Development of a novel model of hypertriglyceridemic acute pancreatitis in mice
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The morbidity rate of hypertriglyceridemic acute pancreatitis (HTG-AP) increased rapidly over the last decade. However an appropriate animal model was lacking to recapitulate this
complicated human disease. We established a novel mice model of HTG-AP by poloxamer 407 (P-407) combined with caerulein (Cae). In our study, serum triglyceride levels of P-407 induced mice
were elevated in a dose-dependent manner, and the pancreatic and pulmonary injuries were much severer in HTG mice than normal mice when injected with conventional dose Cae (50 ug/kg), what’s
more, the severity of AP was positively correlative with duration and extent of HTG. In addition, we found that a low dose Cae (5 ug/kg) could induce pancreatic injury in HTG mice while
there was no obvious pathological injury in normal mice. Finally, we observed that HTG leaded to the increased infiltrations of macrophages and neutrophils in mice pancreatic tissues. In
conclusion, we have developed a novel animal model of HTG-AP that can mimic physiological, histological, clinical features of human HTG-AP and it could promote the development of therapeutic
strategies and advance the mechanism research on HTG-AP. SIMILAR CONTENT BEING VIEWED BY OTHERS LIPROXSTATIN-1 ATTENUATES ACUTE HYPERTRIGLYCERIDEMIC PANCREATITIS THROUGH INHIBITING
FERROPTOSIS IN RATS Article Open access 25 April 2024 FERROPTOSIS EXACERBATES HYPERLIPIDEMIC ACUTE PANCREATITIS BY ENHANCING LIPID PEROXIDATION AND MODULATING THE IMMUNE MICROENVIRONMENT
Article Open access 21 May 2024 ACTIVATION OF AMPK AMELIORATES ACUTE SEVERE PANCREATITIS BY SUPPRESSING PANCREATIC ACINAR CELL NECROPTOSIS IN OBESE MICE MODELS Article Open access 30
September 2023 INTRODUCTION Acute pancreatitis (AP) is a common and devastating inflammatory condition of the pancreas that is considered to have the characteristics of acute onset, rapid
progression and high mortality, and its annual incidence rate is about 700 per million1. What’s more, most AP could involve peripancreatic tissues and other distant organs, and then develop
into serious secondary local and systemic complications, such as infected pancreatic necrosis (IPN), acute respiratory distress syndrome (ARDS), acute kidney injury (AKI) and sepsis. The
main causes of AP include biliary tract disease, alcoholism, mechanical injury, hypertriglyceridemia (HTG), drug and infection2. Clinical researches in Europe have showed that biliary
pancreatitis and alcoholic pancreatitis account for 37.1% and 41% of total incidence respectively3. With further studies for the etiology of AP, it was found that HTG has been the third
major cause of AP following gallstone and alcohol over the last decade, and accounts for about 4–10% of incidence of total AP4,5. Especially in China, the morbidity rate could reach up to
15–20%6. A study has showed that the onset risk of AP was about 5% when serum triglycerides (TG) level >1000 mg/dl, and increased dramatically up to 10–20% when the serum TG level
>2000 mg/dl7. The current international consensus strongly suggests these AP patients with serum triglyceride level >1000 mg/dl to have hypertriglyceridemic acute pancreatitis
(HTG-AP)8. Compared with acute gallstone pancreatitis, HTG-AP has the characteristics of more complications and higher recurrence rate. The current literature on HTG-AP mainly focus on the
analysis of clinical characteristics and there is less mechanism research that may be due to the lack of appropriate animal model for HTG-AP. The Lipoprotein Lipase (LPL) activity of mice
and rats is so high that simply feeding high-fat diet can’t establish ideal animal model of HTG (TG level >1000 mg/dl). what’s more, currently reported genetically modified mice which
used in the study of HTG-AP, such as LPL deficient mice9,10,11, human-apolipoprotein CIII transgenic (ApoCIII-tg) mice12,13 is difficult to get. Therefore, there is an urgent need to develop
new HTG-AP animal models to promote the study of pathogenesis and specific prevention measures of HTG-AP. Poloxamer 407 (P-407) is a hydrophilic triblock copolymer comprised of
polyoxyethylene and polyoxypropylene units and has been reported to induce HTG with little side effects14. P-407 can increase the serum triglyceride concentrations by directly inhibiting the
activity of both LPL and hepatic lipase, which were combined with the capillary wall15,16. Physiological toxicity of P-407 is so low that both short-term and long-term use can induce high
serum triglyceride levels in mice14. Saja _et al_.17 found that serum triglyceride level of mice could rise up to 4000 mg/dl after treated with P-407 for 28 days and long-term HTG can cause
lipid deposition in heart, liver and kidney with infiltration of macrophages and other pathological changes. Therefore we put forward that using P-407 to establish HTG model, then inducing
AP by intraperitoneal injection caerulein (Cae) to build a HTG-AP mice model which provides feasibility for the mechanism study of HTG-AP. RESULTS P-407 INDUCED SEVERE HTG IN MICE Consistent
with the previous outcomes of Professor Saja17, we found that P-407 could elevate the serum ApoCIII levels which affected the metabolism of triglyceride and induced hypertriglyceridemia
(Fig. S1A). The result of fast protein liquid chromatography (FPLC) further validated this phenomenon and indicated that hyperlipidemia in mice induced by P-407 was mainly composed of very
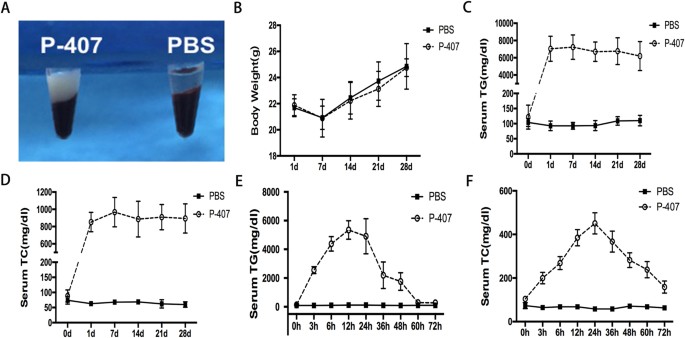
low-density lipoprotein (Fig. S1C,D). After one single intraperitoneal injection of high dose (0.5 g/kg) P-407, serum triglyceride and cholesterol levels of mice increased rapidly and the
peak values appeared around 12 h and 24 h after the injection respectively, then declined slowly and finally returned to normal values after 72 hours (Fig. 1E,F), While after the injections
of low dose (0.1 g/kg, 0.25 g/kg) P-407, the peak values of HTG levels declined and metabolic elimination time had been moved up significantly (Fig. S2A,B). In addition, we observed mice HTG
models by long-term P-407 induction in different doses (0.1 g/kg, 0.25 g/kg, 0.5 g/kg) and found that there was a positive correlation between the severity of hypertriglyceridemia and P-407
doses (Fig. 1C,D and Fig. S2C,D). 28 days after continuous intraperitoneal injection of 0.5 g/kg P-407, the serum of P-407 induced mice became obviously lactescent (milky coloration, Fig.
1A) and the serum triglyceride levels in more than 80% mice were higher than 6000 mg/dl (Fig. 1C), which were more than 50-folds higher than PBS control group. To investigate the safety of
P-407, we examined the liver and kidney functions of the P-407 group, and continuously recorded the body weight of mice of the P-407 group and PBS group. P-407 treatment exerted no effects
on body weight (Fig. 1B), as well as serum alanine transaminase level, serum creatinine level and other liver or kidney function indexes (Fig. S3). HTG AGGRAVATED PANCREATIC INJURY OF AP
Firstly, in order to assess the effects of different extents of HTG exerted on AP, we adopted the above mentioned three doses P-407 to establish mice high triglyceride levels in three
gradients. After induction of AP with standard dose Cae (50 ug/kg). we observed that AP severity was positively associated with serum triglyceride levels rather than the serum amylase levels
(Fig. S5) and this was consistent with the clinical characteristics of HTG - AP18,19. In view of the most remarkable injury of mice AP induced by high dose P-407, hence, we adopted the dose
(0.5 g/kg) as the follow-up experiment research dose. Next, to gain insights into the influence of different durations of HTG on AP, we divided HTG mice into three groups: transient 24 h
HTG group induced by single intraperitoneal injection of P-407; short-term HTG group induced by the 7 days injection of P-407 and long-term HTG group induced by the 28 days injection of
P-407. After the induction of standard dose Cae (50 ug/kg), compared with PBS+Cae group, all three groups of HTG can deteriorate the pancreatic injury degree. Transient HTG mice were mainly
characterized as edema, inflammatory cells infiltration without obvious necrosis, while the necrosis of short-term and long-term HTG mice were significantly severer and the pathological
damage degree of pancreas increased evidently with the prolongation of HTG duration (Fig. 2A,B and Fig. S6). Given the most serious pathological damage took place 28 days post P-407
injection, we chose this long-term HTG model to observe the dynamical changes of pancreas at 4, 8 and 12 h after standard dose Cae (50ug/kg) injection. Indeed, the pathological changes of
P-407 + Cae group mice increased over time and were much higher than PBS + Cae group mice at each time point (Fig. S7). To our surprise, the pancreatic injuries of P-407 + Cae group mice at
8 h after Cae injection were even severer than those of PBS+Cae group mice at 12 h. AP is a kind of disease in which pancreatic local inflammation progresses into severe systemic
inflammatory responses, therefore, local and systemic inflammatory levels are commonly used to assess the severity of AP. We used ELISA to detect the serum levels of inflammatory cytokines
as well as the levels in pancreatic tissue and it turned out to be consistent with the pathological results (Fig. 2C,D). Myeloperoxidase (MPO) is mainly expressed in neutrophils and could be
used as a biomarker of activated neutrophils, Immunohistochemistry examination for MPO of pancreatic tissue indicated that P-407 + Cae group mice had more neutrophils infiltration compared
with PBS + Cae group mice. (Figure 3B,D). Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) staining study, which could detect cell apoptosis, revealed
that pancreatic acinar cells in P-407 + Cae group were involved in more extensive apoptosis process (Fig. 3A,C). In addition, to validate the above findings, we also adopted the Institute of
Cancer Research (ICR) mice strains and the results match that in the C57BL/6 mice, but the inflammation degree of pancreas in ICR mice was severer than that in C57BL/6 mice, implying that
ICR mice are more susceptible to AP than C57BL/6 mice (Fig. S4). HTG EXACERBATED THE SEVERITY OF ACUTE LUNG INJURY IN AP Acute lung injury (ALI) is one of the most common complications of
severe acute pancreatitis (SAP) and patients with SAP are usually required to have mechanical ventilation. Previous studies have indicated that the incidence of acute lung injury in patients
with HTG-AP was significantly higher than that in other types of AP18,20. As expected, there were obvious pathological changes in lungs of P-407 + Cae group mice in comparison with the PBS
+ Cae group mice. A large number of inflammatory cell infiltration and capillary congestion in the alveolar septum were observed in lung of P-407 + Cae group mice and the total pathological
scores of lung in P-407 + Cae group mice were significantly higher than that in PBS + Cae group mice (Fig. 4). HTG INCREASED SUSCEPTIBILITY TO AP To explore the role of HTG in AP, we first
treated normal mice with 10 intraperitoneal injections of Cae at five consecutive gradient doses (1 ug/kg, 2.5 ug/kg, 5 ug/kg, 10 ug/kg and 20 ug/kg b.w) at hourly intervals to induce AP.
Histological examination results showed that pancreas of mice in 10 ug/kg group had obvious inflammatory changes (Fig. S8). While in 5 ug/kg group mice, apart from the slight elevation in
monocyte chemotactic protein-1 (MCP-1) levels in pancreatic tissue (Fig. 5C), we failed to observe the typical pathological changes of AP and there was no significant difference in
pathological scores between the 5 ug/kg group mice and the normal mice (Fig. 5A,B). Next we treated P-407 group mice with Cae at dose of 5 ug/kg b.w and obvious inflammatory cells
infiltration and edema were observed in sections of pancreatic tissue (Fig. 5A,B and Fig. S9) along with the remarkable elevation of inflammatory cytokines levels both in the serum and
pancreatic tissue (Fig. 5C,D), which demonstrated that HTG increases the susceptibility of mice to AP. To strengthen the above findings, we treated P-407 group mice with Cae at doses of 10
ug/kg and 20 ug/kg b.w and PBS group mice with the Cae dose of 50 ug/kg b.w. There is no significant difference in total pathological changes between 20 ug/kg Cae induced P-407group mice and
50 ug/kg Cae induced PBS group mice (Fig. S10), suggesting HTG increases susceptibility to AP from the other side. HTG PROMOTED THE INFILTRATION OF MACROPHAGES AND NEUTROPHILS IN PANCREAS
In order to explore the underlying mechanism of HTG increasing susceptibility to AP, we examined the alterations of immune cell states in pancreatic tissue. It had been observed that the
percentages of CD45+F4/80+, CD45+Gr-1+ cells increased significantly after the long-term P-407 administration, which implying that HTG for a long time caused the infiltration of macrophages
and neutrophils and generated local inflammatory microenvironment in pancreas, which this was in line with the elevated inflammatory cytokines levels in pancreatic tissue (Fig. 6).
DISCUSSION Our study successfully established a novel mice model of HTG-AP with P-407 joint Cae. Through this model, we found that HTG can aggravate pancreas and lung injury under the
condition of AP. Meanwhile, we for the first time put forward that HTG could increase susceptibility to AP from the aspect of animal experiments. AP is common and fatal acute inflammation of
pancreas, whose global incidence increased year by year. Although most cases only feature as mild inflammatory change, there are still about 20% cases developing into critical illness and
the mortality rate exceeds 30%21. HTG is one of the most common etiologies of AP while the present studies concerning the mechanism of HTG-AP are in slow progress worldwide, which may be
related to the lack of appropriate animal models. All the reported animal models of HTG-AP have defects to varying degrees. In 1996, Friess _et al_.22 built a rat model of HTG-AP with
TritonWR 1339 by tail vein injection, and plasma triglyceride level in this model could reach up to about 1000 mg/dl, but only lasting for 24 hours and it was difficult to keep the stable
state of high blood triglycerides, what’s more, TritonWR 1339 was really expensive. LPL genetically deficient mink23,24 and high-fat diet hamster25 both existed HTG, but the absence of
antibodies was not in favor of the immunology and mechanism research on AP25. Along with the development of gene modification technology in recent years, the problem that stable and
effective HTG-AP model can’t be established simply relying on drugs or high fat diet, has been solved by genetically modified animals, such as LPL-deficient mice9,10,11 and ApoCIII-tg
mice12,13. However, genetically modified animals still cannot be used widely because of high cost of breeding, difficult reproduction, mismatch with human plasma lipids and so on. Therefore,
there is an urgent need to establish a novel stable, effective and simple HTG-AP model. Our model simulated mice HTG with intraperitoneal injection of P-407, and then induced AP by
intraperitoneal injection of Cae. Although P-407 is also a non-ionic surface active agent, like TritonWR 1339, but the physiological toxicity of P-407 is low and the plasma triglyceride
level of mice can be maintained stably at 5000 mg/dl after the prolonged stimulation with P-40714. Compared with genetically modified animal models, this novel HTG model has the advantages
of simple operation, low cost and plasma lipid matching with HTG-AP patients, consequently imitating the pathophysiological process of such patients well. The clinical manifestations of
HTG-AP have no significant differences with other types of AP, but generally it has worse prognosis, longer length of stay in hospital and higher morbidity rate of complications. Lindkvist
_et al_.8 showed that HTG was an independent risk factor for persistent organ failure in patients with AP. Pathological examination in our study has demonstrated that severity of pancreatic
injury, inflammatory cell infiltration and other pathological changes in the HTG group were significantly higher than those in the control group and positively correlated with the extents
and durations of serum triglyceride levels. More importantly, for the first time, in our study we proved that HTG could increase the susceptibility to AP in mice. Previous clinical studies
stated that the serum pro-inflammatory cytokines were remarkably elevated in HTG patients26,27, consistently, Liu _et al_.12 who established HTG-AP model with ApoCIII-tg mice joint Cae, also
declared that the monocyte migration and pancreatic injury, along with the expression of inflammatory cytokines, such as TNF-a, IL-6, MCP-1 of HTG group were significantly higher than those
of the wild type mice. Saja _et al_.17 also verified that long-term HTG could cause lipid deposition and macrophages infiltration in heart, liver and kidney. Our results were consistent
with the conclusion of predecessors that we found that the pro-inflammatory cytokines TNF-a, IL-6, MCP-1,IL-1β both in blood and pancreatic tissues of the HTG mice were significantly higher
than normal mice, which indicating that HTG mice possessed local and systemic inflammatory responses. At the same time, Flow Cytometry results manifested that HTG leaded to the increased
infiltrations of macrophages and neutrophils in mice pancreatic tissues. Collectively, the pancreatic local inflammatory microenvironment and systemic inflammation predisposed the HTG mice
to get AP and make AP severer. What’s more, we have sound reasons to believe that severer pancreatic injury, mononuclear cell migration and increasing expression of inflammation cytokines
are common phenomenon of different HTG model, which implies that the pathophysiological changes of pancreas are caused mainly by HTG rather than the toxic effect of P-407. In conclusion,
P-407 joint Cae can build stable and controllable mice model of severe HTG-AP, at the same time, HTG can increase the susceptibility to AP and aggravate the injury of pancreas and lung under
the condition of AP. MATERIALS AND METHODS ANIMALS AND DIETS Male mice in C57BL/6 background weighing approximately 20–25 g were purchased from Model Animal Research Center of Nanjing
University (Nanjing, China). All mice were housed in a SPF standard room under 12/12 h light-dark cycle at 24 °C, given water ad libitum, fed standard laboratory chow and were allowed to
acclimatize for a minimum of 1 week. All methods were carried out in accordance with The Principles of L_aboratory Animal Care_ (NIH publication no. 85Y23, revised 1996); All experimental
protocols were approved by the experimental animal ethics committee of Jinling Hospital affiliated to medical School of Nanjing University (No. 20151008). Meanwhile, in order to verify
whether the model is universal in different mice strains, male mice in ICR background weighing approximately 28–32 g were also used in this research. EXPERIMENTAL DESIGN AND PROCEDURES The
HTG model was developed by administering P-407 (Pluronic F-127, Sigma-Aldrich Co., St. Louis, MO, USA) intraperitoneally to mice each other day at the dose level of 0.1, 0.25,0.5 g/kg body
weight (b.w), the control group was administered PBS equivalently in the same amount. P-407 was mixed with phosphate buffered saline (PBS; pH = 7.4) and refrigerated at 4 °C overnight to
dissolve completely. One single intraperitoneal injection of P-407 could establish the transient HTG model, short-term HTG model was established via 7 consecutive dosing days, and long-term
HTG model is set up via 28 consecutive dosing days. C57BL/6 mice were randomly assigned to 4 groups: PBS, P-407, PBS + Cae and P-407 + Cae. AP was induced by 10 intraperitoneal injections of
Cae (AnaSpec, Inc., Fremont, USA) b.w in PBS at hourly intervals, and the control group injected with PBS in the same way. Blood samples were obtained from the tail veins of
isoflurane-anesthetized mice at different hours after the first Cae injection. Then animals were anaesthetized with an intraperitoneal administration of sodium pentobarbital (50 mg/Kg) and
sacrificed, and pancreatic tissues, along with pulmonary tissues, were taken and fixed in 4% paraformaldehyde in PBS and embedded in paraffin. MEASUREMENT OF PLASMA LIPIDS AND LIPOPROTEIN
Total cholesterol (TC) and triglyceride levels were determined with a commercially available kit (Beijing Zhongsheng Beikong Biochemistry Company, PR China) according to the manufacturer’s
protocol. For determination of the lipids distributed in plasma lipoprotein, FPLC was performed with 200lL of pooled plasma from 10 mice per group, using a Superose 6 column (Amersham
Bioscience) as described previously28. Forty fractions of 0.5 mL each were collected and enzymatically assayed for TC and triglyceride content. PLASMA BIOCHEMICAL ASSAY The plasma of mice
was centrifuged at 28,000 rpm for 30 minutes at 4 °C to remove chylomicrons. Amylase activity was measured by 5-ethylidene-G7PNP as a substrate with a commercial kit (Beijing Zhongsheng
Beikong Biochemistry Company, PR China), and lipase activity was also measured with a commercial kit (Nanjing Jiancheng Biochemistry Company, PR China), as described in the manual from the
manufacturer. Serum alanine-aminotransferase (ALT), total bilirubin (TBIL), creatinine (Cr) and urea nitrogen (BUN) were measured with dry chemistry method in General Surgery Biochemistry
Laboratory of Jinling Hospital (AU680 automatic biochemical analysis system, Beckman Coulter Inc., USA). Serum ApoCIII levels (Cloud-Clone Crop., Wuhan, PR China) and free fatty acids(Wako
Pure Chemical Industries, Ltd., Osaka, Japan) were determined according to the manual from the manufacturer. HISTOLOGICAL EXAMINATION The Paraffin sections of pancreas and lung tissue were
stained with hematoxylin and eosin. Two investigators who were blind to the experimental treatment scored the degree of pancreatic injury by light microscopy, evaluating the severity of
edema, inflammation and necrosis, as we described previously in Table 125,26. We also scored the degree of pulmonary injury by evaluating the severity of neutrophil infiltration, thickness
of alveolar and alveolar congestion, and the scoring standards were described previously as in Table 227. INFLAMMATORY CYTOKINES MEASUREMENT Briefly, we homogenated pancreatic tissue in PBS
and then carried out centrifugation (12000 rpm, 4 °C, 30 min) to get supernatant, the serum TNF-a, IL-6, MCP-1,IL-1β levels were measured with a commercial kit (Affymetrix ebioscience,
Santiago, USA). IMMUNOHISTOCHEMICAL EXAMINATION AND TUNEL STAINING The slices from paraffin-embedded tissues were subjected to immunohistochemical staining for myeloperoxidase (MPO). The
prepared slices were washed in PBS for 10 min and then boiled in 0.01 mmol citrate buffer (pH = 6) for 10 min for antigen retrieval. After incubation with hydrogen peroxide for 10 min, 5%
bovine serum albumin (BSA) was applied as the blocking solution for 20 min at room temperature. Without washing, the sections were incubated with anti-Myeloperoxidase antibody (1:100)
(ab9535, Abcam, Cambridge, UK) overnight at 4 °C. After being rinsed with PBS, the sections were incubated with goat anti-rabbit secondary antibody (1:500) (ab150079, Abcam, Cambridge, UK)
and then visualised using a 3, 3-diaminobenzidine (DAB) kit (AR1022, Boster, Wuhan, China). Finally, images were recorded using a microscope at 100× magnification (IX73, Olympus, Tokyo,
Japan). The TUNEL staining for apoptosis operation was performed with a commercial cell death detection kit purchased from Roche Diagnostics (Indianapolis, USA) according to the
manufacturer’s protocol. The stained slices were observed by microscopy (IX73, Olympus, Tokyo, Japan) and images were recorded. ISOLATION OF PANCREATIC IMMUNE CELLS OF MICE AND FLOW
CYTOMETRY Pancreatic immune cells were isolated using collagenase IV digestion method described by J Xue. _et al_. for flow cytometry analysis29. All antibodies used for flow cytometry were
purchased from BD Biosciences, unless indicated. For surface staining, cells were collected and stained with CD3, CD45, CD 11 C, F4/80, Gr-1 antibodies. The labeled cells were analyzed by
flow cytometry using CellQuest (BD FACSCalibur) or FACS Diva (BD FACSAria software). STATISTICAL ANALYSIS Statistical analysis was performed by SPSS 22.0 software. Results are presented as
mean ± standard deviation (SD). The data of biochemistry measurements were analyzed with a one-way analysis of variance and the Student-Newman-Keuls test. In the histological evaluation, the
results were analyzed by a Mann-Whitney rank sum test, and P < 0.05 was considered statistically significant. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Pan, Y. _et al_.
Development of a novel model of hypertriglyceridemic acute pancreatitis in mice. _Sci. Rep._ 7, 40799; doi: 10.1038/srep40799 (2017). PUBLISHER'S NOTE: Springer Nature remains neutral
with regard to jurisdictional claims in published maps and institutional affiliations. REFERENCES * Tenner, S., Baillie, J., DeWitt, J., Vege, S. S. & American College of, G. American
College of Gastroenterology guideline: management of acute pancreatitis. _The American journal of gastroenterology_ 108, 1400–1415; 1416, doi: 10.1038/ajg.2013.218 (2013). Article CAS
Google Scholar * Karsidag, T., Tuzun, S. & Makine, C. Domino effect from hypertriglyceridemia to sinistral portal hypertension. _Chirurgia (Bucharest, Romania: 1990)_ 104, 219–222
(2009). CAS Google Scholar * Gullo, L. et al. Acute pancreatitis in five European countries: etiology and mortality. _Pancreas_ 24, 223–227 (2002). Article Google Scholar * Scherer, J.,
Singh, V. P., Pitchumoni, C. S. & Yadav, D. Issues in hypertriglyceridemic pancreatitis: an update. _Journal of clinical gastroenterology_ 48, 195–203, doi:
10.1097/01.mcg.0000436438.60145.5a (2014). Article CAS PubMed PubMed Central Google Scholar * Valdivielso, P., Ramirez-Bueno, A. & Ewald, N. Current knowledge of
hypertriglyceridemic pancreatitis. _European journal of internal medicine_ 25, 689–694, doi: 10.1016/j.ejim.2014.08.008 (2014). Article CAS PubMed Google Scholar * Huang, Y. X. et al.
Incidence and clinical features of hyperlipidemic acute pancreatitis from Guangdong, China: a retrospective multicenter study. _Pancreas_ 43, 548–552, doi: 10.1097/MPA.0000000000000069
(2014). Article PubMed Google Scholar * Christian, J. B., Bourgeois, N., Snipes, R. & Lowe, K. A. Prevalence of severe (500 to 2,000 mg/dl) hypertriglyceridemia in United States
adults. _The American journal of cardiology_ 107, 891–897, doi: 10.1016/j.amjcard.2010.11.008 (2011). Article CAS PubMed Google Scholar * Lindkvist, B., Appelros, S., Regner, S. &
Manjer, J. A prospective cohort study on risk of acute pancreatitis related to serum triglycerides, cholesterol and fasting glucose. _Pancreatology : official journal of the International
Association of Pancreatology (IAP) … [et al.]_ 12, 317–324, doi: 10.1016/j.pan.2012.05.002 (2012). Article CAS Google Scholar * Tang, M. et al. A serum metabolomic investigation on
lipoprotein lipase-deficient mice with hyperlipidemic pancreatitis using gas chromatography/mass spectrometry. _Biomedical reports_ 1, 469–473, doi: 10.3892/br.2013.78 (2013). Article CAS
PubMed PubMed Central Google Scholar * Wang, Y. et al. Enhanced susceptibility to pancreatitis in severe hypertriglyceridaemic lipoprotein lipase-deficient mice and agonist-like function
of pancreatic lipase in pancreatic cells. _Gut_ 58, 422–430, doi: 10.1136/gut.2007.146258 (2009). Article CAS PubMed Google Scholar * Yang, F. et al. The role of free fatty acids,
pancreatic lipase and Ca+ signalling in injury of isolated acinar cells and pancreatitis model in lipoprotein lipase-deficient mice. _Acta physiologica_ 195, 13–28, doi:
10.1111/j.1748-1716.2008.01933.x (2009). Article CAS PubMed Google Scholar * Liu, J. et al. FTY720 Attenuates Acute Pancreatitis in Hypertriglyceridemic Apolipoprotein CIII Transgenic
Mice. _Shock_ 44, 280–286, doi: 10.1097/SHK.0000000000000400 (2015). Article CAS PubMed Google Scholar * Ehx, G. et al. Liver proteomic response to hypertriglyceridemia in
human-apolipoprotein C-III transgenic mice at cellular and mitochondrial compartment levels. _Lipids in health and disease_ 13, 116, doi: 10.1186/1476-511X-13-116 (2014). Article CAS
PubMed PubMed Central Google Scholar * Sharyo, S., Kumagai, K., Yokota-Ikeda, N., Ito, K. & Ikeda, M. Amelioration of renal ischemia-reperfusion injury by inhibition of IL-6
production in the poloxamer 407-induced mouse model of hyperlipidemia. _Journal of pharmacological sciences_ 110, 47–54 (2009). Article CAS Google Scholar * Wasan, K. M. et al. Poloxamer
407-mediated alterations in the activities of enzymes regulating lipid metabolism in rats. _Journal of pharmacy & pharmaceutical sciences: a publication of the Canadian Society for
Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques_ 6, 189–197 (2003). CAS Google Scholar * Johnston, T. P. The P-407-induced murine model of dose-controlled
hyperlipidemia and atherosclerosis: a review of findings to date. _Journal of cardiovascular pharmacology_ 43, 595–606 (2004). Article CAS Google Scholar * Saja, M. F. et al.
Triglyceride-Rich Lipoproteins Modulate the Distribution and Extravasation of Ly6C/Gr1(low) Monocytes. _Cell reports_ 12, 1802–1815, doi: 10.1016/j.celrep.2015.08.020 (2015). Article CAS
PubMed PubMed Central Google Scholar * Nawaz, H. et al. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. _The American
journal of gastroenterology_ 110, 1497–1503, doi: 10.1038/ajg.2015.261 (2015). Article CAS PubMed Google Scholar * Zhang, X. L., Li, F., Zhen, Y. M., Li, A. & Fang, Y. Clinical Study
of 224 Patients with Hypertriglyceridemia Pancreatitis. _Chinese medical journal_ 128, 2045–2049, doi: 10.4103/0366-6999.161361 (2015). Article PubMed PubMed Central Google Scholar *
Lechleitner, M. et al. [Hypertriglyceridemia and acute pancreatitis]. _Acta medica Austriaca_ 21, 125–128 (1994). CAS PubMed Google Scholar * Famularo, G., Minisola, G. & De Simone,
C. Acute pancreatitis. _The New England journal of medicine_ 355, 961; author reply 961, doi: 10.1056/NEJMc061618 (2006). Article CAS PubMed Google Scholar * Hofbauer, B. et al.
Hyperlipaemia intensifies the course of acute oedematous and acute necrotising pancreatitis in the rat. _Gut_ 38, 753–758 (1996). Article CAS Google Scholar * Lindberg, A. et al. A
mutation in the lipoprotein lipase gene associated with hyperlipoproteinemia type I in mink: studies on lipid and lipase levels in heterozygotes. _International journal of molecular
medicine_ 1, 529–538 (1998). CAS PubMed Google Scholar * Nordstoga, K. et al. Pancreatitis associated with hyperlipoproteinaemia type I in mink (Mustela vison): earliest detectable
changes occur in mitochondria of exocrine cells. _Journal of comparative pathology_ 134, 320–328, doi: 10.1016/j.jcpa.2006.01.003 (2006). Article CAS PubMed Google Scholar * Hu, G. et
al. Development of a novel model of hypertriglyceridemic acute pancreatitis in hamsters: protective effects of probucol. _Pancreas_ 41, 845–848, doi: 10.1097/MPA.0b013e318247d784 (2012).
Article CAS PubMed Google Scholar * Bosques-Padilla, F. J. et al. Hypertriglyceridemia-induced pancreatitis and risk of persistent systemic inflammatory response syndrome. _The American
journal of the medical sciences_ 349, 206–211, doi: 10.1097/MAJ.0000000000000392 (2015). Article PubMed Google Scholar * Mirhafez, S. R. et al. Association between serum cytokine
concentrations and the presence of hypertriglyceridemia. _Clinical biochemistry_ 49, 750–755, doi: 10.1016/j.clinbiochem.2016.03.009 (2016). Article CAS PubMed Google Scholar * Wei, J.
et al. Characterization of a hypertriglyceridemic transgenic miniature pig model expressing human apolipoprotein CIII. _The FEBS journal_ 279, 91–99, doi: 10.1111/j.1742-4658.2011.08401.x
(2012). Article CAS PubMed Google Scholar * Xue, J. et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. _Nature communications_ 6, 7158, doi:
10.1038/ncomms8158 (2015). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS Supported by National Natural Science Foundation of China (81570584),
the National Natural Science Foundation of Yangzhou city (SQN20140063), the Postdoctoral Science Foundation of China (2014M562664). All authors of this paper have no conflict of interests to
disclose. At last, we want to express our sincere thankfulness to Pro. Gong for her generous help with flow Cytometry experiments and Dr. Dong for his assistance with FPLC examination.
AUTHOR INFORMATION Author notes * Yiyuan Pan, Yong Li and Lin Gao: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Department of General Surgery, Surgical
Intensive Care Unit (SICU), Jinling Hospital, Medical School of Nanjing University, No. 305 Zhongshan East Road, Nanjing, 210002, Jiangsu Province, China Yiyuan Pan, Yong Li, Lin Gao, Zhihui
Tong, Bo Ye, Baiqiang Li, Yizhe Chen, Qi Yang, Lei Meng, Guotao Lu, Weiqin Li & Jieshou Li * Biosciences Division, Center for Immunology and Infectious Diseases, SRI International,
Harrisonburg, 22802, VA, USA Shufeng Liu * Institute of Cardiovascular Science, Key Laboratory of Molecular Cardiovascular Science Ministry of Education, Institute of Cardiovascular Science,
Peking University, Beijing, 100191, China , Yuhui Wang & George Liu * Department of Gastroenterology, The Second Clinical Medical College, Yangzhou University, Yangzhou, China Guotao Lu
Authors * Yiyuan Pan View author publications You can also search for this author inPubMed Google Scholar * Yong Li View author publications You can also search for this author inPubMed
Google Scholar * Lin Gao View author publications You can also search for this author inPubMed Google Scholar * Zhihui Tong View author publications You can also search for this author
inPubMed Google Scholar * Bo Ye View author publications You can also search for this author inPubMed Google Scholar * Shufeng Liu View author publications You can also search for this
author inPubMed Google Scholar * Baiqiang Li View author publications You can also search for this author inPubMed Google Scholar * Yizhe Chen View author publications You can also search
for this author inPubMed Google Scholar * Qi Yang View author publications You can also search for this author inPubMed Google Scholar * Lei Meng View author publications You can also search
for this author inPubMed Google Scholar * Yuhui Wang View author publications You can also search for this author inPubMed Google Scholar * George Liu View author publications You can also
search for this author inPubMed Google Scholar * Guotao Lu View author publications You can also search for this author inPubMed Google Scholar * Weiqin Li View author publications You can
also search for this author inPubMed Google Scholar * Jieshou Li View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS W.L. and G.L. formulated
the idea of the paper and supervised the research, reviewed and revised the manuscript. Y.P., Y.L. and L.G. performed the research and wrote the manuscript. Z.T., B.Y. and S.L. provided
comments and technical advice. B.L., Y.C., Q.Y. and L.M. participated in preparing figures, Tables and data analyzing. J.L., Y.W. and G.L. revised the manuscript and provided comments. All
authors reviewed the manuscript. CORRESPONDING AUTHORS Correspondence to Guotao Lu or Weiqin Li. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (PDF 1474 KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or
other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the
Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Pan, Y., Li, Y., Gao, L. _et al._ Development of a novel model of
hypertriglyceridemic acute pancreatitis in mice. _Sci Rep_ 7, 40799 (2017). https://doi.org/10.1038/srep40799 Download citation * Received: 14 September 2016 * Accepted: 12 December 2016 *
Published: 12 January 2017 * DOI: https://doi.org/10.1038/srep40799 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
