Ubiquitylation directly induces fold destabilization of proteins
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Ubiquitin is a common post-translational modifier and its conjugation is a key signal for proteolysis by the proteasome. Because the molecular mass of ubiquitin is larger than that
of other modifiers such as phosphate, acetyl, or methyl groups, ubiquitylation not only influences biochemical signaling, but also may exert physical effects on its substrate proteins by
increasing molecular volume and altering shape anisotropy. Here we show that ubiquitylation destabilizes the fold of two proteins, FKBP12 and FABP4, and that elongation of the conjugated
ubiquitin chains further enhances this destabilization effect. Moreover, NMR relaxation analysis shows that ubiquitylation induces characteristic structural fluctuations in the backbone of
both proteins. These results suggest that the ubiquitylation-driven structural fluctuations lead to fold destabilization of its substrate proteins. Thus, physical destabilization by
ubiquitylation may facilitate protein degradation by the proteasome. SIMILAR CONTENT BEING VIEWED BY OTHERS SITE-SPECIFIC UBIQUITINATION AFFECTS PROTEIN ENERGETICS AND PROTEASOMAL
DEGRADATION Article 01 June 2020 BREAKING THE K48-CHAIN: LINKING UBIQUITIN BEYOND PROTEIN DEGRADATION Article 16 February 2024 DEUBIQUITINATING ENZYMES AND THE PROTEASOME REGULATE
PREFERENTIAL SETS OF UBIQUITIN SUBSTRATES Article Open access 18 May 2022 INTRODUCTION Ubiquitylation is a common post-translational modification of physiological importance equivalent to
phosphorylation, acetylation, and methylation. In this modification, ubiquitin is covalently conjugated to a lysine residue or the N-terminal residue of a substrate protein via its
C-terminal tail1. The C-terminal tail of ubiquitin can also be covalently conjugated to a lysine residue or the N-terminal residue of another ubiquitin molecule, thereby forming
polyubiquitin chains. (Poly-)ubiquitin molecules attached to a substrate protein are specifically recognized by down-stream ubiquitin-binding proteins2. One of the most well-known cellular
processes associated with ubiquitylation is protein degradation3, in which polyubiquitin-tagged proteins are targeted to the 26S proteasome, where they are unfolded and degraded by the
proteasome in an ATP-dependent manner4. Ubiquitylation also exerts non-proteolytic functions such as the regulation of protein activity and localization1. Thus, similar to other
posttranslational modifications, ubiquitylation participates in many cellular processes by controlling protein function. Ubiquitin (8.6 kDa) and ubiquitin-like modifiers (8–20 kDa)5 are
relatively high-molecular weight entities, as compared with other post-translational modifiers such as acetyl (43 Da), methyl (15 Da), and phosphate (97 Da) groups. This suggests that
conjugation of a ubiquitin molecule to a substrate protein might also affect some physical properties of the substrate such as molecular weight/volume and molecular shape anisotropy. Indeed,
a recent molecular dynamics analysis showed that ubiquitylation might be capable of causing partial unfolding of substrate proteins6. Furthermore, we previously observed a decrease in the
thermodynamic stability of ubiquitin itself due to polymerization7. We therefore hypothesized that the fold of ubiquitylated proteins might be destabilized via a molecular mechanism similar
to that observed for polyubiquitin chains. Furthermore, the ubiquitylation-induced destabilization of substrate proteins might lead to the formation of aggregates or might shorten their
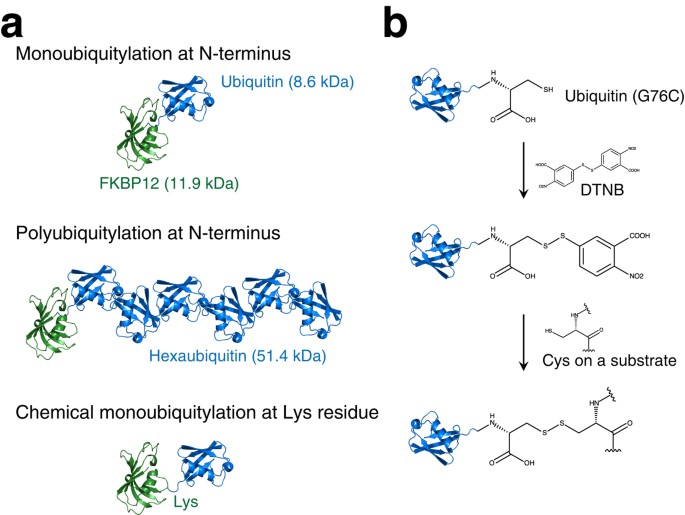
intracellular lifetime. RESULTS We first prepared ubiquitylated proteins with two distinct kinds of linkage between ubiquitin and the target protein: N-terminal ubiquitylation8 and
site-specific ubiquitylation by chemical conjugation at a site where intracellular ubiquitylation has been previously confirmed (Fig. 1a). We used two proteins that have been shown to be
ubiquitylated _in vivo_: human FK506-binding protein (FKBP12) and human fatty acid binding protein 4 (FABP4). According to the PhosphoSite Plus database9, FKBP12 is ubiquitylated at Lys35,
Lys36, Lys48, and Lys53; FABP4 is ubiquitylated at Lys22, Lys32, Lys38, Lys59, Lys80, Lys97, Lys101, Lys113, and Lys121. Each protein is composed of a single-domain structure; therefore, the
physical effect of ubiquitylation on these proteins should be easy to compare. To obtain the N-terminally ubiquitylated form, we fused the gene encoding monoubiquitin (Ub) or tandemly
arranged hexaubiquitin (Ub6) to the gene encoding each protein to express Ub-FKBP12, Ub6-FKBP12, Ub-FABP4, and Ub6-FABP4. To prepare chemically ubiquitylated proteins, we constructed a
simple and efficient ubiquitylation protocol using disulfide conjugation. By activating cysteine thiol groups with 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB)10, a disulfide bridge was
formed specifically between the C-terminus of ubiquitin and the native ubiquitylation site of the substrate protein (Fig. 1b). This disulfide-mediated ubiquitylation has been shown to mimic
native ubiquitylation10,11,12 although it contains an extra carboxyl group (Fig. 1b), implying that the disulfide-mediated ubiquitylation may not cover all the biochemical features of native
ubiquitylation. However, in cases where ubiquitin-conjugating enzymes and ubiquitin ligases for target proteins are not identified, the disulfide-mediated ubiquitylation is the most
straightforward way to prepare sufficient amounts of ubiquitylated protein samples for thermodynamic analysis. In this study, we prepared chemically ubiquitylated FKBP12 at Lys36, Lys48, and
Lys53; and chemically ubiquitylated FABP4 at Lys32, Lys80, and Lys121. Each ubiquitylation site is located in a characteristic secondary structure element: namely, a loop, α-helix, or
β-sheet (Supporting Information (SI), Figure S1). To investigate the physical effect of ubiquitylation, we compared the fold stability of the two ubiquitylated proteins with that of the
non-ubiquitylated form. Native ubiquitin has no tryptophan residues, whereas both substrate proteins (FKBP12 and FABP4) have several. As a result, the tryptophan fluorescence spectrum of the
ubiquitylated protein specifically reflects the chemical environment of tryptophan residues in the substrate proteins. Furthermore, when a tryptophan residue participates in hydrogen
bonding and/or is exposed to water, its fluorescence emission shifts to longer wavelengths; therefore, the emission wavelength changes in response to conformational changes in the substrate
proteins (_e_._g_., heat-denaturation of FKBP12; Fig. 2a). Tryptophan fluorescence analysis indicated that N-terminal mono-ubiquitylation decreased the thermal transition temperature of both
substrate proteins by more than 5 K (Fig. 2b and c). Such ubiquitylation-induced fold destabilization was also observed when inducing chemical denaturation using guanidine hydrochloride
(SI, Figure S2). In stark contrast, a simple mixture of protein and ubiquitin (without covalent conjugation) did not destabilize the fold of those proteins (Fig. 2c and SI, Figure S3). In
particular, the mixture of ubiquitin molecules stabilized the fold of FKBP12. Non-specific weak protein interactions between ubiquitin and FKBP12 might contribute to this thermodynamic
stabilization although such an interaction was undetectable in NMR titration experiments (data not shown). Interestingly, N-terminal poly-ubiquitylation (attachment of linear hexaubiquitin)
further enhanced the unfolding of the substrate proteins to a limited but further extent (_ca_. 1.5 K) (Fig. 2c and SI, Figure S3). Similar to N-terminal ubiquitylation, site-specific
chemical ubiquitylation decreased the transition point of both substrate proteins (Fig. 2d and SI, Figure S4). As compared with N-terminal ubiquitylation, the effect of chemical
ubiquitylation on substrate fold stability seemed to be smaller. Intriguingly, the degree of fold destabilization appeared to depend on secondary structure elements of the substrate protein
at the site of ubiquitylation (SI, Figure S1). Ubiquitylation at a site in a β-sheet (Fig. 2d middle) caused larger fold destabilization than that in loops (Fig. 2d right). On the other
hand, ubiquitylation at a site in an α-helix had no significant effect on fold stability of FABP4 (Fig. 2d lower left). Fold destabilization was not observed for FKBP12 ubiquitylated at
Cys36 (Fig. 2d upper left), which is located in a short β-sheet. Taken together, these results indicate that covalent conjugation of (poly-)ubiquitin to the substrate protein decreases the
fold stability of the substrate and the degree of destabilization depends on the chain length and the site of ubiquitylation. We previously observed that the thermal unfolding of FKBP12 is
reversible; in contrast, N-terminally ubiquitylated FKBP12 displays an irreversible transition7. Similarly, ubiquitin loses its thermal folding reversibility by polymerization or conjugation
to other proteins7; thus, we considered whether FKBP12 in its ubiquitylated form is simply entrapped by insoluble ubiquitin aggregates during heat denaturation, or whether it loses its own
folding reversibility alongside ubiquitin. To probe the folding reversibility of FKBP12 in ubiquitylated form, we monitored its differential scanning calorimetry (DSC) profile after
selective thermal unfolding of FKBP12. Because the thermal transition point of FKBP12 is approximately 20 K lower than that of (poly-)ubiquitin, it is possible to denature only the FKBP12
moiety in Ub-FKBP12 or Ub6-FKBP12, while leaving ubiquitin folded. Although the transition point of free FKBP12 was found to be 335.5 K in previous DSC measurements7, we observed a DSC peak
at a position 10 K lower than the transition of free FKBP12, which we assumed corresponds to the transition of FKBP12 in its (poly-)ubiquitylated form7. This observation is consistent with
the thermodynamic destabilization of FKBP12 by N-terminal ubiquitylation observed in the tryptophan fluorescence experiments (Fig. 2c). After Ub-FKBP12 was heated to 330 K and gently cooled
to room temperature, the DSC peak corresponding to FKBP12 was barely observed in the reheating thermograph. However, the peak of ubiquitin was readily detected (Fig. 3a, upper). Similar to
Ub-FKBP12, the DSC peak corresponding to FKBP12 could not be detected in the reheating thermograph of Ub6-FKBP12, whereas that of linear hexaubiquitin was detected (Fig. 3a, lower). These
results indicate that (poly-)ubiquitylation abolishes the thermal folding reversibility of the modified protein. Interestingly, heat-treated (poly-)ubiquitylated FKBP12 was soluble, and DSC
analysis indicated that heat denaturation of FKBP12 did not affect the folding of (poly-)ubiquitin1. To investigate what structural changes occur during the heat denaturation of FKBP12 in
(poly-)ubiquitylated FKBP12, we measured its 1H-15N hetero-nuclear multiple quantum coherence (HMQC) spectrum. The spectrum of non-heated (poly-)ubiquitylated FKBP12 displayed peaks of both
FKBP12 and ubiquitin in positions corresponding to approximately the sum of those of the unconjugated protein and modifier. In contrast, no peaks corresponding to natively folded FKBP12, but
peaks of (poly-)ubiquitin were detected in the spectrum of (poly-)ubiquitylated FKBP12 that had been heat-treated up to 330 K or 332 K (Fig. 3b and SI, Figure S5). Instead, there were
several new peaks, positioned mainly between 8.5 and 8.0 ppm in the 1H dimension—a region where peaks are often observed for unstructured proteins/peptides. This result implies that the
FKBP12 protein in (poly-)ubiquitylated FKBP12 is irreversibly unfolded by the heat treatment up to 330 K or 332 K, but the ubiquitin moiety is not. To gain insight into the mechanism of
protein destabilization by ubiquitylation, we prepared ubiquitylated proteins in which only the substrate proteins were 15N-labeled in order to selectively observe NMR peaks of the substrate
protein, but not those of the attached ubiquitin, in 1H-15N hetero-nuclear single quantum coherence (HSQC) spectra. The spectra of ubiquitylated FKBP12 and FABP4 showed little chemical
shift perturbation as compared with the respective non-ubiquitylated proteins (SI, Figure S6). This observation indicates that ubiquitylation does not significantly change the overall
average structure of a given substrate protein at moderate temperature. Next, we analyzed the effect of ubiquitylation on protein backbone dynamics at three different frequencies by deriving
spectral density functions, _J_(0), _J_(ωN), and _J_(0.87ωH), from the 15N _T_1, _T_2 relaxation times, and steady-state {1H}-15N heteronuclear NOE values (SI, Figure S7). The spectral
density functions quantitatively report dynamics on a variety of timescales (from pico- to milliseconds)13. We observed more diversity in the spectral density functions of both ubiquitylated
proteins as compared with their non-ubiquitylated counterparts (with the exception of _J_(ωN) of ubiquitylated FKBP12) (SI, Figure S8). The spectral density functions of both of the
non-ubiquitylated proteins were relatively uniform across residues. The most marked difference in function was observed for _J_(0): the mean ± standard deviation values for FKBP12 in the
non-ubiquitylated and ubiquitylated form were 4.2 ± 1.3 ns and 11.4 ± 5.2 ns, respectively; those for FABP4 were 5.2 ± 1.8 ns and 8.0 ± 2.2 ns, respectively. The larger values and deviations
of _J_(0) observed for the ubiquitylated proteins versus non-ubiquitylated proteins may be partly due to the larger molecular mass and more anisotropic shape of ubiquitylated proteins.
However, the magnitude of these differences seems to be beyond what might be assumed by differences in molecular weight and shape anisotropy, in particular for FKBP12 (Fig. 4). _J_(0) is
sensitive to slow protein motions (on the micro- to millisecond timescale), including partial protein folding–unfolding. Thus, the larger values and deviations of _J_(0) observed for the
ubiquitylated proteins might be caused by fluctuations in the protein backbone. 15N relaxation dispersion experiments for the ubiquitylated proteins suggested that ubiquitylation affected
their intrinsic millisecond timescale protein motions to a certain degree (SI, Figures S9 and S10). In addition, rotational diffusion analysis of the observed relaxation parameters (15N
_T_1, _T_2 relaxation times, and 1H-15N hetero-nuclear NOE values) using the program _ROTDIF_14 showed ubiquitylation-induced differences in rotational diffusion anisotropy for the modified
protein (SI, Figure S11 and Table S1). The conjugation of ubiquitin resulted in an exchange of isotropic rotational diffusion to an anisotropic one for FKBP12 and a directional change of the
rotational diffusion tensor for FABP4 (SI, Figure S11 and Table S1). Taken together, these observations suggest that the intrinsic protein motion of the substrate protein is disturbed by
ubiquitylation, and that the resulting increase in global fluctuations may lead to decreased stability of the structural fold of that protein. DISCUSSION In this study, we present
experimental evidence for the ubiquitylation-induced destabilization of the structural fold of proteins. For both of the substrate proteins examined, covalent conjugation of (poly-)ubiquitin
led to a decrease in the temperature of the thermal unfolding of the protein. We also observed that (poly-)ubiquitylation abolishes the thermal folding reversibility of FKBP12 (Fig. 3).
When protein unfolding is thermally irreversible, it is difficult to obtain the Gibbs free energy difference between its folded and unfolded states from the thermal denaturation experiments.
On the other hand, in such a case, the transition midpoints depend on the kinetics of the transitions15,16. Because all our fluorescence experiments were performed in the same manner, it is
possible to compare the stabilities of the protein fold (fold stabilities) using the transition midpoints. In addition, the ubiquitylation-induced destabilization of FKBP12 was observed in
the chemical denaturation experiments using guanidine chloride (SI, Figure S2), where no aggregation occurred. Thus, it is most likely that the ubiquitylation-induced fold destabilization
could be detected in our experiments. The degree of this ubiquitylation-induced fold destabilization depends on the modification site in the substrate protein (Fig. 2c and d). Both FKBP12
and FABP4 possess β-sheets at their N-terminal regions and their ubiquitylation at another β-sheet affected fold stability more severely than ubiquitylation in a loop or α-helix (Fig. 2d).
Thus, secondary structure elements at ubiquitylation sites may be correlated with the degree of resultant destabilization. Because ubiquitin attachment is also used as a non-proteolytic
intracellular signal, it is reasonable that the degree of fold destabilization varies in accordance with the ubiquitylation site. On the other hand, the ubiquitylation-induced structural
fluctuations were not only located at the ubiquitylation site but also distributed rather globally. It will therefore be necessary to further investigate the mechanism underlying the
structural fluctuations and fold destabilization caused by ubiquitylation. Intracellular proteins do not consist exclusively of single-domain structures, but also contain many multi-domain
assemblies. For a multi-domain protein, there may be diverse interactions between domains and thermodynamic properties17,18, and it may be complicated to estimate the effect of
ubiquitylation on its fold stability. On the other hand, the average size of a single protein domain is approximately 100 residues19, which is approximately the size of the model proteins
investigated in this study: FKBP12 and FABP4. This suggests that, in general, ubiquitylation is at least capable of causing fold destabilization of conjugated protein domain units. Indeed,
in support of our hypothesis, we also observed a decrease in the thermodynamic stability of a multi-domain protein by mono-ubiquitylation: Ca2+ -free calmodulin (SI, Figure S12). It will be
intriguing to examine how domain-domain interactions affect ubiquitylation-driven fold destabilization for multi-domain proteins that form more complicated quaternary structures. On the one
hand, a previous molecular dynamics analysis showed that ubiquitylation might stabilize the unfolded state of the substrate protein and that the resultant loss in folding entropy might cause
its thermal instability6. On the other hand, the present study reveals that ubiquitylation induces changes in the dynamics of the folded state of the substrate protein, including structural
fluctuations of the protein backbone. Such backbone fluctuations would directly affect the fold stability and, in fact, conjugation of ubiquitin was found to result in thermal
destabilization of the substrate proteins even though it induces few static structural differences (Fig. 2 and SI, Figure S6). This discussion is consistent with a recent computational
study, which showed that a ubiquitylation-induced increase in entropy in the folded state of the substrate protein causes thermodynamic destabilization20. Furthermore, the induction of more
anisotropic molecular motion by ubiquitylation may be a possible factor that causes these changes in the structural dynamics of the substrate proteins. It will be important to identify the
physical factors that might generate such structural changes and examine their related mechanisms. During the course of proteasomal degradation, (poly-)ubiquitin-tagged proteins recruited to
the 26S proteasome are unfolded by the AAA + ring of the proteasome in a process driven by ATP hydrolysis21. The protein is subsequently translocated to the proteasomal core, where it is
degraded by the core protease machinery of the proteasome. Some intracellular proteins have degron sequences and/or intrinsically disordered tails/regions22, which trigger unfolding of the
global structure; however, a substantial fraction of proteins (about 25%) have few such degrons. When a protein lacking a sufficient amount of such disordered tails/loops is targeted for
degradation by the proteasome, some additional steps or factors to unfold that protein are needed. Therefore, we hypothesize that the direct partial unfolding by ubiquitylation might be one
of the mechanisms that assist in the step of substrate unfolding. This direct partial unfolding by ubiquitylation would work favorably because it would decrease the amount of ATP required
for the proteasome to achieve complete denaturation of a protein. Future studies will need to focus on elucidating the underlying mechanism and on examining the effect of
multi-ubiquitylation of proteins. Furthermore, the present results also suggest that the physical effects of ubiquitylation are related to intracellular aggregate formation. If
(poly-)ubiquitin tagged proteins are not degraded by the proteasome appropriately or their ubiquitin molecules are not cleaved off efficiently, they will accumulate in cells. Given that
polyubiquitin fibril formation is caused by the elongation of ubiquitin chains7, a poly-ubiquitylated protein may form irreversibly insoluble aggregates either via the chain-length-dependent
destabilization of the polyubiquitin chain7 or via the ubiquitylation-induced destabilization of the substrate protein. This indicates that ubiquitylation is closely associated with
intracellular protein aggregation. The relationship between ubiquitylation and human proteinopathies should be further elucidated in future studies. METHODS PROTEIN PREPARATION Human
ubiquitin, human 12-kDa FK506-binding protein (FKBP12) and human fatty acid binding protein 4 (FABP4), and their cysteine mutants were expressed in _Escherichia coli_ strain BL21 (_DE3_)
grown in LB or M9 minimal media containing 99% 15N-labeled ammonium chloride (Cambridge Isotope Laboratories). Human ubiquitin was purified by cation exchange and size-exclusion
chromatography. Human FKBP12 was expressed as a fusion protein with an N-terminal glutathione _S_-transferase and small ubiquitin-like modifier protein (GST-SUMO-1) tag. After cleavage of
the tag by GST-SENP2 protease, FKBP12 was further purified by size-exclusion chromatography. Human FABP4 was expressed as a fusion protein with an N-terminal hexa-histidine (His6) SUMO-1
protein tag. After cleavage of the His6-SUMO tag by GST-SENP2 protease, FABP4 was further purified by size-exclusion chromatography. N-terminally ubiquitylated FKBP12 and FABP4 were
expressed as fusion proteins with an HRV3C-cleavable C-terminal His6-tag and purified by Ni-NTA affinity chromatography. After cleavage of the C-terminal His6-tag by HRV3C protease, the
proteins were further purified by anion exchange and size-exclusion chromatography. CHEMICAL CONJUGATION OF UBIQUITIN TO SUBSTRATE PROTEINS A ubiquitin G76C mutant protein was reduced with 5
mM 2-mercaptoethanol, and then buffer-exchanged into 50 mM sodium phosphate pH 7.5 using a PD-10 desalting column (GE Healthcare). The reduced ubiquitin mutant was mixed with a 20-fold
molar excess of 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB, Tokyo Chemical Industry) and then incubated for 1–3 hours at room temperature with vigorous shaking. The reaction solution was
buffer-exchanged into ligation buffer (20 mM Tris-HCl, 50 mM NaCl and 1 mM EDTA, pH 7.0) using a PD-10 desalting column. The cysteine mutants of the respective substrate proteins were also
reduced, buffer-exchanged into ligation buffer, and mixed with a 3-fold molar excess of activated ubiquitin G76C-DTNB for 1 hour. ISOLATION OF CHEMICALLY UBIQUITYLATED PROTEINS Chemically
ubiquitylated proteins were isolated by hydrophobic interaction chromatography (HIC) using a HiTrap Phenyl HP column (GE Healthcare). The proteins were dissolved in high salt buffer (2 M
potassium phosphate, pH 7.0), applied to the column, washed with more than two column volumes, and eluted by a salt gradient (2 to 0 M potassium phosphate, pH 7.0 in five column volumes).
The purity of the eluted proteins was verified by SDS-PAGE and MALDI-TOF mass spectrometry (Bruker) analysis. FLUORESCENCE SPECTROSCOPY Fluorescence was quantified on a FluoroMax4 (HORIBA)
spectrometer. Tryptophan fluorescence was selectively measured by excitation at 300 nm, and emission spectra were collected over wavelengths of 310 to 400 nm with the slit width set at 5 nm.
All samples were diluted to a final substrate protein concentration of 20 μM in PBS buffer. In the case of (Met1-ubiquitylated-)FKBP12 and FABP4, 0.5 mM TCEP was included in the buffer. The
spectral contribution of the buffer was subtracted from the acquired spectra. The peak shift was evaluated by calculating the barycentric mean of the fluorescence emission spectrum. The
barycentric mean was obtained from the equation , in which F (λ) is the tryptophan fluorescence intensity at λ nm. Using Igor Pro 6 (WaveMetrics), transition points were obtained by fitting
a series of tryptophan emission wavelengths to the sigmoid equation , in which λ is the tryptophan fluorescence emission wavelength observed at temperature T in units of Kelvin, and λN, and
λD are those of the native and the completely denatured proteins, respectively; Thalf is the midpoint temperature of the sigmoidal curve; the _rate_ is a parameter that determines the slope
of the curve. DIFFERENTIAL SCANNING CALORIMETRY Thermal denaturation curves were acquired on a Nano DSC instrument (TA Instruments Inc.). The scan rate was 1 K min−1, and the protein
concentration was 1 mg ml−1. The buffer was PBS (137 mM NaCl, 8.1 mM Na2HPO4, 2.68 mM KCl and 1.47 mM KH2PO4, pH 7.4) containing 0.1 mM TCEP. Reheating experiments were performed in the same
manner after heating the protein to the target temperature, followed by gradual cooling to room temperature. Analysis was performed by using CpCalc (TA Instruments Inc.) and data were
reported as heat capacity (kJ K−1 mol−1). The transition temperature was defined as the temperature corresponding to the transition peak maximum. NMR SPECTROSCOPY All NMR spectra were
acquired at 298 K or 310 K on a Bruker Avance 600 MHz NMR spectrometer equipped with a 5 mm 15N/13C/1H z-gradient triple resonance cryoprobe. Resonance assignments for 1H-15N peaks were
based on previous studies23,24. To probe the folding of native and heat-treated (poly-)ubiquitylated FKBP12, 1H-15N SOFAST-HMQC25 spectra were acquired. Because (non-)heated ubiquitylated
FKBP12 appeared to be unstable, the NMR spectra were obtained in a short amount of time by SOFAST-HMQC. The measurement conditions for SOFAST-HMQC spectra were PBS buffer containing 5 mM
EDTA and 1 mM DTT for Ub-FKBP12, PBS buffer for Ub6-FKBP12, and PBS buffer containing 0.5 mM TCEP for FKBP12. To examine the chemical shift differences caused by ubiquitylation, 1H-15N HSQC
spectra were acquired in phosphate buffer (20 mM potassium phosphate, 5 mM KCl, 1 mM EDTA, 50 mM NaCl, 5 mM DTT, pH 6.8) for the two proteins examined. The 15N relaxation experiments were
also performed in this phosphate buffer. For the 15N _T_1 relaxation experiment, a series of spectra with relaxation delays of 10, 20, 40, 180, 300, 500, and 1000 milliseconds were measured.
For the 15N _T_2 relaxation experiment, a series of spectra with relaxation delays of 10, 30, 50, 70, 90, 110, and 150 milliseconds were acquired. For the 1H-15N NOE, _T_1, and _T_2
relaxation measurements, the recycle delay was set to 3–5 seconds to ensure that there was adequate longitudinal relaxation between acquisitions. Data processing was performed in NMRPipe26
and CCPN27. NMR RELAXATION ANALYSIS In the 15N _T_1 and _T_2 relaxation experiments, the signal intensities _I_(_t_) of each peak with different relaxation delays _t_ were fitted to the
equation to obtain the relaxation time _T_α, where α = 1 or 2. Fitting was performed using the program GLOVE28. 1H-15N heteronuclear NOE (hnNOE) values were calculated by the equation:
(hnNOE value) = _I_sat (_I_eq)−1, where _I_sat and _I_eq are the peak intensities with and without proton saturation, respectively. The values represent the average of two independent
experiments. The respective spectral density functions, _J_(ω), were obtained from the 15N _T_1, _T_2 relaxation times, and hnNOE values13. Errors in the _T_1 and _T_2 relaxation times were
calculated by the Monte Carlo method28, and errors in the hnNOE values were estimated by the standard deviation of two experiments. Errors bars for the spectral density functions were
obtained by error propagation. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Morimoto, D. _et al_. Ubiquitylation Directly Induces Fold Destabilization of Proteins. _Sci. Rep._ 6, 39453;
doi: 10.1038/srep39453 (2016). PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. REFERENCES *
Komander, D. & Rape, M. The ubiquitin code. Annu Rev Biochem 81, 203–229, doi: 10.1146/annurev-biochem-060310-170328 (2012). Article CAS PubMed Google Scholar * Dikic, I., Wakatsuki,
S. & Walters, K. J. Ubiquitin-binding domains - from structures to functions. Nat Rev Mol Cell Biol 10, 659–671, doi: 10.1038/nrm2767 (2009). Article CAS PubMed PubMed Central
Google Scholar * Hershko, A. & Ciechanover, A. The ubiquitin system for protein degradation. Annu Rev Biochem 61, 761–807, doi: 10.1146/annurev.bi.61.070192.003553 (1992). Article CAS
PubMed Google Scholar * Schrader, E. K., Harstad, K. G. & Matouschek, A. Targeting proteins for degradation. Nat Chem Biol 5, 815–822, doi: 10.1038/nchembio.250 (2009). Article CAS
PubMed PubMed Central Google Scholar * Hochstrasser, M. Evolution and function of ubiquitin-like protein-conjugation systems. Nat Cell Biol 2, E153–157, doi: 10.1038/35019643 (2000).
Article CAS PubMed Google Scholar * Hagai, T. & Levy, Y. Ubiquitin not only serves as a tag but also assists degradation by inducing protein unfolding. Proc Natl Acad Sci USA 107,
2001–2006, doi: 10.1073/pnas.0912335107 (2010). Article ADS PubMed PubMed Central Google Scholar * Morimoto, D. et al. The unexpected role of polyubiquitin chains in the formation of
fibrillar aggregates. Nat Commun 6, 6116, doi: 10.1038/ncomms7116 (2015). Article ADS CAS PubMed Google Scholar * Ciechanover, A. & Ben-Saadon, R. N-terminal ubiquitination: more
protein substrates join in. Trends Cell Biol 14, 103–106 (2004). Article CAS PubMed Google Scholar * Hornbeck, P. V. et al. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations.
Nucleic Acids Res 43, D512–520, doi: 10.1093/nar/gku1267 (2015). Article CAS PubMed Google Scholar * Chen, J., Ai, Y., Wang, J., Haracska, L. & Zhuang, Z. Chemically ubiquitylated
PCNA as a probe for eukaryotic translesion DNA synthesis. Nat Chem Biol 6, 270–272, doi: 10.1038/nchembio.316 (2010). Article CAS PubMed Google Scholar * Baker, R. et al. Site-specific
monoubiquitination activates Ras by impeding GTPase-activating protein function. Nat Struct Mol Biol 20, 46–52, doi: 10.1038/nsmb.2430 (2013). Article CAS PubMed Google Scholar *
Chatterjee, C., McGinty, R., Fierz, B. & Muir, T. Disulfide-directed histone ubiquitylation reveals plasticity in hDot1L activation. Nat Chem Biol 6, 267–269, doi: 10.1038/NCHEMBIO.315
(2010). Article CAS PubMed Google Scholar * Farrow, N. A., Zhang, O., Szabo, A., Torchia, D. A. & Kay, L. E. Spectral density function mapping using 15N relaxation data exclusively.
J Biomol NMR 6, 153–162 (1995). Article CAS PubMed Google Scholar * Berlin, K., Longhini, A., Dayie, T. K. & Fushman, D. Deriving quantitative dynamics information for proteins and
RNAs using ROTDIF with a graphical user interface. J Biomol NMR 57, 333–352, doi: 10.1007/s10858-013-9791-1 (2013). Article CAS PubMed PubMed Central Google Scholar * Lumry, R. &
Eyring, H. Conformation changes of proteins. J Phys Chem 58, 110–120, doi: 10.1021/j150512a005 (1954). Article CAS Google Scholar * Sanchez-Ruiz, J. M., Lopez-Lacomba, J. L., Cortijo, M.
& Mateo, P. L. Differential scanning calorimetry of the irreversible thermal denaturation of thermolysin. Biochemistry 27, 1648–1652 (1988). Article CAS PubMed Google Scholar *
Porter, L. L. & Rose, G. D. A thermodynamic definition of protein domains. Proc Natl Acad Sci USA 109, 9420–9425, doi: 10.1073/pnas.1202604109 (2012). Article ADS PubMed PubMed
Central Google Scholar * Bhaskara, R. M. & Srinivasan, N. Stability of domain structures in multi-domain proteins. Sci Rep 1, 40, doi: 10.1038/srep3945340 (2011). Article ADS PubMed
PubMed Central Google Scholar * Wheelan, S. J., Marchler-Bauer, A. & Bryant, S. H. Domain size distributions can predict domain boundaries. Bioinformatics 16, 613–618 (2000). Article
CAS PubMed Google Scholar * Gavrilov, Y., Hagai, T. & Levy, Y. Nonspecific yet decisive: Ubiquitination can affect the native-state dynamics of the modified protein. Protein Sci 24,
1580–1592, doi: 10.1002/pro.2688 (2015). Article CAS PubMed PubMed Central Google Scholar * Sauer, R. T. & Baker, T. A. AAA + proteases: ATP-fueled machines of protein destruction.
Annu Rev Biochem 80, 587–612, doi: 10.1146/annurev-biochem-060408-172623 (2011). Article CAS PubMed Google Scholar * Hagai, T., Azia, A., Tóth-Petróczy, Á. & Levy, Y. Intrinsic
disorder in ubiquitination substrates. J Mol Biol 412, 319–324, doi: 10.1016/j.jmb.2011.07.024 (2011). Article CAS PubMed Google Scholar * Mustafi, S. M., Chen, H., Li, H., Lemaster, D.
M. & Hernández, G. Analysing the visible conformational substates of the FK506-binding protein FKBP12. Biochem J 453, 371–380, doi: 10.1042/BJ20130276 (2013). Article CAS PubMed
Google Scholar * Bai, G., Mo, H. & Shapiro, M. NMR evaluation of adipocyte fatty acid binding protein (aP2) with R- and S-ibuprofen. Bioorg Med Chem 16, 4323–4330, doi:
10.1016/j.bmc.2008.02.092 (2008). Article CAS PubMed Google Scholar * Schanda, P. & Brutscher, B. Very fast two-dimensional NMR spectroscopy for real-time investigation of dynamic
events in proteins on the time scale of seconds. J Am Chem Soc 127, 8014–8015, doi: doi: 10.1021/ja051306e (2005). Article CAS PubMed Google Scholar * Delaglio, F. et al. NMRPipe: a
multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6, 277–293 (1995). Article CAS PubMed Google Scholar * Vranken, W. F. et al. The CCPN data model for NMR
spectroscopy: development of a software pipeline. Proteins 59, 687–696, doi: 10.1002/prot.20449 (2005). Article CAS PubMed Google Scholar * Sugase, K., Konuma, T., Lansing, J. C. &
Wright, P. E. Fast and accurate fitting of relaxation dispersion data using the flexible software package GLOVE. J Biomol NMR 56, 275–283, doi: 10.1007/s10858-013-9747-5 (2013). Article CAS
PubMed PubMed Central Google Scholar * Szep, S., Park, S., Boder, E., Van Duyne, G. & Saven, J. Structural coupling between FKBP12 and buried water. Proteins 74, 603–611, doi:
10.1002/prot.22176 (2009). Article CAS PubMed PubMed Central Google Scholar * González, J. M. & Fisher, S. Z. Structural analysis of ibuprofen binding to human adipocyte fatty-acid
binding protein (FABP4). Acta Crystallogr F Struct Biol Commun 71, 163–170, doi: 10.1107/S2053230X14027897 (2015). Article CAS PubMed PubMed Central Google Scholar Download references
ACKNOWLEDGEMENTS This work was supported by Japan Agency for Medical Research and Development (AMED), by research fellowships from the Japan Society for the Promotion of Science for Young
Scientists (J.S.P.S.), and by the Honjo International Scholarship Foundation. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Molecular Engineering, Graduate School of
Engineering, Kyoto University, Kyoto-Daigaku Katsura, Nishikyo-Ku, 615-8510, Kyoto, Japan Daichi Morimoto, Kenji Sugase & Masahiro Shirakawa * Department of Molecular and Cellular
Physiology, Graduate School of Medicine, Kyoto University, Yoshida Konoe-cho, Sakyo-ku, 606-8501, Kyoto, Japan Erik Walinda * Center for Medical Education, Graduate School of Medicine, Kyoto
University, Yoshida Konoe-cho, Sakyo-ku, 606-8501, Kyoto, Japan Erik Walinda * Graduate School of Life and Environmental Sciences, Osaka Prefecture University, Naka-ku, Sakai, 599-8531,
Osaka, Japan Harumi Fukada Authors * Daichi Morimoto View author publications You can also search for this author inPubMed Google Scholar * Erik Walinda View author publications You can also
search for this author inPubMed Google Scholar * Harumi Fukada View author publications You can also search for this author inPubMed Google Scholar * Kenji Sugase View author publications
You can also search for this author inPubMed Google Scholar * Masahiro Shirakawa View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS D.M. and
E.W. designed the experiments and conducted most experiments. K.S. assisted with NMR analysis. H.F. performed DSC analysis. D.M., E.W., K.S. and M.S. wrote the manuscript. All authors
discussed the results and commented on the manuscript. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL
SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this
article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will
need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Morimoto, D., Walinda, E., Fukada, H. _et al._ Ubiquitylation Directly Induces Fold Destabilization of Proteins. _Sci Rep_ 6, 39453 (2016).
https://doi.org/10.1038/srep39453 Download citation * Received: 22 March 2016 * Accepted: 23 November 2016 * Published: 19 December 2016 * DOI: https://doi.org/10.1038/srep39453 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative
