolyte uptake levels of the PPETA and PDPEPA samples containing the hydroxyl groups (–OH, hydrophilic segments) at the side chain were decreased compared to that of the PPETTA
sample4,5,8. The corresponding electrolyte uptake levels of the PVdF/PPETTA (1:1), PVdF/PPETTA (1:2) and PVdF/PPETTA (1:5) binders were approximately 26.7%, 39.4% and 60.5%. ELECTROCHEMICAL
PROPERTIES OF THE COMPOSITE ELECTRODES Figure 8(b) shows the cycle performance of the PVdF-composite, the PPETA-composite, the PPETTA-composite, the PDPEPA-composite and the
PVdF/PPETTA-composites. The discharge of the PVdF-composite amounted to 2359 mAh g−1, and it decreased by approximately 34% compared to the theoretical specific capacity of Si. In this case,
the charge dropped sharply after one cycle, with only close to 8% (about 202 mAh g−1) of the discharge maintained after 15 cycles. Because the Li-ions migrate through the electrolyte-sorbed
binder matrix5,7, it is expected that the poor electrolyte uptake of the PVdF decreased the discharge by reducing the number of Li-ionic carriers in the binder matrix. This occurred because
the formation of an unstable solid electrolyte interphase (SEI) layer causes uninterrupted electrolyte solution degradation at the surface of the Si during the cycling
process9,10,11,12,13,14,15,16. The abrupt reduction of the charge that occurred after one cycle can be attributed to the considerable volume expansion and the collapse of Si within the
composite electrode1,2,15,16. As shown in Fig. 5, the surface cracks on the morphology of the PVdF-composite were much worse than on the other samples after 10 cycles. The PVdF-composite was
nearly detached from the current collector. On the other hand, the respective discharge amounts for the PPETA-composite, PPETTA-composite and PDPEPA-composite were approximately 1733 mAh
g−1, 1921 mAh g−1 and 1352 mAh g−1, with corresponding decreases of 51%, 46 and 62% compared to the theoretical specific capacity of Si. All cases showed much less discharge than the
PVdF-composite. Despite the high electrolyte uptake of the cross-linked polymer binders, this most likely occurred because the excessive cross-linking networks of the PPETA, PPETTA and
PDPEPA increased the amount of Li trapping by blocking the Li-ion channels in the binder matrix3,5,6,17. In that the cross-linked density increases with an increase in the number of
carbon-carbon double bonds in a functional monomer6,8,13,17, we expect that the discharge of the PDPEPA-composite was decreased compared to those of the PPETA-composite and the
PPETTA-composite. Nevertheless, the charge in these three corresponding cases remained at approximately 47% (about 818 mAh g−1) of the discharge, at approximately 53% (about 1022 mAh g−1) of
the discharge, and at approximately 47% (about 636 mAh g−1) of the discharge for 15 cycles. These values are nearly, 4, 5 and 3 times higher than that of the PVdF-composite. Moreover, the
PPETA-composite, PPETTA-composite and PDPEPA-composite showed much better surface morphologies than the PVdF-composite after 10 cycles (Fig. 5). Accordingly, we believe that the cross-linked
polymer networks of PPETA, PPETTA and PDPEPA as binders played an important role through volume variation of Si and in maintaining the binding strength within the composite electrodes
during the cycling process18,19,20,21,22. This could occur because the robust cross-linking binder system reduced the deformation of SEI layers and the mechanical stress of crystalline
Li15Si4 within the composite electrodes9,10,11,12,13,14,15,16. The discharge amounts of the PVdF/PPETTA-composite (1:1), the PVdF/PPETTA-composite (1:2), and the PVdF/PPETTA-composite (1:5)
were approximately 2739 mAh g−1, 3013 mAh g−1 and 1897 mAh g−1, respectively, showing decreases of approximately 22%, 15 and 47% compared to the theoretical specific capacity of Si.
Specifically, the discharge amounts of the PVdF/PPETTA-composite (1:1) and the PVdF/PPETTA-composite (1:2) improved remarkably by approximately 380 mAh g−1 and 654 mAh g−1 respectively,
compared to that of the PVdF-composite. These outcomes can be attributed to the fact that the numbers of Li traps of the PVdF/PPETTA binder (1:1) and the PVdF/PPETTA binder (1:2) decreased
as the volume of the cross-linked PPETTA domain in the binder matrix was reduced6,18,19. The charge in these respective cases remained at approximately 12% (about 337 mAh g−1) of the
discharge, at about 24% (about 733 mAh g−1) of the discharge, and at nearly 46% (about 884 mAh g−1) of the discharge for 15 cycles, increasing with an increase in the content of the
cross-linked PPETTA in the blending binder matrix. The entire charge pattern for the PVdF/PPETTA-composite (1:5) was similar to that of the PPETTA-composite during the cycling process. The
charge patterns of the PVdF-composite, PPETA-composite, PPETTA-composite, PDPEPA-composite and PVdF/PPETTA-composites during the cycling process are shown in Table 3. According to work by
Dong _et al_.11 the discharge amount of micro-Si negative electrodes for LIBs with sodium carboxymethyl cellulose (Na-CMC) as a binder was approximately 2150 mAh g−1, showing a decrease of
40% compared to the theoretical specific capacity of Si. The charge in this case was approximately 1770 mAh g−1 after one cycle. Park _et al_.7 also showed that (poly)vinyl alcohol (PVA) as
a binder maintained excellent cyclic retention of Si/graphites due to its numerous hydroxyl groups. The discharge amount for their Si/graphites negative electrode was approximately 1500 mAh
g−1. Koo _et al_.6 reported that the discharge amounts of Si composite electrodes with cured PAA-CMC, PAA and PVdF binders were approximately 2850 mAh g−1, 2200 mAh g−1 and 300 mAh g−1
respectively, at a current density of 300 mA g−1. As mentioned earlier, these studies depended only on a linear-polymer as a binder. The lower electrochemical performances reported in those
studies may be due to the weak linear-polymeric binding system used or the poor electrolyte uptake levels of the binders within the Si negative electrodes in comparison to our study. In
conclusion, despite the fact that the charge of the PPETA-composite, the PPETTA-composite and the PDPEPA-composite as investigated here increased sharply during the cycling process, the
discharge in these cases dropped significantly compared to that of the PVdF-composite. These outcomes were improved considerably by blending a linear-polymer binder and a cross-linked
polymer binder through a curing process. These results could stem from the precise manipulation of the electrolyte uptake and cross-linking level of the binder within the composite
electrodes. METHODS MATERIALS Si was purchased from Aldrich and used as received (powder, −325 mesh, 99% trace metals basis). PETA (molecular formula: C14H18O7, Mw: 298.24, CAS number:
3524-68-3, density: 1.18 g/mL at 25 °C (lit.), Refractive index: _n_20/D 1.483 (lit.), Flash point: >230 °F), PETTA (molecular formula: C17H20O8, Mw: 352.34, CAS number: 4986-89-4,
density: 1.19 g/mL at 25 °C (lit.), Refractive index: _n_20/D 1.487 (lit.), Flash point: >230 °F), DPEPA (molecular formula: C25H32O12, Mw: 524.52, CAS number: 60506-81-2, density: 1.155
g/mL at 25 °C (lit.), Refractive index: _n_20/D 1.49 (lit.), Flash point: >230 °F), CB (Denka black) and NMP (molecular formula: C5N9NO2, Mw: 115.13, CAS number: 41194-00-7) were also
purchased from Aldrich and used as received. AIBN (molecular formula: C8H12N4, Mw: 164.21, CAS number: 78-67-1) was purchased from DEEJUNG CHEMICALS & METALS CO., LTD. COIN HALF-CELL
MEASUREMENTS Coin half cells (CR2032) were manufactured in a dry glove box with ethylene carbonate (EC)/ethyl methyl carbonate (EMC) (3:7 vol. ratio) as an electrolyte containing 1.3 M of
LiPF6 and Celgard® commercial trilayer PP/PE/PP separators. Lithium metal was used as a counter electrode. The galvanostatic cycle was carried out in a voltage range of 0~2.0 V with a
current density of 100 mA/g (WBCS 3000 cycler, Wonatech Co., Korea). EU MEASUREMENT The EU of the prepared raw-binders was determined by measuring the change in the weight between the wet
and dry binder. The raw-binders were soaked in an EC/EMC (3:7 vol. ratio) electrolyte solution containing 1.3 M of LiPF6 at room temperature for 48 h. The external electrolyte was wiped off,
and the binders were weighed. The electrolyte uptake amounts of the binders were obtained by the following equation: Here, _W_dry and _W_wet are the weight of the dried and the
electrolyte-sorbed binder, respectively. MORPHOLOGY MEASUREMENT Dispersed electrode particle images of the prepared electrodes were confirmed by a field emission scanning electron microscope
(FE-SEM, Hitachi Co. Japan). ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Jang, S.-Y. and Han, S.-H. Fabrication of Si negative electrodes for Li-ion batteries (LIBs) using cross-linked
polymer binders. _Sci. Rep._ 6, 38050; doi: 10.1038/srep38050 (2016). PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. REFERENCES * Ito, K. Novel cross-linking concept of polymer Network: synthesis, structure, and properties of slide-ring gels with freely movable junctions. Polym.
J. 39, 489–499 (2007). Article CAS Google Scholar * Tillet, G., Boutevin, B. & Ameduri, B. Chemical reactions of polymer crosslinking and post crosslinking at room and medium
temperature. Prog. Polym. Sci. 36, 191–217 (2011). Article CAS Google Scholar * Kim, J. Y. et al. Preparation of organic–inorganic nanocomposite membrane using a reactive polymeric
dispersant and compatibilizer: Proton and methanol transport with respect to nano-phase separated structure. J. Membr. Sci. 283, 172–181 (2013). Article Google Scholar * Kim, J. Y. et al.
Synthesis of CdS nanoparticles dispersed within solutions and polymer films using amphiphilic urethane acrylate chains. J. Ind. Eng. Chem. 15, 103–109 (2009). Article CAS Google Scholar *
Jang, S. Y. & Han, S. H. Characterization of sulfonated polystyrene-block-poly(ethyl-ran-propylene)-block-polystyrene copolymer for proton exchange membranes (PEMs). J. Membr. Sci. 444,
1–8 (2013). Article CAS Google Scholar * Koo, B. et al. A highly cross-linked polymeric binder for high-performance silicon negative electrodes in lithium ion batteries. Angew. Chem.
Int. Ed. 51, 8762–8767 (2012). Article CAS Google Scholar * Park, H. K. et al. Effect of high adhesive polyvinyl alcohol binder on the anodes of lithium ion batteries. Electrochem.
Commun. 13, 1051–1053 (2011). Article CAS Google Scholar * Ahn, S. H. et al. Synthesis and characterization of soluble polythiophene derivatives containing electron-transporting moiety.
Macromolecules 34, 2522–2527 (2001). Article ADS CAS Google Scholar * Kovalenko, I. et al. A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 334,
75–79 (2011). Article ADS CAS Google Scholar * Ryu, J. H. et al. The failure modes of silicon powder negative electrode in lithium secondary batteries. Electrochem. Solid-State Lett. 7,
A306–A309 (2004). Article CAS Google Scholar * Ding, N. et al. Improvement of cyclability of Si as anode for Li-ion batteries. J. Power Sources 192, 644–651 (2009). Article ADS CAS
Google Scholar * Ji, X. et al. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nature Materials 3, 500–506 (2009). Article ADS Google Scholar *
Chen, Z. et al. Large-volume-change electrodes for Li-ion batteries of amorphous alloy particles held by elastomeric tethers. Electrochem. Commun. 5, 919–923 (2003). Article CAS Google
Scholar * Li, J. et al. Lithium polyacrylate as a binder for tin–cobalt–carbon negative electrodes in lithium-ion batteries. Electrochim. Acta. 55, 2991–2995 (2010). Article CAS Google
Scholar * Buqa, H. et al. Study of styrene butadiene rubber and sodium methyl cellulose as binder for negative electrodes in lithium-ion batteries. J. Power Sources 161, 617–622 (2006).
Article ADS CAS Google Scholar * Komab, S. et al. Polyacrylate modifier for graphite anode of lithium-ion batteries. Electrochem. Solid-State Lett. 12, A107–A110 (2009). Article Google
Scholar * Sato, K. & Koinuma, H. Highly cross-linked polymer networks of amorphous silicon alloys quantum chemical studies on plasma and photochemical processes. Prog. Polym. Sci. 21,
255–297 (1996). Article CAS Google Scholar * Wu, M. et al. Toward an ideal polymer binder design for high-capacity battery anodes. J. Am. Chem. Soc. 135, 12048–12056 (2013). Article CAS
Google Scholar * Sagar, R. U. R. et al. High capacity retention anode material for lithium ion battery. Electrochim. Acta. 211, 156–163 (2016). Article ADS Google Scholar * Nguyen, M.
H. T. & Oh, E. S. Application of a new acrylonitrile/butylacrylate water-based binder for negative electrodes of lithium-ion batteries. Electrochem. Commun. 35, 45–48 (2013). Article
CAS Google Scholar * Xue, Z., Zhang, Z. & Amine, K. Cross-linkable urethane acrylate oligomers as binders for lithium-ion battery. Electrochem. Commun. 34, 86–89 (2013). Article CAS
Google Scholar * Tsao, C. H., Hsu, C. H. & Kuo, P. L. Ionic conducting and surface active binder of poly(ethylene oxide)-block-poly(acrylonitrile) for high power lithium-ion battery.
Electrochim. Acta. 196, 41–47 (2016). Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Graduate School of Knowledge Based Technology and
Energy, Korea Polytechnic University 237 Sangidaehak-Ro (2121 Jungwang-Dong) Siheung-Si, Gyeonggi-Do, 429–450, Republic of Korea Suk-Yong Jang * Department of Chem. Eng. & Biotech.,
Korea Polytechnic University, 237 Sangidaehak-Ro, 2121 Jeongwang-Dong, Siheung-Si, 429-793, Gyeonggi-Do, Republic of Korea Sien-Ho Han Authors * Suk-Yong Jang View author publications You
can also search for this author inPubMed Google Scholar * Sien-Ho Han View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS First author (Dr.
Suk-Yong Jang) wrote the main manuscript text and corresponding author (Prof. Dr. rer. nat. Sien-Ho Han) prepared figures. All authors reviewed the manuscript. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing financial interests. RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or
other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the
Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jang, SY., Han, SH. Fabrication of Si negative electrodes for Li-ion batteries
(LIBs) using cross-linked polymer binders. _Sci Rep_ 6, 38050 (2016). https://doi.org/10.1038/srep38050 Download citation * Received: 13 July 2016 * Accepted: 03 November 2016 * Published:
19 December 2016 * DOI: https://doi.org/10.1038/srep38050 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
