The hepatic and skeletal muscle ovine metabolomes as affected by weight loss: a study in three sheep breeds using nmr-metabolomics
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Sheep are a valuable resource for meat and wool production. During the dry summer, pastures are scarce and animals face Seasonal Weight Loss (SWL), which decreases production
yields. The study of breeds tolerant to SWL is important to understand the physiological mechanisms of tolerance to nutritional scarcity, and define breeding strategies. Merino, Damara and
Dorper sheep breeds have been described as having different levels of tolerance to SWL. In this work, we assess their liver and muscle metabolomes, and compare the responses to feed
restriction. Ram lambs from each breed were divided into growth and feed restricted groups, over 42 days. Tissue metabolomes were assessed by 1H-NMR. The Dorper restricted group showed few
changes in both tissues, suggesting higher tolerance to nutritional scarcity. The Merinos exhibited more differences between treatment groups. Major differences were related to fat and
protein mobilization, and antioxidant activity. Between the Damara groups, the main differences were observed in amino acid composition in muscle and in energy-related pathways in the liver.
Integration of present results and previous data on the same animals support the hypothesis that, Dorper and Damara breeds are more tolerant to SWL conditions and thus, more suitable breeds
for harsh environmental conditions. SIMILAR CONTENT BEING VIEWED BY OTHERS THE METABOLOMICS PROFILE OF GROWTH RATE IN GRAZING BEEF CATTLE Article Open access 15 February 2022 COMMON AND
DIET-SPECIFIC METABOLIC PATHWAYS UNDERLYING RESIDUAL FEED INTAKE IN FATTENING CHAROLAIS YEARLING BULLS Article Open access 21 December 2021 CHANGES IN THE BLOOD METABOLOME OF WAGYU CROSSBRED
STEERS WITH TIME IN THE FEEDLOT AND RELATIONSHIPS WITH MARBLING Article Open access 04 November 2020 INTRODUCTION Small ruminants like sheep and goats are particularly important in the
tropics and the Mediterranean regions, and are a major source of income and food in small-scale subsistence farming systems. Additionally, in some countries in the Southern hemisphere, such
as Australia, New Zealand, Argentina or South Africa, sheep production, particularly for wool, is one of the major commercial products, historically playing an important role in such
economies1,2. Animal production in the tropics and the Mediterranean is strongly affected by pasture scarcity and quality during the dry summer and autumn months, leading to Seasonal Weight
Loss (SWL), as we have demonstrated in South Africa3,4, Western Africa5,6, Western Australia7 and the Canary Islands8,9. To counter the effects of SWL, farmers use supplementation to balance
the nutritional needs of the animals. Supplementation is expensive and difficult to implement in extensive production systems in developing countries or remote locations8. An alternative
method for addressing the effects of SWL is the use of sheep breeds that are naturally adapted to this constraint and are able to thrive and more effectively produce in such difficult
environments. Understanding the biochemical and physiological mechanisms by which such breeds are able to cope with SWL is therefore very important. The Australian Merino breed is the basis
of the wool, ovine meat, and live animal export markets for that country. The Merino has a long history in Australia after being introduced in 17972, but in the last two decades, market
changes and animal welfare policies have led to an increased interest for breeds with other characteristics, particularly regarding the absence of wool (shedding hair sheep), heat tolerance
and a natural adaptation to SWL7. Recently, two breeds from South Africa, the Damara and the Dorper, have been introduced to Australia. The Damara is a large fat-tailed, hair sheep breed,
native to the fringes of the Kalahari Desert in Namibia and South Africa. This breed is well adapted to arid climatic conditions and water scarcity2. The Dorper is also a hair sheep breed
native to Southern Africa. It was selected by combining the hardiness of the Blackhead Persian indigenous breed with the carcass and meat traits of British Dorset Horn breed2. These sheep
breeds have been reviewed by Almeida _et al_.2. In a previous work, our team conducted a productive characterization of these breeds and their reaction to SWL. We have studied SWL effect on
live weight10, carcass and meat characteristics7,11, in gene expression of regulatory enzymes in the liver12, and more recently on the skeletal muscle proteome13, and fatty acid composition
of muscle14 and the Damara fat tail adipose tissue15. However, no broad characterization of the muscle and liver metabolomes of these three breeds has ever been conducted. Recent studies, by
our team used Nuclear Magnetic Resonance (NMR) to characterize the mammary gland and milk metabolome of goats under SWL16. The study, demonstrated the potential of this approach to help
create a more systematic metabolome analysis16. Other recent studies in farm animals confirm the potential of NMR technique to metabolomics approaches17,18,19,20. The aim of this work was to
characterize the metabolome of the muscle and liver of Merino, Damara and Dorper sheep breeds, and study the effect of feed restriction in these tissues, which are important from the
productive and metabolic perspectives. We used an NMR-metabolomics based approach, which, to the best of our knowledge was for the first time applied to these breeds. The results will be of
importance to understand which biochemical pathways are associated with SWL tolerance in sheep and that may benefit breeding programs. MATERIAL AND METHODS ANIMAL EXPERIMENT The trial was
carried out at the Merredin Research Station in Western Australia, following the experimental design and nutritional treatments previously described10. Briefly, a total of 72 six-month-old
ram lambs from each of the Merino, Dorper and Damara breeds were divided into the experimental diet groups (12 animals per group: Merino growth, Merino restricted, Dorper growth, Dorper
restricted, Damara growth and Damara restricted). All animals were fed on commercial pellets and had free access to drinking water as described10. Individual nutritional treatments were
calculated so that animals in the growth groups gained weight (100 g/day) and animals in the restricted groups lost weight (100 g/day). The trial lasted 42 days, after which animals were
slaughtered in a commercial abattoir, following commercial practices. For further information, kindly refer to Scanlon _et al_.10 and Almeida _et al_.21. By the end of the nutrition trial,
gastrocnemius muscle and liver tissues were sampled and preserved at −80 °C for further analysis. SAMPLE PROCESSING For muscle tissue we analysed 11 samples in all experimental groups, with
the exception of the Merino Restricted group where 10 samples were used. For the liver tissue we used 12 samples for all groups, with the exception of the Merino Restricted group where 11
samples were used. Frozen tissues were powdered individually with porcelain mortar and pestle with liquid nitrogen. Metabolites were extracted following the Bligh and Dyer method22 with
adaptations. Muscle samples were processed as previously described for goat mammary gland samples16. For liver samples the solvent volumes used were modified as follows: an initial 3 ml cold
chloroform/methanol mixture (1:2, v/v) was added to the tissue and mixed, followed by the addition and mixing of 2 ml of cold chloroform. Then 1 ml of cold water was added and mixed by
vortexing. For both tissues, the mixture was finally centrifuged and the methanol/water fraction was separated and dried as previously described16. NMR SPECTROSCOPY The aqueous fraction of
the muscle samples was re-suspended in 600 μl phosphate buffer (150 mM, pH 7.0/pD 7.4, with 1 mM sodium-2,2-dimethyl-2-silapentane-5-sulfone (DSS), in D2O), while the water-soluble fraction
of the liver samples was dissolved in 800 μl phosphate buffer (100 mM, pH 7.4/pD 7.8, with 0.5 mM DSS, in D2O). Samples were transferred into 5 mm NMR tubes. Proton (1H) NMR spectroscopy was
conducted on an 800 MHz Bruker AvanceII+ (Ettlingen, Germany) spectrometer, with a triple resonance HCN Z-gradient probe, at 298 K. 1H 1D-NOESY spectra were collected for each sample using
the “noesypr1d” pulse sequence (spectral width: 12 ppm; mixing time: 0.1 s; relaxation delay: 1 s; acquisition time: 4 s), following the parameters for profiling recommended from Chenomx NMR
Suite software (Chenomx Inc., Edmonton, Canada). All spectra were processed with a line broadening (lb) of 0.5 Hz and a final number of 128 K points. Additional J-resolved spectra were
collected to assist with assignment. All spectra were acquired, processed and analysed using TopSpin 3.2 (Bruker, Ettlingen, Germany). METABOLITE PROFILING Metabolite identification and
quantification was carried out using Chenomx NMR Suite 8.12 software (Chenomx Inc., Edmonton, Canada), using the internal reference library (Version 10), and with support of published data
for other animals23,24,25. DATA ANALYSIS Both univariate and multivariate analysis were performed for the obtained metabolite concentrations, following the approach previously described16.
Briefly, for univariate analysis we performed a t-test with 2 tails and test type 3, considering _p_ < 0.05 to reject null-hypothesis (equal means between groups), using Microsoft Excel.
Multivariate analysis was performed using the SIMCA 13.0.3.0 software (Umetrics AB, Umeå, Sweden) for unsupervised Principal Components Analysis (PCA) and supervised Partial Least Squares
Discriminant Analysis (PLS). In PLS analysis, Q2 (predictive ability of the model) and R2 (goodness of the fit) were considered as quality parameters of the model. Results were accepted for
Q2 above 0.526. For PLS models, a permutation test was additionally performed, using 100 permutations and accepting the model as “valid” when R2Y-intercept < 0.4 and Q2Y-intercept <
0.05. All ellipses in the scores plots were drawn at the 95% confidence level. ANIMAL WELFARE DISCLAIMER All work involving animals was conducted according to relevant international
guidelines (European Union procedures on animal experimentation—Directive 2010/63/EU) that regulate the use of production animals in animal experimentation. These define that in the case of
experiments carried out under standard production conditions, no approval from an ethics committee is required. Nevertheless, this experiment was conducted with the approval of the Ethics
Committee of the Department of Agriculture and Food Western Australia (DAFWA, Perth, WA, Australia) registered as process 07ME06. The entire trial was conducted under the supervision of the
veterinary authority in the State of Western Australia. Author AM Almeida holds a FELASA (Federation of European Laboratory Animal Society Associations) grade C certificate that enables
designing and carrying out animal experimentation under European Union regulations. Animal management, handling, transport and slaughter were all conducted replicating approved standard
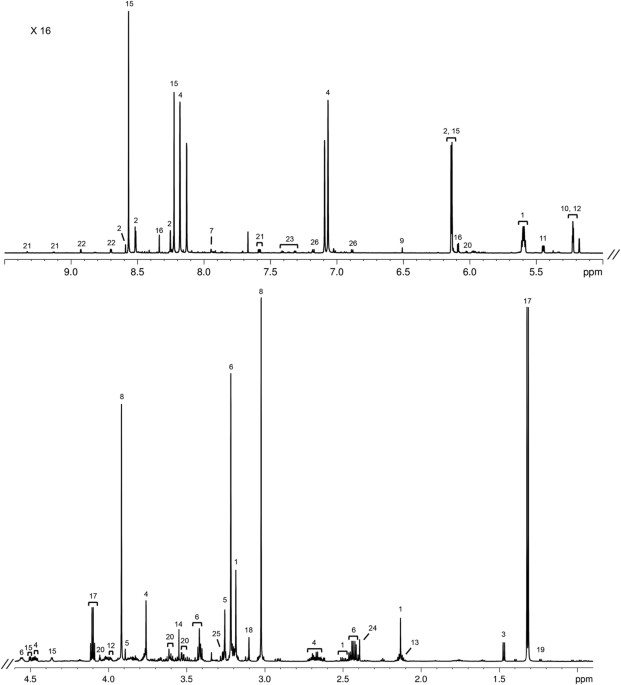
commercial practices in the Commonwealth of Australia and in the State of Western Australia. RESULTS MUSCLE TISSUE A representative 1H 1D NOESY spectrum of the sheep gastrocnemius muscle
(Merino breed, growth group) is shown in Fig. 1. A total of 51 metabolites were identified and quantified in the aqueous fractions of the muscle, and the average metabolite concentrations
from the six experimental groups are shown in Supplementary Table S1. The most abundant metabolites are lactate and creatine/creatine-phosphate in all breeds, followed by taurine, anserine,
carnitine and glutamine in the Dorper and Damara breeds; and carnitine, malonate, taurine and anserine in the Merino breed. Significant differences (_p_ < 0.05) between growth and
restricted groups for each breed are presented in Table 1. Among these, the more marked differences were observed in glycerophosphocholine in the Merino breed which decreased 4.1 times
between growth and restricted groups; and adenine in the Dorper breed, which increased 2.5 times from growth to restricted groups. In the Merino breed, we identified differences between
growth and restricted groups in citrate, glucose-6-phosphate, glutathione, glycerophosphocholine, glycine, acetyl-L-carnitine, taurine and tyrosine. In the Damara breed, differences between
growth and restricted groups were observed in glucose-1-phosphate, inosine monophosphate (IMP), isoleucine, leucine, tyrosine, valine, phenylalanine and taurine. In the Dorper breed only
four metabolites show significant differences between groups: adenine, formate, glycine and taurine. Multivariate analysis was applied to metabolite concentrations. PCA scores plot of all
groups does not show any specific separation by group (Supplementary Figure S1A), however it is possible to see some distinction by breed. In the PCA scores plot of the growth groups (Fig.
2A) it is possible to see some separation among breeds, especially between Merino and Dorper. On the other hand, the PCA scores plot of the restricted groups revealed no specific separation
between breeds (Supplementary Figure S1B). However, it is noteworthy that the Damara restricted groups show a less broad distribution when compared to the distributions of the other two
breeds. Multivariate analysis was also applied per breed. PCA scores of Merino growth and restricted groups revealed the two groups could be separated by the second principal component (PC2)
(Fig. 2B). Analysis of the loadings (Supplementary Table S2) indicate that the metabolites that contribute more to this separation were glycerophosphocholine, citrate, acetyl-L-carnitine,
myo-inositol, glutathione and glucose-6-phosphate. For Damara and Dorper breeds it was only possible to separate their growth and restricted groups applying PLS analysis. Damara growth and
restricted groups were separated by the first principal component (PC1) with acceptable quality parameters (Supplementary Figure S1C). However, the permutation test (Supplementary Figure
S1D) failed the model validation. Concerning the Dorper growth and restricted groups, PLS was not able to separate the groups with acceptable quality parameters (Supplementary Figure S1E).
Furthermore, permutation test (Supplementary Figure 1F) do not validate the model. LIVER TISSUE A representative 1H 1D NOESY spectrum of the sheep liver (Merino breed, growth group) was
selected and is shown in Fig. 3, with examples of some of the identified metabolites. A total of 46 metabolites were identified in the aqueous fraction of sheep liver and the metabolite
concentration of all experimental groups are presented in Supplementary Table S3. The most abundant metabolites are glucose, lactate, glycerophosphocholine and glutamate in the three breeds,
followed by taurine, glycine, glutathione and alanine in different orders depending on the breed. Univariate analysis revealed differences between growth and restricted groups (Table 2) of
the Merino breed in 3-hydroxybutyrate, acetate, alanine, ascorbate, creatine/creatine-phosphate, lactate, sarcosine, succinate, glutathione, and UDP-glucose/UDP-glucoronate. In the Damara
breed, differences between growth and restricted groups are identified in acetate, adenine, alanine, ascorbate, choline/acetylcholine/phosphocholine, citrate, fomate, lactate and
UDP-glucose/UDP-glucoronate. It is noteworthy to mention that in the Dorper breed only two metabolites: carnitine and sarcosine, show differences between growth and restricted groups.
Concerning the multivariate analysis, albeit the PCA scores plot of the six experimental groups (Fig. 4A) does not reveal a clear separation of the groups, some tendencies may be noted.
Dorper growth and restricted group have indistinguishable distribution, whereas the Merino groups seem to be separated into clusters influenced by the PC2. This separation of the Merino
groups from the other groups could be due to variations in benzoate, glycine and succinate, as shown in loadings list (Supplementary Table S4). PCA scores plot of the three restricted groups
(Fig. 4B) show a distinct clustering of the Merino restricted samples away from the other two restricted groups of Damara and Dorper breeds. Loadings list of this model indicates that
separation is due to variations in succinate, formate, and xanthine (Supplementary Table S5). Merino growth and restricted groups show separation in the PCA scores plot by the PC2 (Fig. 4C).
Loading list of this model (Supplementary Table S6) indicate variations in glycine, formate, lactate and succinate. PLS analysis for this breed (Merino growth and restricted groups) has
good quality parameters but the permutation test does not validate the model. PCA scores plot of the Damara growth and restricted groups (Fig. 4D) show a slight separation in the PC1. The
growth group have less disperse distributions whereas the restricted group is more spread-out along the plot area. Separation of the two groups could be due to differences in glucose,
tyrosine, isoleucine, inosine and leucine (Supplementary Table S7). PLS analysis for the two groups of Damara breed has unacceptable quality parameters and cannot be validate by the
permutation test. The Dorper growth and restricted groups (Supplementary Figure S2A) were only separated by the PLS analysis. Although the quality parameters are acceptable, the permutation
test does not validate the model (Supplementary Figure S2B). DISCUSSION To the best of our knowledge, this work is the first to apply the NMR technique to studies on the effects of SWL in
muscle and liver metabolome on the Merino, Damara and Dorper sheep breeds. The approach proved to be adequate for the study, allowing the identification and quantification of 51 metabolites
in muscle, and 46 in liver. As suggested for other metabolomics studies27, we analysed the data using both univariate and multivariate analyses. PCA of the different groups did not reveal a
clear separation between groups, neither for muscle nor for liver, although the muscle samples could be discriminated by breed. In the per-breed analysis, some tendencies are noted,
especially in the Dorper breed, where it was impossible to discriminate between the two nutritional treatment groups with good quality parameters, in both liver and muscle tissues. Such
separation was possible for the Merino and Damara in both tissue samples. These results could be an indicator of a more pronounced reaction of the muscle to feed-restriction in Merino and
Damara, and a general different response in the Dorper breed. Univariate analysis results of the Dorper growth and restricted groups revealed that few metabolite variations were found in
both tissues, four in muscle and two in liver. In the Merino and Damara breeds, the extent of metabolite differences in both tissues was comparable, with half of them coinciding in both
breeds. The Merino had eight metabolites with variations in muscle and 10 in liver tissue, while the Damara had eight in muscle and nine in liver. The distinct variations among breeds,
identified by both statistical approaches, could be indicative of breed-specific response to the feed-restriction treatment. Interestingly, we observed that some muscle metabolites could
explain differences between treatment groups in both statistical analyses in Merino and Damara. In the liver, the same was observed for the Merino breed. However, a more detailed analysis of
these variations is needed to understand the physiological significance of these observations. Figure 5 summarises the major results for each breed and includes some results from previous
studies on these animals, to help integrate and evaluate all the information. MERINO BREED METABOLOMES In muscle samples, decreased levels of amino acids like tyrosine, glycine and taurine
during restriction could be an indication of a reduction of muscle growth. Taurine levels can also be affected by a reduction in dietary cysteine levels28, making it difficult to separate
the effect of diet restriction and the inner response of the animal. Glycine also has an additional role as a precursor of glucagon in glycogenolysis29, and can affect glutathione
production30,31 in the antioxidant defence mechanism. However, glutathione synthesis can also be limited by diet related cysteine intake32, mixing the effects of the restricted feed with
those of the metabolic response. Since glutathione is produced in liver and released to the muscle33, its decreased levels in the liver could be related with its increase in muscle of the
same animals. The lower levels of ascorbate (Vitamin C) in liver could also be related to glutathione levels34. However, ruminants are able to synthesize it from glucose, and its reduction
could also be related to the diet restriction. Low levels of glutathione and ascorbate could be indicative of oxidative stress in the tissue35 likely as a consequence of weight loss.
Sarcosine is a precursor of creatine, so variations in their concentrations in liver could be related with this pathway36,37. Levels of creatine/creatine-phosphate, two metabolites related
to energy production in muscle, are in fact increased in the restricted merino group as well as in the other breeds. As previously mentioned, glycine is a precursor of glucagon, which,
during low glucose levels and under stress conditions, promotes gluconeogenesis and glycogenolysis. Indeed, significant differences in some metabolites related with such these metabolic
pathways were observed. Glucose-6-phosphate and UDP-glucose/UDP-glucoronate are intermediate products in the glycogenolysis pathway. Their lower levels in muscle of the restricted group
could be due to the depletion of glucose and glycogen stock in these animals, due to the diet limitation. Previous proteome analysis of the same muscle samples revealed an increased
expression of phosphoglucomutase, an enzyme involved in glycogenolysis and glycogenesis13. The over expression occurs only in the Merino breed and not in the SWL tolerant breeds, confirming
that this breed is more susceptible to feed restriction and has a specific response to this treatment. van Harten _et al_.12 determined the gene expression of regulatory enzymes in the liver
of the same animals (Dorper and Merino), and no changes were observed in the enzymes of the gluconeogenesis (phosphoenolpyruvate carboxylase) and glycolysis (phosphofructokinase and
pyruvate kinase) pathways. The expression level of glucose-6-phosphatase, essential in glucose supply during feed-restriction, was determined and its value decreased in restricted groups.
During diet restriction animals tend to reduce the glycolytic pathway and promote gluconeogenesis12. These results could then be indicative of a minor adaptation to the feed-restriction. In
the livers of the restricted group we also observed lower levels of alanine and lactate. These two metabolites are related in both muscle and liver to the Cori and the Alanine-Glucose
Cycles. A decrease on their concentrations could be an indirect consequence of the lower levels of glucose in both tissues. Alanine is also a structural amino acid and its concentration is
usually low during diet restriction when muscle breakdown occurs. Glucose levels in the liver of the same animals were determined in a previous study and was observed a significant decrease
between growth and restricted animals12, supporting this hypothesis. Energy from nutrients in the diet could be obtained through processes other than glycolysis, especially during
feed-restriction periods. Variations in some metabolites are indicative of such process. The increased levels of acetyl-L-carnitine in the muscle of the restricted merino group is an
indication of fat mobilization. This metabolite is responsible for the transport of fatty acids into mitochondria to be oxidized and used as energy sources during high energy demanding or
glucose starvation periods. Previous results showed lower expression levels of fatty acid synthase, an enzyme responsible for fatty acids synthesis, in the liver of restricted animals12.
These results suggest that restricted-fed animals are using fatty acids as an energy source. During fasting periods and low carbohydrate diets, ketone bodies are produced in liver through
gluconeogenesis. We present some indicators of ketone body production in the liver of the restricted group, as 3-hydroxybutyrate and acetate, derived respectively from acetone and
acetoacetate. However, in ruminant both compounds could be a result of rumen activity16,38 due either to changes in microflora profile or in the diet composition. Succinate and citrate show
variations between groups in liver and muscle, respectively. Both metabolites are intermediates in the Krebs cycle with associations to other metabolic pathways. These variations could be
indicative of variations in the Krebs cycle or regulation of other secondary pathway, as the inhibition of glycolysis and promotion of gluconeogenesis by elevated levels of citrate. The
increased levels of glycerophosphocholine in the muscle could also be related with some regulatory process, since this metabolite is a storage form of choline in cytosol, with functions as
muscle control and source of methyl group39. It is noteworthy that, as wool producers these animal will use nutritional resources mainly or wool production, making them unavailable to be
used in other pathways in harsh conditions. Also, the wool production continued during the trial, channelling nutritional resources to it and influencing the general pathways. DAMARA BREED
METABOLOMES Levels of isoleucine, leucine, tyrosine, valine, phenylalanine and taurine decreased in muscle of the restricted group. All these amino acids are related to muscle development
and their lower concentrations could be directly linked to muscle production decrease. However, a previous study on muscle of these animals revealed a unique individual response of this
breed, when compared with the Merino and Dorper breeds13. In the Damara breed the levels of desmin, a muscle-specific protein responsible for cell architecture, increased, ensuring the
structure and function of the muscle even if some tissue mobilization occurs13. Isoleucine and leucine are also related with other metabolic pathways, related to ketonic bodies production
and cell growth regulator respectively, that can influence their concentrations. The reduction of UDP-glucose/UDP-gucoronate and glucose-1-phosphate in liver and muscle respectively, as
observed in the Merino breed, is indicative of changes in the glycogenolysis/glycogenesis pathway. IMP and adenine levels are both lower in restricted group, in muscle and liver
respectively. IMP is a nucleoside and an intermediate in purine metabolism, from which adenine is one of the examples. This result could be a consequence of a slower muscle development or an
imbalance in these tissues. Choline could also be connected with cell development and balance39, and it is also reduced in the liver of the restricted-fed animals. Variations in Krebs cycle
intermediates (citrate), in rumen-related metabolite (formate), and in ascorbate was observed in this breed, similarly to the Merino breed. Considering the special adaptation of this breed
to store fat in the tail, it is interesting that changes observed in the metabolism are in general not directly related to fat metabolism. Previous studies with the same animal groups
suggest that the Damara breed has a unique lipid metabolism, mostly due to the putative contribution of the fat tail as supplier of odd and branched-chain fatty acids (BCFA) to the muscle40.
However, it is also suggested that this tolerance to feed restriction could also be due to some kind of peculiarities in rumen activity40. Specifically, if the Damara breed has some
digestive adaptation that can increase the efficiency of fibre digestion, the acetate-propionate ratio will be affected40,41. Indeed, in the present study, levels of acetate in the liver of
the restricted group was lower than in the growth group. DORPER BREED METABOLOMES Variations in glycine and sarcosine in this breed show the same pattern as observed in Merino breed. Higher
levels of sarcosine in liver of the restricted groups could be indicative of an increase glutathione production. At the same time, lower levels of glycine in muscle of restricted groups
suggest a decrease of glycogenolysis. Previous results12 on the enzyme expression levels show that enzymes related with glycolysis (phosphofructokinase and pyruvate kinase) have lower
expression in the restricted group. Simultaneously, enzyme related to glycogenesis (glycogen synthase) did not vary between treatments. Moreover, the same study showed that levels of glucose
in liver of restricted group did not differ from the values of growth group12, suggesting an efficient response of this breed to feed restriction condition. Since carnitine is essential for
fat mobilization and energy production during fasting periods and feed restriction, higher levels of this metabolite in the liver could help explain the glucose homeostasis of this breed.
Previous results on the enzymes related with fatty acid synthesis (fatty acid synthase)12 revealed a decrease on its expression in the restricted group, reinforcing the hypothesis of fatty
acids request for energy production. As observed in the other two breeds, taurine levels are lower in the muscle of the restricted group, being indicative of lower muscle production and
development. However, previous results on enzymes involved in protein catabolism (glutamate dehydrogenase) show a decrease in restricted groups12. These results suggest that in the Damara
muscle production could be reduced due to feed restriction, while the Dorper breed can maintain tissue function and structure. Adenine concentration was higher in muscle of the restricted
group, opposite to what was observed in the liver of the Damara breed. This metabolite is a nucleoside with functions in protein synthesis and energy production. The Dorper breed also showed
differences in rumen-related metabolites (formate) between the restricted and growth groups that could be indicative of adaptations in rumen microbiota as response to the restriction-fed
regime. METABOLOMICS RESULTS, GROWTH, CARCASS TRAITS, LEPTIN AND INSULIN CONCENTRATIONS In general, we observed a decrease in muscle development, an increase in antioxidant activity and
differences in energy production pathways between the different breeds as each breed cope with feed restriction. Dorper and Damara breeds seem to be more tolerant to feed restriction.
Previous studies on these animals7,13 (Supplementary Table S8) already suggested this tendency. Variations in the carcass weight and yields and in the dimensions of eye muscle were similar
in Dorper and Damara breed, and both different of what was observed in Merino. Concerning plasma parameters, Damara breed presented differences in leptin and insulin concentrations, when
compared with the other breeds. Higher levels of leptin and insulin in Damara could be justified by the existence of the tail fat depot. Leptin is produced by adipose cells and is highly
correlated with body fat, whereas insulin stimulates lipogenesis and fatty acids esterification42. In ruminants, insulin stimulates the leptin expression43, which together with the body fat
content, could explain the higher level of leptin concentrations in this breed. CONCLUSIONS AND FUTURE PROSPECTS In general, the Dorper breed seems to be more adapted to SWL, showing few
changes in both tissues when subjected to feed restriction. This tolerance could be a result of the breed-selection history. The Merino, probably due to its selection for wool production,
showed more marked changes both tissues and seems to be the less adapted to SWL. The Damara presented a specific set of adaptations, reflecting the physiology of its major body
characteristic, the fat-tail. In the context of the breed selection towards SWL tolerance, our results confirm that the Dorper and Damara breeds have performed better under SWL conditions.
Their adaptation seems to be linked to a more efficient metabolic adaptation to feed-restriction, which change the nutritional energy source without compromising the overall muscle
structure. A possible adaptation at the rumen level should also be considered in these breeds, since they presented some variations related with rumen microflora composition and activity.
ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Palma, M. _et al_. The hepatic and skeletal muscle ovine metabolomes as affected by weight loss: a study in three sheep breeds using
NMR-metabolomics. _Sci. Rep._ 6, 39120; doi: 10.1038/srep39120 (2016). PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. REFERENCES * Boutonnet, J. P. Perspectives of the sheep meat world market on future production systems and trends. Small Rum Res 189–195, doi:
10.1016/S0921-4488(99)00072-3 (1999). * Almeida, A. The Damara in the context of Southern Africa fat-tailed sheep breeds. Trop Anim Health Pro 43, 1427–1441 (2011). Article Google Scholar
* Almeida, A. M., Schwalbach, L. M. J., Cardoso, L. A. & Greyling, J. P. C. Scrotal, testicular and semen characteristics of young Boer bucks fed winter veld hay: The effect of
nutritional supplementation. Small Rum Res 216–220, doi: 10.1016/j.smallrumres.2007.02.001 (2007). * Almeida, A., Schwalbach, L., Waal, H., Greyling, J. & Cardoso, L. The effect of
supplementation on productive performance of Boer goat bucks fed winter veld hay. Trop Anim Health Prod 38, 443–449 (2006). Article CAS Google Scholar * De Almeida, A. & Cardoso, L.
Animal production and genetic resources in Guinea Bissau: I – Northern Cacheu Province. Trop Anim Health Prod 529–536, doi: 10.1007/s11250-008-9130-9 (2008). * Almeida, A. & Cardoso, L.
Animal production and genetic resources in Guinea Bissau: II–Tombali province. Trop Anim Health Pro 40, 537–543 (2008). Article Google Scholar * Almeida, A. M. et al. Assessing carcass and
meat characteristics of Damara, Dorper and Australian Merino lambs under restricted feeding. Trop Anim Health Prod 45, 1305–11 (2013). Article Google Scholar * Lérias, J. R. et al. Body
live weight and milk production parameters in the Majorera and Palmera goat breeds from the Canary Islands: influence of weight loss. Trop Anim Health Prod 45, 1731–6 (2013). Article Google
Scholar * Lérias, J. R. et al. Establishment of the biochemical and endocrine blood profiles in the Majorera and Palmera dairy goat breeds: the effect of feed restriction. J. Dairy Res.
82, 416–25 (2015). Article Google Scholar * Scanlon, T. et al. Live weight parameters and feed intake in Dorper, Damara and Australian Merino lambs exposed to restricted feeding. Small
Ruminant Res 109, 101–106 (2013). Article Google Scholar * Tshabalala, P. A., Strydom, P. E., Webb, E. C. & de Kock, H. L. Meat quality of designated South African indigenous goat and
sheep breeds. Meat Sci. 65, 563–70 (2003). Article CAS Google Scholar * Van Harten, S. et al. Gene expression of regulatory enzymes involved in the intermediate metabolism of sheep
subjected to feed restriction. Animal 7, 439–45 (2013). Article CAS Google Scholar * Almeida, A. M. et al. The Effect of Weight Loss on the Muscle Proteome in the Damara, Dorper and
Australian Merino Ovine Breeds. PLoS ONE 11, e0146367 (2016). Article Google Scholar * Van Harten, S. et al. Fatty acid composition of the ovine longissimus dorsi muscle: effect of feed
restriction in three breeds of different origin. J. Sci. Food Agric. 96, 1777–82 (2016). Article CAS Google Scholar * Webb, E. C., Casey, N. H. & Van Niekerk, W. A. Fatty acids in the
subcutaneous adipose tissue of intensively fed SA Mutton Merino and Dorper wethers. Meat Sci. 38, 123–31 (1994). Article CAS Google Scholar * Palma, M. et al. NMR-metabolomics profiling
of mammary gland secretory tissue and milk serum in two goat breeds with different levels of tolerance to seasonal weight loss. Mol Biosyst 12, 2094–107 (2016). Article CAS Google Scholar
* Xu, C. et al. (1)H-Nuclear Magnetic Resonance-Based Plasma Metabolic Profiling of Dairy Cows with Fatty Liver. Asian-australas. J. Anim. Sci. 29, 219–29 (2016). Article CAS Google
Scholar * Scano, P. et al. (1)H NMR brain metabonomics of scrapie exposed sheep. Mol Biosyst 11, 2008–16 (2015). Article CAS Google Scholar * Mickiewicz, B. et al. Metabolic profiling of
synovial fluid in a unilateral ovine model of anterior cruciate ligament reconstruction of the knee suggests biomarkers for early osteoarthritis. J. Orthop. Res. 33, 71–7 (2015). Article
CAS Google Scholar * Wang, L. F. et al. The effects of acute lipopolysaccharide challenge on dairy goat liver metabolism assessed with (1) HNMR metabonomics. J Anim Physiol Anim Nutr
(Berl). doi: 10.1111/jpn.12439 (2016). * Almeida, A. M. et al. Assessing carcass and meat characteristics of Damara, Dorper and Australian Merino lambs under restricted feeding. Trop Anim
Health Prod 45, 1305–1311 (2013). Article Google Scholar * Bligh, E. G. & Dyer, W. J. A Rapid Method of Total Lipid Extraction and Purification. Can J Biochem Physiol 37, 911–917
(1959). Article CAS Google Scholar * Nicholas, P., Kim, D., Crews, F. & Macdonald, J. 1H NMR-based metabolomic analysis of liver, serum, and brain following ethanol administration in
rats. Chem Res Toxicol 21, 408–20 (2007). Article Google Scholar * Serkova, N. et al. Metabolic profiling of livers and blood from obese Zucker rats. J Hepatol 44, 956–62 (2005). Article
Google Scholar * Martínez-Granados, B. et al. Metabolic profile of chronic liver disease by NMR spectroscopy of human biopsies. Int J Mol Med 27, 111–7 (2010). PubMed Google Scholar *
Triba, M. N. et al. PLS/OPLS models in metabolomics: the impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol Biosyst 11, 13–9 (2015). Article CAS
Google Scholar * Saccenti, E., Hoefsloot, H., Smilde, A., Westerhuis, J. & Hendriks, M. Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics, doi:
10.1007/s11306-013-0598-6 (2014). * Stipanuk, M. H. Role of the liver in regulation of body cysteine and taurine levels: a brief review. Neurochem. Res. 29, 105–10 (2004). Article CAS
Google Scholar * Li, C. et al. Regulation of glucagon secretion in normal and diabetic human islets by γ-hydroxybutyrate and glycine. J Biol Chem 3938–51, doi: 10.1074/jbc.m112.385682
(2012). * Jackson, A. A. The glycine story. Eur J Clin Nutr 45, 59–65 (1991). CAS PubMed Google Scholar * Ruiz-Ramírez, A., Ortiz-Balderas, E., Cardozo-Saldaña, G., Diaz-Diaz, E. &
El-Hafidi, M. Glycine restores glutathione and protects against oxidative stress in vascular tissue from sucrose-fed rats. Clin. Sci. 126, 19–29 (2014). Article Google Scholar * Stipanuk,
M. H., Coloso, R. M., Garcia, R. A. & Banks, M. F. Cysteine concentration regulates cysteine metabolism to glutathione, sulfate and taurine in rat hepatocytes. J. Nutr. 122, 420–7
(1992). Article CAS Google Scholar * Griffith, O. W. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 27, 922–35 (1999). Article CAS
Google Scholar * Linster, C. L. & Van Schaftingen, E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 274, 1–22 (2007). Article CAS Google Scholar * Meister,
A. Glutathione-ascorbic acid antioxidant system in animals. J. Biol. Chem. 269, 9397–400 (1994). CAS PubMed Google Scholar * De Vogel, S. et al. Sarcosine and other metabolites along the
choline oxidation pathway in relation to prostate cancer–a large nested case-control study within the JANUS cohort in Norway. Int. J. Cancer 134, 197–206 (2014). Article Google Scholar *
Beyer, C., Alting, I. H. & Backer, E. T. Measurement of creatine in urine by creatinase, sarcosine oxidase, and peroxidase reevaluated. Clin. Chem. 39, 1743–4 (1993). CAS PubMed Google
Scholar * McNeil, N. I. The contribution of the large intestine to energy supplies in man. Am. J. Clin. Nutr. 39, 338–42 (1984). Article CAS Google Scholar * Zeisel, S. H. &
Blusztajn, J. K. Choline and human nutrition. Annu. Rev. Nutr. 14, 269–96 (1994). Article CAS Google Scholar * Alves, S. P. et al. Does the fat tailed Damara ovine breed have a distinct
lipid metabolism leading to a high concentration of branched chain fatty acids in tissues? PLoS ONE 8, e77313 (2013). Article CAS ADS Google Scholar * Mirzaei-Aghsaghali, A. &
Maheri-Sis, N. Importance of ‘physically effective fibre’ in ruminant nutrition: A review. Ann Biol Res 2, 262–270 (2011). CAS Google Scholar * Ren, M. et al. Comparing mRNA levels of
genes encoding leptin, leptin receptor, and lipoprotein lipase between dairy and beef cattle. Domest Anim Endocrinol 371–381, doi: 10.1016/S0739-7240(02)00179-0 (2002). * Eryavuz, G., Avci,
I., Kucukkurt, I. & Fidan, A. F. Comparison of plasma leptin, insulin and thyroid hormone concentrations and some biochemical parameters between Fat-tailed and Thin-tailed sheep breeds.
Rev Med Vet 5, 244–249 (2007). Google Scholar Download references ACKNOWLEDGEMENTS Animal work was supported by the Department of Agriculture and Food of the Government of Western Australia
(Perth, WA, Australia) to which author AM Almeida acknowledges a visiting scientist research grant. The contribution of the technical staff of the Merredin Research Station: Alan Harrod,
Leanne Young, Nicky Stanwyck, Elmer Kidson and Dr. Roy Butler, is appreciated. Author M Palma is funded by grant SFRH/BD/85391/2012, from _FCT - Fundação para a Ciência e a Tecnologia_
(Lisbon, Portugal). The NMR spectrometer is part of The National NMR Facility (RECI/BBB-BQB/0230/2012). This work was financially supported by: Project LISBOA-01-0145-FEDER-007660
(Microbiologia Molecular, Estrutural e Celular) funded by FEDER funds through COMPETE2020 - _Programa Operacional Competitividade e Internacionalização_ (POCI) and national FCT funds. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * ITQB NOVA, Instituto de Tecnologia Química e Biológica António Xavier, Universidade Nova de Lisboa, Oeiras, Portugal Mariana Palma & Manolis
Matzapetakis * DAFWA – Department of Agriculture and Food Western Australia, Perth, WA, Australia Tim Scanlon, Tanya Kilminster, Chris Oldham & Johan Greeff * UWA – University of Western
Australia, Perth, WA, Australia John Milton * IBET – Instituto de Biologia Experimental e Tecnológica, Oeiras, Portugal André M. Almeida * RUSVM – Ross University School of Veterinary
Medicine, Basseterre, St. Kitts and Nevis André M. Almeida Authors * Mariana Palma View author publications You can also search for this author inPubMed Google Scholar * Tim Scanlon View
author publications You can also search for this author inPubMed Google Scholar * Tanya Kilminster View author publications You can also search for this author inPubMed Google Scholar * John
Milton View author publications You can also search for this author inPubMed Google Scholar * Chris Oldham View author publications You can also search for this author inPubMed Google
Scholar * Johan Greeff View author publications You can also search for this author inPubMed Google Scholar * Manolis Matzapetakis View author publications You can also search for this
author inPubMed Google Scholar * André M. Almeida View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.M.A., T.S., T.K., J.M., C.O. and J.G.
designed and conducted animal experiment. A.M.A., M.M. and M.P. designed Metabolomics analysis. M.P. and M.M. conducted NMR and statistical analysis. A.M.A. and M.M. designed and supervised
laboratory work. M.P., M.M. and A.M.A. wrote the manuscript and prepared the figures. A.M.A. and M.M. designed and supervised the experiments, corrected the manuscript and coordinated the
project. All authors reviewed the manuscript. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY
MATERIAL RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in
the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain
permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Palma, M., Scanlon, T., Kilminster, T. _et al._ The hepatic and skeletal muscle ovine metabolomes as affected by weight loss: a study in three sheep breeds using
NMR-metabolomics. _Sci Rep_ 6, 39120 (2016). https://doi.org/10.1038/srep39120 Download citation * Received: 12 August 2016 * Accepted: 17 November 2016 * Published: 14 December 2016 * DOI:
https://doi.org/10.1038/srep39120 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
