Parallel Metabolomic Profiling of Cerebrospinal Fluid and Serum for Identifying Biomarkers of Injury Severity after Acute Human Spinal Cord Injury
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Suffering an acute spinal cord injury (SCI) can result in catastrophic physical and emotional loss. Efforts to translate novel therapies in acute clinical trials are impeded by the SCI
community’s singular dependence upon functional outcome measures. Therefore, a compelling rationale exists to establish neurochemical biomarkers for the objective classification of injury
severity. In this study, CSF and serum samples were obtained at 3 time points (~24, 48, and 72 hours post-injury) from 30 acute SCI patients (10 AIS A, 12 AIS B, and 8 AIS C). A differential
chemical isotope labeling liquid chromatography mass spectrometry (CIL LC-MS) with a universal metabolome standard (UMS) was applied to the metabolomic profiling of these samples. This
method provided enhanced detection of the amine- and phenol-containing submetabolome. Metabolic pathway analysis revealed dysregulations in arginine-proline metabolism following SCI. Six CSF
metabolites were identified as potential biomarkers of baseline injury severity, and good classification performance (AUC > 0.869) was achieved by using combinations of these metabolites in
pair-wise comparisons of AIS A, B and C patients. Using the UMS strategy, the current data set can be expanded to a larger cohort for biomarker validation, as well as discovering biomarkers
for predicting neurologic outcome.
Acute spinal cord injury (SCI) often causes paralysis and severe disability, for which there are few treatments that even marginally improve neurologic outcome. In Canada, the incidence of
acute traumatic SCI is over 1,500 per year1, and the estimated annual economic impact of SCI is over $2 billion2. As such, SCI has emerged as a major public health issue in modern society
and has prompted considerable efforts to develop therapeutic strategies to improve neurologic outcome and reduce the burden of disability. Many of these efforts have focused on the acutely
injured patient, as it is expected that interventions applied at this early stage of injury afford the opportunity to reduce secondary injury and, through such ‘neuroprotection’, promote
improved outcome.
Efforts to validate potential interventions in the acute SCI setting have been hampered by the reliance upon clinical assessments to quantify neurologic impairment after SCI and to stratify
the severity of injury. These assessments are imprecise predictors of neurologic recovery and are often impossible to even obtain in the acute setting due to intoxication/sedation or
concomitant injuries3. Biomarkers that objectively characterize injury severity and more precisely predict neurologic recovery would be extremely helpful in facilitating the evaluation of
novel treatments in acute SCI clinical trials.
Additionally, the scientific development of novel therapies for acute SCI depends upon an understanding of the complex pathophysiologic mechanisms that are triggered after acute SCI.
Traumatic injury to the spinal cord induces multiple disturbances in the human metabolic network, including oxidative stress, glycolysis, and amino acid and lipid metabolism4,5,6,7.
Metabolomics is an emerging field for high-throughput global profiling of the collection of all metabolites in a biological system (i.e., the metabolome). Such profiling may also identify
metabolite candidates that can be utilized as potential biomarkers. While almost all of our understanding of the metabolite changes after SCI has come from small animal models of
SCI8,9,10,11, a global profiling of the metabolic network in response to acute human SCI has not been previously reported.
In this study, we describe a parallel metabolomic profiling of cerebrospinal fluid (CSF) and serum from acute SCI patients using a newly developed chemical isotope labeling liquid
chromatography mass spectrometry (CIL LC-MS) platform with a universal metabolome standard (UMS)12. Our objectives were to characterize the metabolite changes that occur in these two
biofluids in response to injury, and to identify potential biomarkers of injury severity. We utilized dansylation LC-MS to enable relative quantification of the amine- and phenol-containing
submetabolome with high coverage. With the UMS, the data set reported herein can be expanded in the future by analyzing additional samples for biomarker validation as well as discovering
prognostic biomarkers for predicting neurologic recovery.
We prospectively enrolled 30 acute traumatic SCI patients who had suffered a cervical or thoracolumbar injury (C3-L1) in whom a valid baseline neurologic examination could be performed in
accordance with the International Standards for Neurologic Classification of SCI (ISNCSCI). This clinical examination was done typically within 24 hours of injury. The ISNCSCI examination
and its scoring conventions are available from http://asia-spinalinjury.org/wp-content/uploads/2016/02/International_Stds_Diagram_Worksheet.pdf and this form is also available from the
corresponding authors upon request. The baseline American Spinal Injury Association (ASIA) Impairment Scale (AIS) grade was A for 10 patients, B for 12, and C for 8. In general terms, AIS A
denotes those with complete motor and sensory paralysis (the most severe neurologic impairment), AIS B denotes those with complete motor paralysis but some preserved sensation, and AIS C is
assigned when there is some preserved motor and sensory function. A lumbar intrathecal catheter was inserted at the time of surgery and maintained for 3–5 days post-injury for the
acquisition of serial CSF samples. CSF and serum samples were obtained at 3 time points: approximately 24, 48, and 72 hours post-injury. The CSF and blood samples were drawn within a few
minutes of one another and were processed, centrifuged, and frozen at the bedside. The patients were examined clinically at 6 months post-injury to determine AIS grade and motor score
recovery.
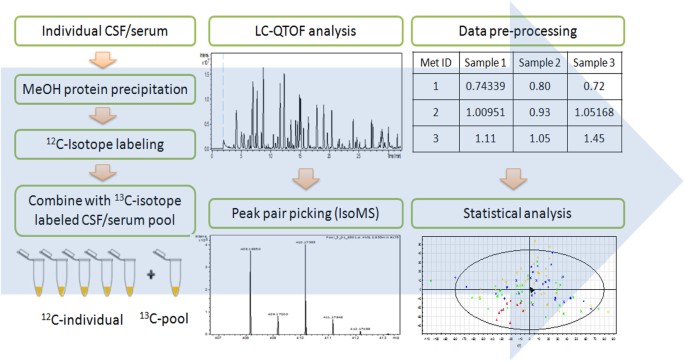
Figure 1 shows the overall workflow of CIL LC-MS for profiling the submetabolomes of CSF and serum in this cohort of 30 acute SCI patients. Prior to LC-MS analysis, each 12C-labeled
individual CSF or serum sample was combined with an equal mole amount of the corresponding 13C-labeled pooled sample which served as the UMS. The relative concentration of each metabolite in
an individual sample to that of the corresponding metabolite in UMS was measured using the intensity ratio of the 12C/13C peak pair. Since the same UMS was spiked into all the comparative
samples, the peak ratio values of a given metabolite in individual samples reflected their concentration differences in these samples. The use of 13C-labeled UMS as internal standards
enables more accurate quantification of the 12C-labeled metabolites. In addition, any future samples could be 12C-labeled and then compared to the 13C-labeled UMS, thereby allowing expansion
of the current dataset to a larger cohort. Dansylation LC-MS has the advantage of improved chromatographic separation and enhanced electrospray ionization (ESI) response, resulting in 10-
to 1000-fold increase in detection sensitivity13. A detailed discussion of this analytical platform, including the workflow, evaluation of analytical variability and peak detectability, as
well as comparison between the CSF and serum submetabolomes is included in Supplemental Note S1. Using this method of targeting the amine/phenol-containing metabolites, we were able to
detect 1213 and 2316 12C/13C peak pairs from CSF and serum, respectively. By matching accurate mass and retention time with the dansyl standard library14 (mass error
