Voltage fluctuation in a supercapacitor during a high-g impact
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Supercapacitors (SCs) are a type of energy storage device with high power density and long lifecycles. They have widespread applications, such as powering electric vehicles and
micro scale devices. Working stability is one of the most important properties of SCs, and it is of significant importance to investigate the operational characteristics of SCs working under
extreme conditions, particularly during high-_g_ acceleration. In this paper, the failure mechanism of SCs upon high-_g_ impact is thoroughly studied. Through an analysis of the intrinsic
reaction mechanism during the high-_g_ impact, a multi-faceted physics model is established. Additionally, a multi-field coupled kinetics simulation of the SC failure during a high-_g_
impact is presented. Experimental tests are conducted that confirm the validity of the proposed model. The key factors of failure, such as discharge currents and discharging levels, are
analyzed and discussed. Finally, a possible design is proposed to avoid the failure of SCs upon high-_g_ impact. SIMILAR CONTENT BEING VIEWED BY OTHERS FATIGUE ANALYSIS OF AN ENERGY STORAGE
SUPERCAPACITOR BOX UNDER RANDOM VIBRATION LOADING Article Open access 04 March 2025 IMPACT-RESISTANT SUPERCAPACITOR BY HYDROGEL-INFUSED LATTICE Article Open access 01 August 2024 A
HIGH-SPEED CURRENT SOURCE FOR MAGNETORHEOLOGICAL APPLICATIONS Article Open access 16 October 2023 INTRODUCTION With the increasing concern for the worldwide energy crisis and environmental
pollution due to the depletion and burning of fossil fuels, there is an urgent demand for efficient, clean and sustainable energy sources. Supercapacitors or electrochemical capacitors
represent a promising approach to meet the increasing power needs of both macro and microelectronic devices1,2. Compared with other energy storage methods, supercapacitors offer advantages
such as high power density, good environmental adaptability and long cycle life3,4,5,6,7. To date, supercapacitors have been widely used as energy supplies for electric cars, subway trains
and satellites. For these practical applications, supercapacitors may suffer many extreme working conditions, and their reliability is a serious concern that needs to be taken into
consideration in application design and development. For example, the high-_g_ acceleration (high acceleration up to tens of thousands times that of gravity) often occurs during the
operation of electric cars, subway trains and satellites when they are speeding up, slowing down, turning, etc. As a result, the influences of high-_g_ acceleration must be considered in
order to insure normal and safe operation of these systems. There have been reports about the reliability of supercapacitors and other electrochemical energy storage devices, such as lithium
batteries, under high-_g_ acceleration impacts. According to these studies, supercapacitors may suffer voltage fluctuations while discharging during a high-_g_ impact8. However, the
previous reports for supercapacitor failure under high-_g_ impact are only based on experimental tests. Due to the lack of theoretical analysis, the kinetic mechanism of this failure
phenomenon is still unclear. To improve the reliability of supercapacitors during high-_g_ impacts, it is highly necessary to study the kinetic mechanism of this failure phenomenon. In this
paper, we focus on the electrical-chemical mechanism of the supercapacitor voltage fluctuation during a high-_g_ impact process. For the first time, a multi-field coupled kinetics model is
proposed to describe the failure mode during such a process. In this model, the electrolyte flow and ion redistribution caused by the high-_g_ impact account for the voltage fluctuation, and
the ion concentration field and electrical field during the impact process can be worked out completely by the model. Simulations of supercapacitor voltage and ion concentration response
processes are realized, and experimental tests are conducted using a Machete Hammer, which verify the model. Based on the failure mechanism characterized by the model, an optimized design is
proposed to improve supercapacitor reliability during high-_g_ impact. This paper reveals the fundamental nature of the high-_g_ impact failure of supercapacitors and has guiding
significance for avoiding the supercapacitor failure in practical applications. HIGH-_G_ RESPONSE MODEL OF SUPERCAPACITORS A supercapacitor or electrochemical capacitor is composed of two
electrodes, which are usually separated by an ion-permeable membrane, and an electrolyte ionically connecting both electrodes. It can be divided into two types: electrical double-layer
capacitors and pseudo capacitors. The regular double-layer capacitance results from the potential-dependence of the surface density of charges stored electrostatically at the interfaces of
the two electrodes. The pseudocapacitance results from Faradaic electron charge transfer with redox reactions, intercalation or electrosorption related to the electroactive materials. For
supercapacitors in practical applications, the double-layer capacitors usually exhibit perhaps 1–5% of their capacitance as pseudocapacitance owing to the Faradaic reactivity of surface
oxygen-functionalities. In contrast, pseudocapacitors usually exhibit 5–10% of their capacitance as electrostatic double-layer capacitance due to the ion adsorption/desorption in the
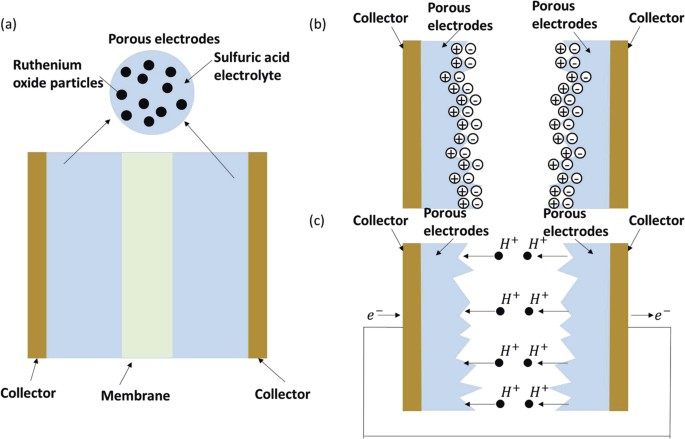
interfacial areas. With this in mind, we established a dynamic model based on a typical supercapacitor, which is schematically shown in Fig. 1(a). The porous electrodes are usually made of
materials with large surface areas, such as ruthenium oxide. A liquid electrolyte fully fills the pores of the electrodes and membrane. The energy storage mechanism of the supercapacitor is
based on a combination of the electrical double layer effect and the Faraday process. The electric double layer effect stores energy at the surface of the electrodes. As shown in Fig. 1(b),
the electrical double layer structure results from the electrical attraction between positive ions and negative ions. The Faraday process is a highly reversible redox reaction of the
electroactive species, and energy storage can be realized through the resulting electron transfer, as shown in Fig. 1(c). DYNAMIC MODEL FOR ENERGY STORAGE IN A SUPERCAPACITOR The energy
storage mechanism of a typical supercapacitor is shown in Fig. 1(b) and (c). Modeling and simulations are useful for theoretical research and optimizing designs of energy storage devices9.
For example, a dynamic modeling simulation is the most accurate and effective way to study the mechanism and characteristics of the charging and discharging processes10,11,12,13,14,15,16,17,
and it establishes a multi-faceted physics model composed of an electric field and an ion concentration field for the electrodes and the membrane, respectively. First, in the porous
electrode, the total current from the electrode phase to the electrolyte phase can be decomposed into the electric double layer current and the Faraday process current, where _i_l represents
the surface current density of the electrolyte phase, and _i_F and _i_DL represent the current density that result from the Faraday process and the electric double layer effect,
respectively. For the electrode phase, the relation between the current and the potential is given by Ohm’s law, where _i_s, _σ_s,eff and Φs represent the surface current density, the
effective conductivity and the potential of the electrode phase. For the porous materials, all effective parameters are derived from their material parameters using the Bruggeman method. For
example, , where _σ_s represents the electrical conductivity of ruthenium oxide and _ε_s represents the volume ratio of solid to total volume. However, for the electrolyte phase of the
electrode, Ohm’s law needs to be modified in light of the concentrated solution effect, where _i_l, _σ_l,eff and Φl represent the surface current density, the effective conductivity, and the
potential of the electrolyte phase of the electrode, respectively. _t_+ and _c_l are the transference number and ion concentration in the solution. _F, R_ and _T_ represent the Faraday
constant, Universal gas constant, and the temperature in Kelvin, respectively. The potential equation of the electrolyte phase can be obtained by combining equations (1) and (3), The
relation ∇ · _i_s + ∇ · _i_l = 0 must hold due to charge conservation. The potential equation of the electrode phase can then be obtained by combining this conservation equation with
equation (2), For the Faraday process, the binding and release of hydrogen ions at the surface of the ruthenium oxide is governed by classical porous electrode theory13. As shown in equation
(6), the ion concentration field is influenced by the current and the transfer of ions, where _ε_l and _D_l,eff represent the porosity of the electrode and the hydrogen ions diffusion
coefficient in the electrolyte, respectively. In the membrane, _i_s = 0 since it is an insulator. Thus, ∇ · _i_l = 0. One can write the potential equation for the electrolyte phase in the
membrane as, Similar to equation (6), the ion concentration equation for the electrolyte phase in the membrane can be obtained, since there is no Faraday current there. According to
electrochemical theory, the Faraday current can be calculated as: where _a_v represents the specific surface area of the electrodes and _j_loc represents the Faraday transfer current
density. The _a_v depends on porosity _ε_l, and the ruthenium oxide particle radius _r_p, The _j_loc term can be calculated by equation (10), according to the Butler-Volmer equation, where
_i_0 represents the Faraday exchange current density, _α_a and _α_c represent the transfer coefficients of the anode and cathode, and _U_oc is the open-circuit potential of the Faraday
process. The _U_oc for the positive and negative electrodes are both dependent on the extent of the redox reactions, where _θ_ represents the extent of the redox reactions, which can be
represented by the ratio between the hydrogen ion concentration _c_s of the electrode phase and the maximum achievable concentration _c_s,max, The _c_s,max depends on the microstructure of
ruthenium oxide, where _L_A represents Avogadro’s number and _h_ represents the lattice constant of ruthenium oxide. According to the theory proposed by Kim and Popov13, we can obtain the
conservation equations inside the ruthenium oxide particles (0 < _r_ < _r_p), The boundary condition for equation (15) is, The current density _i_DL created by the electric double
layer effect is, where _C_dl represents the capacitance associated with the electric double layer effect. Equations (1),(2),(3),(4),(5),(6),(7),
(8),(9),(10),(11),(12),(13),(14),(15),(16),(17) fully describe the mechanism of the supercapacitor’s charging and discharging processes, and constitute a dynamic model for energy storage in
the supercapacitor. A multi-faceted physics simulation for these processes can be realized by solving the model equations. DYNAMIC MODEL FOR SUPERCAPACITOR RESPONSE BASED ON THE FLUID AND
CONCENTRATION FIELDS To characterize the impact response of supercapacitors, the dynamic model for energy storage needs to be modified to account for the effects of a high-_g_ process. In
fact, an inhomogeneity in the hydrogen ion concentration always exists during the charging and discharging of the supercapacitor18, as shown in the left part of Fig. 2. The electrolyte will
flow under the influence of a high-_g_ impact, and this flow causes the hydrogen ions to redistribute homogeneously in a very short time, as shown in the right part of Fig. 2. The ion
distribution caused by a high-_g_ impact process has a significant influence on the performance of supercapacitors, which is discussed and modeled in the following section. The inhomogeneity
in the hydrogen ion concentration can be derived from a dynamic model proposed above. From equation (6) in the energy storage model of the supercapacitor, we can now consider the initial
state before the supercapacitor begins charging or discharging. At the beginning, there is no Faraday current in the supercapacitor, and the hydrogen ion concentration in the electrolyte is
homogeneous due to steady-state diffusion, As the supercapacitor begins to charge or discharge, there are opposite Faraday currents in the positive electrode and negative electrodes, and
hydrogen ions become bound to or released from these two electrodes. For the charging process, While for the discharging process, It can be concluded that the hydrogen ion concentrations of
the two electrodes change in an opposing manner. As a result, the homogeneity of the ion concentration no longer holds once the charging or discharging process begins, Fluid mechanics
dictates that the liquid will flow due to the effects of gravity or acceleration19, and electrochemistry predicts that the electric field of the system may be affected by the fluid nature of
the electrolyte20,21,22. The fluid field is controlled by the Navier-Stokes equations, where _u, ρ, p, μ_ and _a_ represent the flow velocity, the density of the liquid, the pressure of the
liquid, the dynamic viscosity, and the acceleration, respectively. The ion concentration in the electrolyte not only is influenced by the Faraday current and the diffusion effect but also
changes based on electrolyte flow. During an impact process, for example, a constant acceleration _a_ lasts for a period of time _t_g. Equation (6) cannot accurately describe the change of
ion concentration, and must be modified to include the fluid flow occurring during that time. For the electrodes, equation (6) should be modified as: For the membrane, After replacing
equations (6) and (8) with equations (24) and (25), a new model can be constructed with the electric field, ion concentration field, fluid field, and acceleration field. According to this
model, the primary mechanism of the impact response is the ion redistribution process shown in Fig. 2. During the discharging process of the supercapacitor, the hydrogen ion concentration is
larger at one electrode and smaller at the other due to ion release and binding during the Faraday process, as shown in the left part of Fig. 2. As the impact occurs, the electrolyte begins
to flow and causes the ions to redistribute homogeneously, as shown in the right part of Fig. 2. Thus, it can be concluded that acceleration can directly influence the fluid field of the
electrolyte and further change the ion concentration field and electric field of the supercapacitor, revealing the microscopic dynamic mechanism of the voltage fluctuations during high-_g_
impact. The details of the response are discussed in the following section using simulation results. An equivalent circuit diagram is also proposed to help understand the impact response
characteristics of supercapacitors intuitively. For a typical qualitative and semi-quantitative analysis of the charging and discharging process of a supercapacitor, the equivalent circuit
model in Fig. 3(a) is commonly used23,24,25. According to the equivalent circuit model shown in Fig. 3(a), the equivalent series capacitance _C_i0, _C_i1 and internal resistance _R_i
describe the fast transient response of the charging and discharging processes. In addition, the hysteresis loop composed of _C_d and _R_d characterizes the relaxation process of the
supercapacitor caused by the ion redistribution effect. For practical supercapacitors, the relaxation process is relatively slow and can be ignored during charging and discharging, which
means that the switch _k_1 between the hysteresis circuit and the main circuit will not close until the charging or discharging process is complete. According to the mechanism discussed in
equations (24) and (25), the impact process influences the supercapacitor through ion redistribution. The self-discharging theory of supercapacitors states that the relaxation process is
also caused by ion redistribution. Therefore, in order to take the supercapacitor’s response to acceleration into account, we should modify the typical equivalent circuit model, similar to
modeling the relaxation process, by adding another hysteresis circuit, as shown in Fig. 3(b). The impact response is modeled by the hysteresis circuit composed of _C_d and _R_s. This new
hysteresis circuit has a small response time, which means that and that the relaxation time constant _R_s_C_d is sufficiently small. When a high-_g_ impact happens, the switch _k_2 closes,
and this hysteresis circuit will be connected to the main circuit, resulting in a fast hysteresis process. The redistribution of charges between capacitors _C_d and _C_i0, _C_i1 will result
in a hysteresis current in the main circuit and fluctuate the output voltage _V_out. The simulations in this paper are based on the dynamic model proposed above because it analyzes the micro
scale impact response mechanism of the supercapacitor and is more accurate than the equivalent circuit model shown in Fig. 3(b). SIMULATION AND EXPERIMENT RESULTS Based on the dynamic model
for the impact response of the supercapacitor that takes into consideration the fluid field and the concentration field, a multi-faceted physics dynamic simulation is implemented. The
parameters in the simulations are shown in Table 1. Furthermore, to verify the validity of the dynamic model and the simulation, we tested the most important simulation conclusions
experimentally by the Machete hammer test, which can provide accelerations of up to 30000 _g_. The Machete hammer test is commonly used in high-_g_ impact experiments26 and can realize
high-_g_ impact by a violent collision process, with is caused by the huge kinetic energy of the counterweight, as shown in Fig. 4. We used a CHI 660 D electrochemical workstation to
establish a supercapacitor high-_g_ impact test platform. In the following part, we discuss the simulation and experimental results in detail. Two typical impact response processes are
simulated and their voltage responses are shown in Fig. 5(a) and (b). For case 1, the supercapacitor is charged with a large current, and the discharging process begins immediately after the
supercapacitor is fully charged. Then, the supercapacitor suffers high-_g_ impact. Figure 5(a) shows that the voltage of the supercapacitor fluctuates downward when the impact occurs under
this condition; in other words, Δ_V_ < 0, where Δ_V_ represents the voltage increase during the impact process. For case 2, the supercapacitor begins to discharge after a long
self-discharging process, and the high-_g_ impact occurs during the discharging process. Figure 5(b) shows that the voltage of the supercapacitor fluctuates upward (Δ_V_ > 0) when impact
occurs in this condition, in contrast to case 1 in Fig. 5(a). The experimental results also verify the two opposite voltage fluctuations for case 1 and case 2. Corresponding to case 1 in the
simulation, the supercapacitor is first charged with a 20 mA current. The discharging process begins immediately after the supercapacitor is fully charged, and the high-_g_ impact occurs
shortly after the discharge begins. The experimental results clearly show the voltage drop (Δ_V_ < 0) in Fig. 5(c). Corresponding to case 2 of the simulation, the supercapacitor is
maintained at a constant voltage for a long time after it is fully charged. After the long period of constant voltage, the supercapacitor begins to discharge with a 20 mA current. The
experimental results clearly show the voltage increase (Δ_V_ > 0) in Fig. 5(d). The experimental phenomena shown in Fig. 5(c) and (d) are highly consistent with the simulation phenomenon
shown in Fig. 5(a) and (b) and verify the validity of the proposed model. To understand the nature of the two different failure phenomenon shown in Fig. 5, it is necessary to further study
the changes of hydrogen ion concentration distribution in the electrolyte during the impact process. It is difficult to measure the ion concentration distribution experimentally during the
impact process because the distribution changes dramatically during a short process and the ion concentration measurements are challenging to implement under such extreme conditions. Thus,
we calculated the ion concentration distribution by simulation based on the proposed model. For case 1, while charging with a large current, the Faraday reaction releases hydrogen ions much
faster than the rate of ion diffusion in the electrolyte. As a result, a large number of hydrogen ions are released and accumulated on the positive electrode, while hydrogen ions on the
negative electrode are consumed at the same time. Before the high-_g_ impact occurs, the hydrogen ion concentration at the positive electrode is much greater than at the negative electrode,
as shown by the black line in Fig. 6(a). After the impact begins, the electrolyte flow quickly changes the distribution of the hydrogen ions in the electrolyte, and the ion concentration
returns to a homogeneous state in a short time. This process is shown in Fig. 6(a), where _t_s represents the time from the beginning of the impact. According to equation (5), the ion
concentration _c_l directly influences the electrolyte potential Φl, which impacts the output voltage of supercapacitor. This voltage fluctuation can be attributed to the electrolyte ions
redistribution effect18. During the discharging process of the supercapacitor, hydrogen ions are bound to the positive electrode. The impact process makes the hydrogen ion concentration more
homogeneous and reduces the hydrogen ion concentration at the positive electrode. The decrease of hydrogen ions at the positive electrode reduces the discharging capacity of the
supercapacitor and makes the output voltage drop. In contrast, the ion concentration distribution of case 2, as shown in Fig. 6(b), has already become homogeneous after a long
self-discharging process, according to the self-discharging theory of supercapacitors18. Then, as the supercapacitor discharges with a large current, the ion concentration at the negative
electrode significantly exceeds that of the positive electrode, as shown by the black line in Fig. 6(b). During the impact process, the ion concentration becomes homogeneous in a short time.
According to equation (5), the influence of the ion concentration _c_l on the electric field depends on the value of ∇ln(_c_l). Due to the opposite values of ∇ln(_c_l) in Fig. 6(a) and (b),
the voltage fluctuations in case 2 are opposite to that in case 1. As shown in Fig. 5(b), there is an upward voltage fluctuation, rather than a downward one, during the impact process.
Based on the electrolyte ion redistribution, the electrolyte flow resulting from a high-_g_ impact makes the hydrogen ion concentration more homogeneous and increases the hydrogen ion
concentration at the positive electrode. As the electrolyte provides more hydrogen ions for the Faraday process, the discharge capacity is improved, and the output voltage increases. To
summarize, the voltage fluctuation is determined by the ion concentration distribution at the time of the impact. If the hydrogen ions are concentrated at the positive electrode at the
initial time (_c_l|positive > _c_l|negative), the voltage fluctuates downward (Δ_V_ < 0) during the impact process, as shown in the Figs 5(a) and 6(a). Conversely, as shown in Figs
5(b) and 6(b), when the hydrogen ions are concentrated at the negative electrode at the initial time (_c_l|positive < _c_l|negative), the voltage fluctuates upward (Δ_V_ > 0) during
the impact process. The analysis above demonstrates that the voltage fluctuation generated by the impact process depends on the hydrogen ion concentration of the electrolyte. Further
simulation results will elaborate on this conclusion in detail. Considering the supercapacitors used in practical applications, most of them will not begin to discharge until they are fully
self-discharged. For this reason, the following simulation will focus on the case 2 discussed above. Figure 7(a) shows the voltage fluctuations with different discharging currents and
different discharging levels when the high-_g_ impacts occur. We define the discharging level as the ratio of the output voltage when the high-_g_ impact occurs to the maximum voltage when
the supercapacitor is fully charged. The results in Fig. 7(a) show the character of the impact response. First, for the same discharging current, as the discharging level increases, the
voltage fluctuation becomes larger. Second, for a larger discharging current, the voltage increase is larger. Third, for relatively small discharging currents, when the discharging level is
low, the voltage increase is approximately a linear function of the discharging level. However, as the discharging level continues to increase and approaches 1, the amplitude of voltage
fluctuation increases relatively slowly and finally approaches a constant value. Corresponding to the simulation results in Fig. 7(a), the amplitudes of voltage fluctuation under different
discharge currents and discharging levels are experimentally measured. The experimental results in Fig. 7(b) show that the amplitude of voltage fluctuation increases as the discharging level
and discharging current increase. According to the experimental results in Fig. 7(b), when the discharging current is small, as the discharging level increases, the amplitude of the voltage
fluctuation increases slower and slower, similar to the convergence phenomenon illustrated by the black line of Fig. 7(a). The only difference between the simulation and experimental
results is that the experimentally measured voltage fluctuation amplitudes are always larger than the simulated ones under certain discharging current and discharging levels. This phenomenon
is due to the piezoresistive effect of the supercapacitor electrodes. The porous electrode is piezoresistive and has less resistance during the high-_g_ impact due to the huge pressure. The
decrease of the electrode resistance reduces the voltage of the electrode. As a result, the output voltage of the supercapacitor will increase. This output voltage increase caused by the
piezoresistive effect of the electrode accounts for the difference between the simulated and experimentally measured voltage fluctuation amplitudes. The qualitative consistency between the
simulation (Fig. 7(a)) and experiment (Fig. 7(b)) further proves the validity of the proposed model. These important findings from simulations and experiments can be explained by the ion
concentration simulations in Fig. 7(c) and (d). First, the concentration difference results from the accumulation of Faraday current. Thus, as the discharging level increases, more ions
accumulate at the negative electrode, and the concentration difference becomes larger. As a result, homogenization of the concentration field caused by the high-_g_ impact process will
influence the electric field more significantly. Second, a concentration difference arises from the fact that the ion release rate in the Faraday reaction exceeds the diffusion rate. As the
discharging current becomes larger, the Faraday reaction becomes more dramatic, and the concentration difference becomes larger. Third, according to equation (18), the rate of diffusion
depends on the concentration difference. As the concentration difference becomes sufficiently large, the diffusion effect can offset the ion release in the Faraday reaction and keep the
concentration stable, as shown in Fig. 7(c). Thus, the voltage fluctuation approaches a constant value, although the discharging level continues to increase. The simulation and experimental
results both show that supercapacitors suffer voltage fluctuations during high-_g_ impact processes. The inhomogeneity of the ion concentration and the electrolyte flow are the two primary
reasons for these voltage fluctuations. To avoid this failure during high-_g_ impact, it is necessary to reduce the inhomogeneity of ion concentration or the electrolyte flow, which can be
achieved by using a large diffusion-coefficient electrolyte and a solid electrolyte, respectively. To reduce the inhomogeneity of the ion concentration, it is worthwhile to use an
electrolyte with a large diffusion coefficient. According to equations (24) and (25), such an electrolyte will allow ions to diffuse more quickly and reduce the ion inhomogeneity, resulting
in a smaller effect by a high-_g_ impact. It is also helpful to use a solid electrolyte, which does not flow when the impact occurs, unlike a liquid one, so that the ion concentration and
output voltage can remain stable during a short duration impact process. CONCLUSIONS This paper investigates the failure of a supercapacitor during a high-_g_ impact. The impact response
mechanism of the supercapacitor is analyzed, and a dynamic model for the voltage response is established. Based on this model, a multi-faceted physics simulation of the supercapacitor under
a high-_g_ impact is achieved, and the experimental results verify the proposed model and simulation. According to this model, the high-_g_ impact causes the electrolyte to flow and results
in a change of ion concentration distribution, which further influences the electric field and makes the supercapacitor’s voltage fluctuate. Simulation results indicate that the
inhomogeneity of ion concentration and the electrolyte flow are the two primary factors that control these voltage fluctuations. There will be larger fluctuations when there is a larger
inhomogeneity of the ion concentration. To avoid this failure during high-_g_ impacts, it is suggested that electrolytes with a large diffusion coefficient or a solid electrolyte be used for
supercapacitors. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Dai, K. _et al_. Voltage Fluctuation in a Supercapacitor During a High-_g_ Impact. _Sci. Rep._ 6, 38794; doi:
10.1038/srep38794 (2016). PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. REFERENCES * Yuan, Z.,
Zhang, Y., Fu, W., Li, Z. & Liu, X. Investigation of a small-volume direct methanol fuel cell stack for portable applications. Energy 51, 462–467 (2013). Article CAS Google Scholar *
Yi, F. et al. Stretchable-rubber-based triboelectric nanogenerator and its application as self-powered body motion sensors. Advanced Functional Materials 25, 3688–3696 (2015). Article CAS
Google Scholar * Wang, X., Yin, Y., Hao, C. & You, Z. A high-performance three-dimensional micro supercapacitor based on ripple-like ruthenium oxide-ccarbon nanotube composite films.
Carbon 82, 436–445 (2015). Article CAS Google Scholar * Wang, X.-f., Ruan, D.-b. & You, Z. Application of spherical ni(oh)(2)/cnts composite electrode in fp asymmetric supercapacitor.
Transactions Of Nonferrous Metals Society Of China 16, 1129–1134 (2006). Article CAS Google Scholar * Wang, X., Yin, Y., Li, X. & You, Z. Fabrication of a symmetric micro
supercapacitor based on tubular ruthenium oxide on silicon 3d microstructures. Journal of Power Sources 252, 64–72 (2014). Article ADS Google Scholar * Yu, L., Zhang, G., Yuan, C. &
Lou, X. W. Hierarchical nico2o4@mno2 core-shell heterostructured nanowire arrays on ni foam as high-performance supercapacitor electrodes. Chemical Communications 49, 137–139 (2013). Article
CAS Google Scholar * Chen, H., Hu, L., Chen, M., Yan, Y. & Wu, L. Nickel-cobalt layered double hydroxide nanosheets for high-performance supercapacitor electrode materials. Advanced
Functional Materials 24, 934–942 (2014). Article Google Scholar * Jing, P. _High-g impact test value of the research of key technologies_. Master’s thesis, North University of China
(2009). * Niu, S. et al. Theoretical study of contact-mode triboelectric nanogenerators as an effective power source. Energy & Environmental Science 6, 3576–3583 (2013). Article Google
Scholar * Lin, C., Ritter, J. A., Popov, B. N. & White, R. E. A mathematical model of an electrochemical capacitor with double-layer and faradaic processes. Journal of the
Electrochemical Society 146, 3168–3175 (1999). Article CAS Google Scholar * Farsi, H. & Gobal, F. A mathematical model of nanoparticulate mixed oxide pseudocapacitors; part i: model
description and particle size effects. Journal Of Solid State Electrochemistry 13, 433–443 (2009). Article CAS Google Scholar * Farsi, H. & Gobal, F. A mathematical model of
nanoparticulate mixed oxide pseudocapacitors; part ii: the effects of intrinsic factors. Journal Of Solid State Electrochemistry 15, 115–123 (2011). Article CAS Google Scholar * Kim, H.
& Popov, B. N. A mathematical model of oxide/carbon composite electrode for supercapacitors. Journal of the Electrochemical Society 150, A1153–A1160 (2003). Article CAS Google Scholar
* Johnson, A. M. & Newman, J. Desalting by means of porous carbon electrodes. Journal of the Electrochemical Society 118, 510–517 (1971). Article CAS Google Scholar * Srinivasan, V.
& Weidner, J. W. Mathematical modeling of electrochemical capacitors. Journal of the Electrochemical Society 146, 1650–1658 (1999). Article CAS Google Scholar * Lin, C., Popov, B. N.
& Ploehn, H. J. Modeling the effects of electrode composition and pore structure on the performance of electrochemical capacitors. Journal of the Electrochemical Society 149, A167–A175
(2002). Article CAS Google Scholar * Farsi, H. & Gobal, F. Theoretical analysis of the performance of a model supercapacitor consisting of metal oxide nano-particles. Journal of Solid
State Electrochemistry 11, 1085–1092 (2007). Article CAS Google Scholar * Hao, C., Wang, X., Yin, Y. & You, Z. Analysis of charge redistribution during self-discharge of double-layer
supercapacitors. Journal of Electronic Materials 45, 2160–2171 (2016). Article CAS ADS Google Scholar * Evett, J. B. & Liu, C. Fundamentals of fluid mechanics (McGraw-Hill College,
New York, 1987). * Chen, D., Li, G., Wang, J., Chen, J. & He, W. A micro electrochemical seismic sensor based on mems technologies. Sensors and Actuators A: Physical 202, 85–89 (2013).
Article CAS Google Scholar * Deng, T., Chen, D., Wang, J., Chen, J. & He, W. A mems based electrochemical vibration sensor for seismic motion monitoring. Journal of
Microelectromechanical Systems 23, 92–99 (2014). Article Google Scholar * Sun, Z. & Agafonov, V. M. 3d numerical simulation of the pressure-driven flow in a four-electrode rectangular
micro-electrochemical accelerometer. Sensors and Actuators B: Chemical 146, 231–238 (2010). Article CAS Google Scholar * He, Y., You, Z. & Wang, X. Modelling of a hybrid energy system
for autonomous application regular paper. International Journal of Advanced Robotic Systems 10, 356 (2013). Article Google Scholar * Yang, H. & Zhang, Y. Evaluation of supercapacitor
models for wireless sensor network applications. In _Signal Processing and Communication Systems (ICSPCS_)_, 2011 5th International Conference on_, 1–6 (IEEE, 2011). * Zubieta, L. &
Bonert, R. Characterization of double-layer capacitors for power electronics applications. IEEE Transactions on Industry Applications 36, 199–205 (2000). Article Google Scholar * Li, Y.,
Chen, J. & Wang, Y. The study on anti-high-g technology of missile body attitude measurement system. In _Automatic Control and Artificial Intelligence (ACAI 2012_)_, International
Conference on_, 2195–2198 (IET, 2012). Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Precision Instrument, Collaborative Innovation Center for Micro/Nano
Fabrication, Device and System, State Key Laboratory of Precision Measurement Technology and Instruments, Tsinghua University, Beijing, 100084, China Keren Dai, Xiaofeng Wang, Yajiang Yin,
Chenglong Hao & Zheng You Authors * Keren Dai View author publications You can also search for this author inPubMed Google Scholar * Xiaofeng Wang View author publications You can also
search for this author inPubMed Google Scholar * Yajiang Yin View author publications You can also search for this author inPubMed Google Scholar * Chenglong Hao View author publications You
can also search for this author inPubMed Google Scholar * Zheng You View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Keren Dai proposed the
multi-field coupled kinetics model, Keren Dai and Chenglong Hao conceived the simulation, Keren Dai and Xiaofeng Wang conceived the experiments, Keren Dai and Yajiang Yin conducted the
experiments, Keren Dai and Zheng You analysed the results. All authors reviewed the manuscript. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests.
RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission
from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Dai, K., Wang, X., Yin, Y. _et al._ Voltage Fluctuation in a Supercapacitor During a High-_g_ Impact. _Sci Rep_ 6, 38794 (2016). https://doi.org/10.1038/srep38794 Download citation *
Received: 13 September 2016 * Accepted: 14 November 2016 * Published: 13 December 2016 * DOI: https://doi.org/10.1038/srep38794 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
