Unconscious processing of invisible visual stimuli
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Unconscious processing of subliminal visual information, as illustrated by the above-chance accuracy in discriminating invisible visual stimuli, is evident in both blindsight
patients and healthy human observers. However, the dependence of such unconscious processing on stimulus properties remains unclear. Here we studied the impact of stimulus luminance and
stimulus complexity on the extent of unconscious processing. A testing stimulus presented to one eye was rendered invisible by a masking stimulus presented to the other eye, and healthy
human participants made a forced-choice discrimination of the stimulus identity followed by a report of the perceptual awareness. Without awareness of the stimulus existence, participants
could nevertheless reach above-chance accuracy in discriminating the stimulus identity. Importantly, the discrimination accuracy for invisible stimuli increased with the stimulus luminance
and decreased with the stimulus complexity. These findings suggested that the input signal strength and the input signal complexity can affect the extent of unconscious processing without
altering the subjective awareness. SIMILAR CONTENT BEING VIEWED BY OTHERS A NASAL VISUAL FIELD ADVANTAGE IN INTEROCULAR COMPETITION Article Open access 17 March 2022 DISSOCIATING CONSCIOUS
AND UNCONSCIOUS INFLUENCES ON VISUAL DETECTION EFFECTS Article 04 January 2021 DECODING DYNAMIC FACES AND SCENES WITHOUT AWARENESS UNDER DIS-CONTINUOUS FLASH SUPPRESSION Article Open access
31 January 2025 INTRODUCTION Visual information outside of awareness can affect conscious experience1,2, motor response3,4, and even goal pursuit5,6. To understand the power and limits of
subliminal visual information, it is necessary to address the degree to which supraliminal visual information can be processed. If observers can correctly “guess” the identity of visual
stimuli despite being unaware of the stimuli, it is intuitive that such subliminal visual information may affect behavior in a fashion similar to the way supraliminal visual information
influences behavior7. The unconscious processing of subliminal visual information has been reported in blindsight patients, who, due to the lesions in primary visual cortex, cannot
consciously perceive visual stimuli in their defect visual field, but can nonetheless correctly discriminate the stimuli8. This unconscious processing of subliminal visual information is
also evident in healthy human observers, where visual stimuli rendered invisible can be discriminated at above-chance accuracy9. Despite these established dissociations between perceptual
awareness and correct discrimination of visual stimuli, it remains unclear how the unconscious processing of invisible visual stimuli is dependent on the stimulus properties. We suggest that
the extent of unconscious processing may be affected by the strength and the complexity of the signal carrying subliminal visual information, which may in turn determine the degree towards
which subliminal visual information can influence behavior. To test this hypothesis, we investigated the influence of stimulus luminance and stimulus complexity on the extent of unconscious
processing. Using the paradigm of continuous flash suppression, we rendered a testing stimulus invisible by presenting it to one eye while presenting a masking stimulus to the other eye3,10.
This created situations where the same testing stimulus was totally invisible in some trials yet fully or partially visible in other trials2, possibly due to the fluctuations in cortical
signal evoked by the testing stimulus. Such induction of different perceptual awareness scales by the same physical stimulus allowed us to compare the discrimination accuracy between trials
where the testing stimulus was invisible and visible, respectively. We found that in trials where the testing stimulus was invisible, participants could nonetheless reach above-chance
discrimination accuracy. Based on this observation, we explored how the discrimination accuracy of invisible testing stimulus changed with the luminance and the complexity of the stimulus.
To vary the stimulus complexity, we compared simple, low-level visual stimuli such as oriented grating11, with complex, high-level visual stimuli such as face or house12. We found that while
the testing stimulus remained invisible, the discrimination accuracy increased with the stimulus luminance and decreased with the stimulus complexity. Our findings suggested that the
strength and the complexity of the signal carrying subliminal visual information can affect the extent of unconscious processing without altering the subjective awareness. MATERIAL AND
METHODS PARTICIPANTS AND APPARATUS Twelve healthy volunteers gave written informed consent to participate in this study that was approved by the Biomedical Research Ethics Committee of the
Shanghai Institutes for Biological Sciences. The study and methods were carried out in accordance with the guidelines of the Biomedical Research Ethics Committee of the Shanghai Institutes
for Biological Sciences and the guidelines of the declaration of Helsinki. The participants were young adults (aged 19 to 25, 8 females, 4 males) with normal or corrected-to-normal vision
and no neurological history. Apart from one of the authors (CS), all participants were naive to the aims of this study and received payment for participation. The experiments were programmed
in MATLAB using Psychtoolbox13 and conducted in a darkened room with the monitor providing the only significant source of light. STIMULI AND PROCEDURE We measured the discrimination
accuracy of visual stimulus in conditions where participants were unaware, partially aware, or fully aware of the stimulus. For this purpose, we presented a low-luminance gray-scale testing
stimulus to one eye and a high-contrast colorful mask to the other eye, while the eye-of-presentation was random and counter-balanced across trials. To test the influence of stimulus
luminance and stimulus complexity, we conducted three separate experiments using simple or complex stimuli at one of five possible luminance levels. The testing stimulus was a gray-scale
sinusoidal grating (spatial frequency: 2.8 cycles per degree of visual angle) oriented at 45 degree towards left or right in experiment one, a gray-scale female face or house in experiment
two, and a gray-scale happy or sad cartoon face in experiment three. The masking stimulus was a high-contrast colorful Mondrian pattern flashed at a frequency of 33.33 Hz in all three
experiments. The testing stimulus (size: 3 × 3 degree of visual angle) and the masking stimulus (size: 4 × 4 degree of visual angle) were presented in a black background on the two halves of
a calibrated CRT monitor (Viewsonic P225, size 22″, spatial resolution of 1024 × 768 pixels, refresh rate of 100 Hz, viewing distance of 81.2 cm). To aid binocular convergence, each
stimulus was placed in a white square frame (size: 4 × 4 degree of visual angle) with a red fixation cross at its center, and viewed through a mirror stereoscope with a chin rest. The
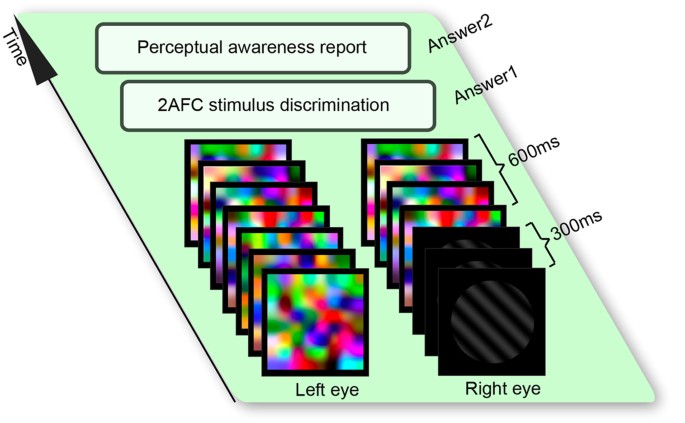
testing stimulus and the masking stimulus were presented for 300 ms, after which the testing stimulus was replaced by the masking stimulus and the same masking stimulus was presented to the
two eyes for 600 ms (Fig. 1). Following the stimulus offset, participants made an unspeeded two-alternative forced choice judgment as to whether the testing stimulus was a left-oriented or a
right-oriented grating (experiment one), a face or a house (experiment two), and a happy or a sad face (experiment three). Participants then made an unspeeded perceptual awareness report as
to whether the testing stimulus was seen clearly and discriminable (fully visible), or seen vaguely and un-discriminable (partially visible), or not seen at all (invisible). To control the
physical differences between different testing stimuli, we adjusted them to have the same mean luminance and the same luminance distribution. Thus, any difference in discrimination accuracy
was not the artifact of some testing stimuli being physically more similar and consequently less discriminable. The mean luminance of the testing stimulus was 4.75%, 9.5%, 19%, 28.5%, 38% of
the maximum luminance of the monitor (2.4 cd/m2, 4.8 cd/m2, 9.7 cd/m2, 14.5 cd/m2, 19.4 cd/m2). The mean luminance of the masking stimulus was 50% of the maximum luminance (25.5 cd/m2). For
each experiment, participants completed 1250 trials that were divided into 5 blocks of 250 trials (5 luminance values x 50 trials). Within each experiment, the luminance and the identity of
the testing stimulus was randomized but counter-balanced across trials. The order of the experiments was counter-balanced across participants. DATA ANALYSIS In each experiment, the trials
of each luminance scale were divided into three sets where the testing stimulus was invisible, partially visible, and fully visible, respectively. For each of these sets, we calculated the
discrimination accuracy (i.e., the percentage of correct answers), and the percent invisible (i.e., the percentage of trials in which the testing stimulus was invisible). The percent
invisible reflected the effectiveness of the masking stimulus. It decreased with the luminance of the testing stimulus. In particular, the percent invisible for the five luminance levels was
[96% ± 1.5%, 77% ± 2.1%, 54% ± 2.1%, 21% ± 2.7%, 11% ± 0.6%] (mean ± SEM) in experiment one, [98% ± 0.7%, 74% ± 2.3%, 49% ± 3.2%, 26% ± 3.1%, 12% ± 1.1%] (mean ± SEM) in experiment two, and
[99% ± 0.5%, 75% ± 3.8%, 48% ± 1.9%, 22% ± 2.9%, 11% ± 0.8%] (mean ± SEM) in experiment three. This proximity in percent invisible across different testing stimuli (i.e., different
experiments) allowed us to study the influence of stimulus complexity on the extent of unconscious processing. RESULTS We performed a three-way analysis of variance test (ANOVA) with the
stimulus visibility, the stimulus luminance, and the stimulus complexity as three factors-of-interest. We found that the discrimination accuracy of the testing stimulus varied with the
stimulus visibility (F(2, 42) = 351.8, p < 10−13), the stimulus luminance (F(4, 40) = 75.9, p < 10−9), and the stimulus complexity (F(2, 42) = 15.7, p < 10−3). Moreover, the
analysis revealed a significant interaction between the stimulus visibility and the stimulus luminance (F(8, 36) = 23.13, p < 10−6), as well as a significant interaction between the
stimulus visibility and the stimulus complexity (F(4, 40) = 4.65, p < 0.05), but no interaction between the stimulus luminance and the stimulus complexity (F(8, 36) = 1.15, p = 0.39).
These results suggested that both the luminance and the complexity of the testing stimulus influenced the discrimination accuracy, whereas the exact pattern of influence might differ across
different conditions of stimulus visibility. We therefore explored the exact dependency of the discrimination accuracy on the luminance and the complexity of the testing stimulus, and
addressed whether this pattern of dependency varied with the visibility of the testing stimulus. We first investigated how the overall discrimination accuracy changed with the visibility of
the testing stimulus. We found that without awareness of the stimulus existence, participants could nevertheless reach above-chance accuracy (>50%) in discriminating the testing stimulus,
regardless of its luminance or complexity (t-test; experiment one: t(11) = 44.2, p < 10−13; experiment two: t(11) = 35.1, p < 10−11; experiment three: t(11) = 57.1, p < 10−14).
This discrimination accuracy of invisible testing stimulus, however, was significantly lower than the discrimination accuracy where the same testing stimulus was partially visible (t-test;
experiment one: t(11) = 10.7, p < 10−6; experiment two: t(11) = 6.7, p < 10−4; experiment three: t(11) = 8.1, p < 10−5) or fully visible (t-test; experiment one: t(11) = 18.6, p
< 10−8; experiment two: t(11) = 18.1, p < 10−8; experiment three: t(11) = 33.1, p < 10−11). Moreover, the discrimination accuracy of partially visible stimulus was lower than the
discrimination accuracy of fully visible stimulus (t-test; experiment one: t(11) = 3.1, p < 0.01; experiment two: t(11) = 3.3, p < 0.01; experiment three: t(11) = 5.1, p < 10−3).
These results suggested that participants could form unconscious knowledge of subliminal visual information, although not as accurate as the conscious knowledge of supraliminal visual
information. We then plotted the discrimination accuracy of the testing stimulus against the stimulus luminance and the stimulus complexity, separately for different conditions of stimulus
visibility. In trials where the testing stimulus was partially or fully visible, we did not observe a significant dependency of the discrimination accuracy (averaged across stimuli with
different complexity) on the stimulus luminance (Fig. 2A; one-way ANOVA with FDR correction for multiple-comparison; partially visible, F(2, 33) = 4.1, p = 0.08; fully visible, F(2, 33) =
1.1, p = 0.81). Moreover, the discrimination accuracy (averaged across stimuli with different luminance) did not change significantly from left/right oriented grating to face/house (Fig. 2B;
t-test with FDR correction for multiple-comparison; partially visible, T(11) = 0.9, p = 0.36; fully visible, T(11) = 0.6, p = 0.81), or from left/right oriented grating to happy/sad cartoon
face (Fig. 2B; t-test with FDR correction for multiple-comparison; partially visible, T(11) = 2.1, p = 0.09; fully visible, T(11) = 0.1, p = 0.91). By contrast, in trials where the testing
stimulus was invisible, the discrimination accuracy (averaged across stimuli with different complexity) increased with the stimulus luminance, even when the stimulus luminance changed mildly
from level one to level three (Fig. 3A; one-way ANOVA with FDR correction for multiple-comparison; F(2, 33) = 17.8, p < 10−4). Moreover, the discrimination accuracy (averaged across
stimuli with different luminance) decreased from left/right oriented grating to face/house (Fig. 3B; t-test with FDR correction for multiple-comparison; T(11) = 4.8, p < 10−3) or
happy/sad cartoon face (Fig. 3B; t-test with FDR correction for multiple-comparison; T(11) = 5.8, p < 10−3). These results suggested that the extent of unconscious processing was
dependent on the strength and the complexity of the signal carrying subliminal visual information. DISCUSSION Unconscious processing of subliminal visual information was first observed in
blindsight patients7,14,15. Later on, by using transcranial magnetic stimulation (TMS) to create artificial scotoma16 or using binocular rivalry to render monocular stimulus invisible8, it
was found that healthy human participants could also reach above-chance accuracy in discriminating invisible visual stimuli. Importantly, such subliminal visual information influences
behavior in a fashion similar to the influenced exerted by supraliminal visual information. For example, subliminal visual stimulus can induce visual illusion just as supraliminal visual
stimulus does1,2,17. Moreover, the influence of subliminal visual stimulus is not limited to low-level sensory domains but also evident in high-level cognitive domains, where subliminal
stimulation of achievement-related words was found to influence goal pursuits and improve task performances3,5,6,18,19. The widespread influence of subliminal visual information raises the
question of what is its limit. One way to address this question is to test the extent towards which participants can unconsciously process subliminal visual stimulus. The extent of such
unconscious processing is likely to be affected by the strength and the complexity of the signal carrying subliminal visual stimulus and may in turn indicate the degree towards which
subliminal visual stimulus can influence behavior. As such, studying how the extent of unconscious processing depends on stimulus properties is an important step towards understanding
unconscious processing of subliminal visual stimulus. Whereas the existence of unconscious processing of subliminal visual stimulus is well established, its dependence on stimulus properties
remains unclear. Here we explored how the extent of unconscious processing depends on the properties of subliminal visual stimulus. It is plausible that subliminal visual stimulus of
different complexity or from different categories was processed by visual cortical regions at different hierarchical levels20,21,22. As such, we compared simple, low-level visual stimulus
(e.g., oriented grating) with complex, high-level visual stimulus (e.g., face or house), in order to explore how the complexity of the signal carrying subliminal visual stimulus might affect
the extent of unconscious processing. Moreover, we used visual stimulus with different luminance23, in order to explore how the strength of the signal carrying subliminal visual stimulus
might affect the extent of unconscious processing. We found that the extent towards which participants processed subliminal visual stimulus (i.e., the discrimination accuracy of invisible
testing stimulus) increased with stimulus luminance and decreased with stimulus complexity. These results suggested that the strength and the complexity of the signal carrying subliminal
visual stimulus affected the extent of unconscious processing. It will be of interest for future studies to address, using neuroimaging techniques, the exact cortical processing for
subliminal visual information. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Song, C. and Yao, H. Unconscious processing of invisible visual stimuli. _Sci. Rep._ 6, 38917; doi:
10.1038/srep38917 (2016). PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. REFERENCES * Clifford,
C. W. G. & Harris, J. A. Contextual modulation outside of awareness. Current Biology 15, 574–578 (2005). Article CAS Google Scholar * Harris, J. J., Schwarzkopf, D. S., Song, C.,
Bahrami, B. & Rees, G. Contextual illusions reveal the limit of unconscious visual processing. Psychological Science 22, 399–405 (2011). Article Google Scholar * Dehaene, S., Naccache,
L., ClecH, G. L., Koechlin, E., Mueller, M., Dehaene-Lambertz, G. et al. Imaging unconscious semantic priming. Nature 395, 597–600 (1998). Article ADS CAS Google Scholar * Gaal, S. van,
Ridderinkhof, K. R., Fahrenfort, J. J., Scholte, H. S. & Lamme, V. A. F. Frontal cortex mediates unconsciously triggered inhibitory control. Journal of Neuroscience 28, 8053–8062
(2008). Article Google Scholar * Pessiglione, M., Schmidt, L., Draganski, B., Kalisch, R., Lau, H., Dolan, R. et al. How the brain translates money into force: A neuroimaging study of
subliminal motivation. Science 316, 904–906 (2007). Article ADS CAS Google Scholar * Custers, R. & Aarts, H. The unconscious will: How the pursuit of goals operates outside of
conscious awareness. Science 329, 47–50 (2010). Article ADS CAS Google Scholar * Dehaene, S. & Changeux, J. P. Experimental and theoretical approaches to conscious processing. Neuron
70, 200–227 (2011). Article CAS Google Scholar * Weiskrantz, L., Barbur, J. & Sahraie, A. Parameters affecting conscious versus unconscious visual discrimination with damage to the
visual cortex (v1). Proceedings of the National Academy of Sciences 92, 6122–6126 (1995). Article ADS CAS Google Scholar * Kolb, F. C. & Braun, J. Blindsight in normal observers.
Nature 377, 336–338 (1995). Article ADS CAS Google Scholar * Tsuchiya, N. & Koch, C. Continuous flash suppression reduces negative afterimages. Nature Neuroscience 8, 1096–1101
(2005). Article CAS Google Scholar * Yacoub, E., Harel, N. & Ugurbil, K. High-field fMRI unveils orientation columns in humans. Proceedings of the National Academy of Sciences 105,
10607–10612 (2008). Article ADS CAS Google Scholar * Grill-Spector, K. & Malach, R. The human visual cortex. Annual Review Neuroscience 27, 649–677 (2004). Article CAS Google
Scholar * Brainard, D. H. The psychophysics toolbox. Spatial Vision 10, 433–436 (1997). Article CAS Google Scholar * Gelder, B. de, Vroomen, J., Pourtois, G. & Weiskrantz, L.
Non-conscious recognition of affect in the absence of striate cortex. Neuroreport 10, 3759–3763 (1999). Article Google Scholar * Overgaard, M., Fehl, K., Mouridsen, K., Bergholt, B. &
Cleeremans, A. Seeing without seeing? Degraded conscious vision in a blindsight patient. PLoS One 3, e3028 (2008). Article ADS Google Scholar * Boyer, J. L., Harrison, S. & Ro, T.
Unconscious processing of orientation and color without primary visual cortex. Proceedings of the National Academy of Sciences 102, 16875–16879 (2005). Article ADS CAS Google Scholar *
Mudrik, L., Breska, A., Lamy, D. & Deouell, L. Y. Integration without awareness: Expanding the limits of unconscious processing. Psychological Science 22, 764–770 (2011). Article Google
Scholar * Strahan, E. J., Spencer, S. J. & Zanna, M. P. Subliminal priming and persuasion: Striking while the iron is hot. Journal of Personality and Social Psychology 38, 556–568
(2002). Google Scholar * Hart, W. & Albarracn, D. The effects of chronic achievement motivation and achievement primes on the activation of achievement and fun goals. Journal of
Personality and Social Psychology 97, 1129–1141 (2009). Article Google Scholar * Fang, F. & He, S. Cortical responses to invisible objects in the human dorsal and ventral pathways.
Nature Neuroscience 8, 1380–1385 (2005). Article CAS Google Scholar * Jiang, Y. & He, S. Cortical responses to invisible faces: Dissociating subsystems for facial-information
processing. Current Biology 16, 2023–2029 (2006). Article CAS Google Scholar * Sterzer, P., Haynes, J. D. & Rees, G. Fine-scale activity patterns in high-level visual areas encode the
category of invisible objects. Journal of Vision 8, 1–12 (2008). Article Google Scholar * Haynes, J. D., Lotto, R. B. & Rees, G. Responses of human visual cortex to uniform surfaces.
Proceedings of the National Academy of Sciences 101, 4286–4291 (2004). Article ADS CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by Sigma Xi
Grants-in-Aid of Research Award G200803150480 (CS) and National Natural Science Foundation of China 31571079 (HY). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute of Cognitive
Neuroscience, University College London, London WC1N 3AR, UK Chen Song * Wellcome Trust Centre for Neuroimaging, University College London, London WC1N 3BG, UK Chen Song * Department of
Psychiatry, University of Wisconsin - Madison, Madison 53719 WI, USA Chen Song * Institute of Neuroscience and State Key Laboratory of Neuroscience, Shanghai Institutes for Biological
Sciences, Chinese Academy of Sciences, Shanghai 200031, China Chen Song & Haishan Yao Authors * Chen Song View author publications You can also search for this author inPubMed Google
Scholar * Haishan Yao View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS C.S. designed the research. C.S. participated in data collection.
C.S. analyzed the data. All authors participated in the writing of the manuscript and preparation of figures. All authors reviewed the paper. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing financial interests. RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party
material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons
license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Song, C., Yao, H. Unconscious processing of invisible visual stimuli. _Sci Rep_ 6, 38917 (2016). https://doi.org/10.1038/srep38917
Download citation * Received: 04 August 2016 * Accepted: 15 November 2016 * Published: 12 December 2016 * DOI: https://doi.org/10.1038/srep38917 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative
