Deep brain stimulation for treatment-resistant major depressive disorder: a comparison of two targets and long-term follow-up
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT We previously found that electrical stimulation in the anterior limb of the internal capsule/bed nucleus of the stria terminalis (IC/BST) alleviates depressive symptoms in severe
treatment-resistant obsessive-compulsive disorder (OCD) patients. Here we tested the hypothesis that electrical stimulation in either IC/BST or in the inferior thalamic peduncle (ITP)
effectively reduces depressive symptoms in treatment-resistant major depressive disorder (TRD). In a double-blind crossover design, the effects of electrical stimulation at both targets were
compared in TRD patients. The 17-item Hamilton Depression Rating scale (HAM-D) was the primary outcome measure. During the first crossover, patients received IC/BST stimulation versus no
stimulation in random order (2 × 1 weeks). During the second crossover (3 × 2 months), patients received IC/BST versus ITP versus no stimulation. Patients and evaluators were blinded for
stimulation conditions. All patients (_n_=7) were followed up for at least 3 years (3–8 years) after implantation. Six patients completed the first crossover and five patients completed the
second. During the first crossover, mean (s.d.) HAM-D scores were 21.5 (2.7) for no stimulation and 11.5 (8.8) for IC/BST stimulation. During the second crossover, HAM-D scores were 15.4
(7.5) for no stimulation, 7.6 (3.8) for IC/BST stimulation and 11.2 (7.5) for ITP stimulation. The final sample size was too small to statistically analyze this second crossover. At last
follow-up, only one patient preferred ITP over IC/BST stimulation. Two patients, with a history of suicide attempts before implantation, committed suicide during the follow-up phases of this
study. Our data indicate that, in the long term, both ITP and IC/BST stimulation may alleviate depressive symptoms in patients suffering from TRD. SIMILAR CONTENT BEING VIEWED BY OTHERS A
RANDOMISED, DOUBLE-BLIND, SHAM-CONTROLLED TRIAL OF DEEP BRAIN STIMULATION OF THE BED NUCLEUS OF THE STRIA TERMINALIS FOR TREATMENT-RESISTANT OBSESSIVE-COMPULSIVE DISORDER Article Open access
29 March 2021 BILATERAL HABENULA DEEP BRAIN STIMULATION FOR TREATMENT-RESISTANT DEPRESSION: CLINICAL FINDINGS AND ELECTROPHYSIOLOGICAL FEATURES Article Open access 03 February 2022
ELECTROCONVULSIVE THERAPY-INDUCED VOLUMETRIC BRAIN CHANGES CONVERGE ON A COMMON CAUSAL CIRCUIT IN DEPRESSION Article Open access 20 November 2023 INTRODUCTION Major depressive disorder (MDD)
is defined as the presence of depressed mood or anhedonia for more than 2 weeks, accompanied by additional symptoms (for example, suicidal thoughts, sleep disturbances and so on). MDD is a
burdensome disorder with a substantial and increasing impact on society,1, 2 and a lifetime prevalence of up to 17%,3 with recent findings suggesting an even higher prevalence.4 Treatment
strategies include pharmacotherapy,5 psychotherapy,6 repetitive transcranial magnetic stimulation (rTMS)7 and electroconvulsive therapy (ECT).8 Up to 30% of patients with MDD however do not
adequately respond to treatment. In these treatment-resistant MDD (TRD) patients, the disorder often takes a chronic, disabling course.9, 10 Functional imaging studies have resulted in a
better understanding of the brain regions involved in the pathology of MDD.11 This in turn has led to a search for treatment options targeting this dysfunctional neurocircuitry. Several
potential targets for deep brain stimulation (DBS) have been investigated: the subcallosal cingulate (SCC, Cg 25),12, 13, 14, 15, 16 nucleus accumbens (NAc),17 ventral capsule/ventral
striatum (VC/VS),18, 19 and supero-lateral medial forebrain bundle (MFB)20 might all be valuable in the treatment of TRD. Since 1998, we have been investigating DBS in the anterior limb of
the internal capsule and the bed nucleus of the stria terminalis (IC/BST), for obsessive-compulsive disorder (OCD).21, 22, 23, 24 A sustained, and often immediate, reduction in obsessions,
compulsions, anxiety and depressive mood was demonstrated in these OCD patients, particularly when the active stimulation contacts were situated in or near the BST.22 The BST is located in
the immediate vicinity of the VC/VS and NAc region that has been used in other DBS trials for psychiatric disorders, but meticulous neuroanatomical analyses have indicated that the
stimulated region in our patients does not or only partially overlaps with the target in VC/VS studies that provided details about their exact stimulation target.25 The BST is part of the
limbic system26, 27 and, for the present study, this target was mainly chosen because of the beneficial effects on comorbid depressive symptoms in our OCD patients.24 Moreover, a strong
theoretical argument can be made for the BST as a target for treating TRD, as it has projections to many of the above-mentioned structures (MFB, NAc)28 and might function as a relay center
in the processing of reward, stress and anxiety.29 The potential usefulness of this target is further underlined by the correlation of local field potential power in this area and symptom
severity in TRD patients.30 A potential disadvantage of DBS at IC/BST is the rather high charge density compared to typical stimulation parameters used for DBS in Parkinson’s disease,31
necessitating frequent replacements of the neurostimulators or the use of rechargeable batteries.22 To address this issue, we further explored the inferior thalamic peduncle (ITP) as a DBS
target. Bilateral ITP stimulation was reported to induce beneficial effects in one TRD patient.32 ITP connects the dorsomedial thalamus with the orbitofrontal cortex, which both show
hypermetabolism in depressed patients.33 ITP is a small structure and might require lower charge densities to be stimulated effectively (for a more detailed description of ITP as a target in
MDD patients, we refer to Velasco _et al._33) Because of the limited experience with ITP stimulation in TRD patients, we opted to directly compare IC/BST and ITP stimulation. We
hypothesized that electrical stimulation at both targets would be equally safe and effective, but that ITP stimulation would require lower charge densities, implying longer battery life. To
our knowledge, until now, there are no studies comparing different psychiatric DBS targets in the same patient. MATERIALS AND METHODS ELIGIBILITY CRITERIA FOR PARTICIPANTS Patients were
initially screened by a comprehensive review of their psychiatric history, obtained by interviewing the patient, family and treating psychiatrist and/or psychologist, as well as by examining
all the available records of previous psychiatric treatment. Inclusion criteria ensured the severity, treatment-refractoriness and incapacitating nature of MDD. Patients were considered
candidates for this trial if they were 18–65 years old, had MDD, unipolar type, diagnosed by the Structured Clinical Interview for DSM-IV (SCID-IV)34 and judged to be of disabling severity,
with a 17-item Hamilton Depression Rating scale (HAM-D) score of at least 19 and Global Assessment of Function (GAF) score of 45 or less. MDD had to be recurrent (>4 episodes) or chronic
(episode duration >2 years). Other requirements were a minimum of 5 years since the onset of the first depressive episode, with documented major impairment in functioning or potentially
severe medical outcomes (repeated hospitalizations, serious suicidal ideation or a history of previous suicide attempts or other self-injurious behavior). Treatment history was required to
prove failure in response to adequate trials of pharmacotherapy and ECT. For more detailed inclusion and exclusion criteria, see Supplementary Information. Medication was tapered off to a
bearable minimum and maintained on a stable regimen throughout the first year of DBS. Patients were recruited from Belgium and The Netherlands, and were screened and followed up both at the
psychiatric department of the University Hospital of Antwerp (until June 2007) and the University Hospitals of Leuven (from July 2007 onwards), and at the neurosurgical department of the
University Hospitals of Leuven. The protocol was approved by the medical ethics committees of the University of Antwerp and the University Hospitals of Leuven, and all patients provided
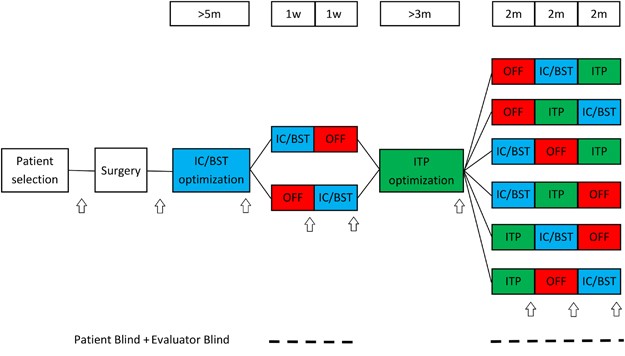
witnessed informed consent. CLINICAL TRIAL DESIGN For a schematic overview, see Figure 1. Four quadripolar leads (Medtronic, Minneapolis, MN, USA) were implanted; two bilaterally in IC/BST
and two bilaterally in ITP. Electrical stimulation was initiated 2–4 weeks after implantation. During a subsequent period of ~5 months, stimulation parameters (voltage, pulse width,
frequency and contacts used) of the anterior IC/BST electrodes were optimized. Clinical evaluation by the psychiatrist, considering both beneficial and adverse effects, was used to adjust
parameters. If a stable reduction of 50% was found on HAM-D scores for a period of several weeks, patients entered the first crossover (if after a period of 1 year no stable reduction in
HAM-D scores was found, patients continued to the crossover anyway). The first crossover consisted of 1 week of stimulation OFF and 1 week of IC/BST stimulation in randomized order. Our
prior experience with longer (that is, 6 months) blinded crossover studies in OCD patients learned that a substantial portion of the patients might refuse to enter or drop out during such a
long blinded crossover period.22 Therefore, the first crossover was included to optimize the chances of having an initial, brief double-blind evaluation of the hypothesized
depression-reducing effects of IC/BST stimulation in as many patients as possible. After the first crossover, patients received a second optimization period, during which parameters for ITP
stimulation were determined. After ITP optimization, patients continued to the second crossover. This crossover consisted of 2 months of IC/BST stimulation, 2 months of ITP stimulation and 2
months of stimulation OFF, again in a randomized order. As the change in stimulation condition at the start of each crossover phase may provoke a change in mood and potential appearance of
suicidal ideation, the patient could (if necessary) be hospitalized in a psychiatric ward, without breaking the blind. An escape procedure was implemented for the second crossover, because
of its longer, 2-month phases: if during one of these periods, mood deteriorated beyond baseline scores, or if both the patient and psychiatrist decided that the suffering of the patient
necessitated abbreviation of that period, this was discussed in the team, and after full psychiatric evaluation, the neurosurgeon started the next condition without unblinding patient or
evaluators. After the second crossover, patients could continue stimulation if they wished to do so using the target that provided the best result for the patient. Psychiatric visits were
scheduled every 2 weeks during the optimization periods, and at the end of each crossover phase. If necessary, patients could request additional consults at any time throughout the study.
After the crossover period, follow-up visits were planned according to clinical necessity. INTERVENTION After mounting the CRW stereotactic frame, T2-weighted magnetic resonance (MR) images
without contrast medium and MPRAGE images with contrast medium were acquired together with a computed tomography (CT) of the brain. Implantation of all four electrodes was performed in a
single session. Using routine stereotactic techniques under local anesthesia with sedation, the patients received quadripolar 3887-28 (patients C1–2), 3387 (C3–5) or 3391 (C6–7) leads
(Medtronic) in left and right IC/BST22, 35 and 3389 DBS electrodes (Medtronic) in left and right ITP. The ITP target was determined using the stereotactic atlas of Mai _et al._36 A
postoperative CT (C4, C6 and C7) or MR scan (1.5-T system with a send/receive head radiofrequency coil only and specific absorption rate limited to 0.4 W kg−1) confirmed the absence of
important intracranial hemorrhage and fusion with preoperative MRI allowed comparison between planned and actual trajectory of the leads. Short pulsed sequences were avoided and low specific
absorption rate was used on a 1.5 T MR to preclude potentially dangerous temperature rises around the electrodes. In C1, a 3389 lead could only be implanted in the right ITP as due to
vascularization, no trajectory that safely reached the left ITP could be found. In all other patients, four leads were implanted. OUTCOME MEASURES Extensive well-validated and reliable
measures (both clinician- and patient-rated) were taken at baseline and throughout the different stages of the study. The 17-item HAM-D37 was used to quantify the severity of depressive
symptoms and was the primary outcome measure of this study. Response to DBS was defined as a 50% decrease on the HAM-D compared to baseline17 and a score of 7 or less was defined as
remission.38 We also included secondary psychiatric measures and neuropsychological tests (for details see Supplementary Information). Evaluation time points of psychiatric outcome measures
are indicated with arrows in Figure 1. Neuropsychological measures were taken at baseline and at the end of each optimization and crossover phase. After the second crossover, psychiatric
outcome measures were evaluated regularly with a formal evaluation at least every 12 months including all secondary psychiatric measures. In addition, charge densities (per phase, per
contact) were evaluated for both stimulation targets.31 They were calculated using the contact surface area, voltage and pulse width from the second crossover and the impedances measured at
the start of each crossover phase. ELECTRODE POSITION To investigate the precise stimulation target in more detail, we transferred the center of the active contacts (cathodes) on Mai’s brain
atlas.22, 36 Using pre- and postoperative MR and CT, we localized the exact position of the cathodes for each patient during both crossover phases and at follow-up. For C4–C6–C7, we merged
preoperative MR and postoperative CT scans using Medtronic FrameLinkTM Software (Medtronic). For the remaining four patients, postoperative MR scans were used. All digital images were
manually reformatted along the intercommissural plane. Finally, contact positions were determined by two observers (LL & BN) taking into account all the available neuroanatomical
information and marked on the atlas plates. STATISTICAL METHODS The sample size for this study was calculated based upon the effects of DBS on comorbid depressive symptoms in six OCD
patients.24 Assuming an effect size of 2 and a within-patient standard deviation of 6.5 on the HAM-D scale, a sample size of seven patients in a crossover study with two conditions (ON and
OFF), results in 80% power at an alpha level of 0.05. We aimed to include 10 patients in this first exploratory trial, to account for dropout. However, the study was aborted before this
original aim was reached and the final sample size during crossovers was quite small (six and five patients). The originally proposed analysis was therefore not feasible. Instead, we
analyzed the primary outcome (HAM-D) using a paired _t_-test for the first crossover and follow-up periods and a repeated measures ANOVA for the second crossover, assuming the absence of
carry-over effects. Normality of residuals was tested for using a Shapiro–Wilk test. Because of the limited group sizes, we refrained from using any other inferential statistics. Descriptive
statistics are given for other outcome measures. IBM SPSS Statistics version 22 was used for all analyses. RESULTS PATIENTS Inclusion for the study started on 1 January 2005 and ended on 31
December 2009. During these 5 years, 53 patients were screened and 11 patients were eligible. Ultimately, 4 men and 3 women were willing to participate in this study (Table 1 and
Supplementary Table S1). Mean (s.d.) age at implantation was 50.0 (5.6). Participant flow is shown in Supplementary Figure S1. Patients required intensive follow-up by the neurosurgeon and
psychiatrist, with frequent patient contacts in the outpatient clinic, not only during the optimization and crossover periods, but also during follow-up. Mean number of outpatient visits to
the psychiatrist (including optimization of stimulation parameters) was 21.9 per year (range 14.4–32.0). Mean number of outpatient visits to the neurosurgeon (including replacement of
neurostimulators due to end of life of battery) was 4.2 per year (range 2.1–6.3) during follow-up. Patients required battery replacement on average every 14 months. To reduce the burden of
these surgeries, patients were implanted with a rechargeable system (Activa RC, Medtronic). OUTCOMES POSTOPERATIVE PERIOD Immediately after surgery and before neurostimulators were turned
on, mean (s.d.) HAM-D dropped to 14.4 (6.7). This effect was temporary and after a period of 2–4 weeks mean (s.d.) HAM-D scores increased to 22.3 (6.8). IC/BST OPTIMIZATION At the end of the
open optimization for the IC/BST leads, six out of seven patients were responders, and five were in remission. Stimulation parameters and HAM-D (mean (s.d.): 7.2 (3.6)) scores at the end of
the IC/BST optimization period are given in Supplementary Table S2. ITP OPTIMIZATION Two patients aborted the ITP optimization period prematurely due to the lack of beneficial effect and
were considered non-responders to DBS in ITP. They were not willing to participate in the second crossover. At the end of the open optimization for the ITP leads, four out of seven patients
were responders, and one of these was in remission. Parameters and HAM-D scores (mean (s.d.): 8.8 (2.7)) at the end of the ITP optimization period are given in Supplementary Table S2.
CROSSOVERS During the first crossover (_n_=6), mean (s.d.) HAM-D scores were 21.5 (2.7) for no stimulation (stimulator OFF) and 11.5 (8.8) for IC/BST stimulation (Figure 2). Four out of six
patients were responders to DBS in IC/BST. Remission was achieved in three of those. The difference between both stimulation conditions approached significance (_t_(5)=−2.38, _P_=0.06). Five
patients were able to correctly guess if they were being stimulated. Notably, C7 who showed a deterioration with DBS during the first crossover was aware of the stimulation condition.
During the second crossover (_n_=5), mean (s.d.) HAM-D scores were 15.4 (7.5) for no stimulation, 7.6 (3.8) for IC/BST stimulation and 11.2 (7.5) for ITP stimulation (Figure 2), without a
main effect of stimulation condition (F(2,8)=1.68, _P_=0.25). During the IC/BST stimulation phase, four patients were responders to DBS, two of them being in remission. Three patients were
responders to DBS in ITP and two of them were in remission. Four patients correctly guessed whether they were being stimulated, three patients were able to differentiate between ITP and
IC/BST stimulation. This partial unblinding resulted from a combination of both clinical response and/or recurrence of some mild side effects which they had already experienced during the
unblinded optimization periods. The stimulation OFF phase was shortened to 4 weeks in two patients because of severe deterioration of depressive symptoms. Average charge densities were
calculated for ITP stimulation (42.8 μC cm−2 per phase) and IC/BST stimulation (34.3 μC cm−2 per phase) for the five patients completing both optimization periods. The difference was not
statistically significant (_t_(7)=1.08, _P_=0.32). Secondary outcomes for both crossovers are shown in Table 2. Both the Montgomery–Åsberg Depression Rating Scale (MADRS) and the Inventory
of Depressive Symptoms (IDS) confirmed the decrease in depressive symptoms when stimulation was turned on. When comparing IC/BST stimulation with ITP stimulation, IC/BST generally seemed to
have better effects. Descriptive statistics for neuropsychological tests during the second crossover are summarized in Supplementary Table S3. No evidence was found for cognitive decline
during stimulation in IC/BST or ITP. On the contrary, stimulation seemed to improve neuropsychological test scores. The effect was usually greatest for IC/BST stimulation. Due to the small
sample size and multiple tests, we opted not to analyze these data statistically. THREE YEARS AND LAST FOLLOW-UP Three years after DBS implantation all patients were stimulated at IC/BST.
Mean (s.d.) HAM-D had dropped from 24.9 (3.6) at baseline to 9.7 (5.4), that is, an average reduction of 61%. This decrease was statistically significant (_t_(6)=6.96, _P_<0.001). Five
out of seven patients were responders and two were in remission. Secondary measures are shown in Supplementary Table S4. At last follow-up, on average 63 months (range 36–97) after DBS
implantation, the mean (s.d.) HAM-D was 10.6 (4.2) (significant reduction compared to baseline, _t_(6)=7.11, _P_<0.001), and only one patient (C2) preferred DBS in ITP. Secondary outcomes
are shown in Supplementary Table S3. During follow-up, fluctuations in the severity of depressive symptoms were observed in most patients. Two patients committed suicide respectively after
39 and 80 months of DBS. One of these patients (C1) was a responder with a 56% decrease in HAM-D score at 79 months follow-up. The other patient (C4) had a reduction of 42% at 36 months.
Four out of five remaining patients (C2, C3, C5 and C6) were responders, and two of them were in remission. C7 had a reduction of 36% at last follow-up. Mean (s.d.) general Quality of Life
Enjoyment and Satisfaction Questionnaire (Q-Les-Q) scores increased from 34.9 (9.5) to 56.0 (10.9) at last measurement (21–81 months after surgery). Before surgery, patients took an average
of 4.7 different psychotripic agents. At last follow-up, one patient required no additional medication to remain in remission. One patient only used a benzodiazepine for sleeping
difficulties. On average, patients took 3.4 different psychotropic drugs. ELECTRODE POSITION A detailed examination of the cathodes’ position (Figure 3) showed that, for the IC/BST target,
active contacts were indeed typically located in the BST or IC. All patients had bilateral contacts in or bordering the BST during crossover and/or at follow-up. C2 and C3 were stimulated
rather dorsally and anterior in the anterior limb of the IC, but they had additional active contacts, situated more ventrally in the IC and BST. For the ITP target, which is very small,
three patients had cathodes centered in this brain region (C3 bilaterally, C2 and C5 unilaterally), while all other active contacts were located adjacent to the ITP. ADVERSE EVENTS A total
of 75 different adverse events (AEs) and 11 serious AEs were reported during the course of this trial. A full list can be found in Supplementary Table S5. There were four device-related
serious adverse events (conversely labeled leads, two infections around neurostimulator site, damage of IC/BST electrode) leading to additional surgical procedures. The number of AEs
reported during IC/BST stimulation is higher than during ITP stimulation, but the total time during which patients were stimulated in ITP is also shorter (total of 98 months versus a total
of 341 months for IC/BST stimulation). Most common were psychiatric AEs (for example, increase in depressive symptoms, sleep disturbances). We noticed the emergence of extrapyramidal-like
symptoms (hypomimia, micrographia, hesitant walking, less fluent movement) during ITP stimulation in one patient, which disappeared when stimulation was turned off. We have not yet
encountered such symptoms in our experience with DBS in IC/BST. An increase in depressive symptoms was reported by all patients at some point. Some patients also reported an increase of
suicidal thoughts. One patient (C5) showed, after a period of remission, a deterioration of depressive symptoms and was hospitalized. During this hospitalization, he committed suicide, 39
months after DBS implantation. No new stressful life events were mentioned in the period preceding the suicide. Another patient (C1) responded well to DBS but had frequent relapses with
suicidal ideations and suicide attempts. After 79 months of stimulation, the patient was a responder to DBS, but committed suicide a month after the last evaluation. Both patients had a
history of suicide attempts before DBS implantation. Note that CT scans were performed to evaluate a potential electrode position shift in these patients (respectively 34 and 51 months after
electrode implantation), but no electrode deviation was found. DISCUSSION In this study, two stimulation targets were compared directly, to our knowledge for the first time in a psychiatric
population. Although we clearly observed clinically relevant effects in our patients, predominantly for stimulation in IC/BST, these findings could not be substantiated statistically in the
crossovers, probably due to a lack of power, given the limited sample size, which is the main limitation of this study. Therefore the data and conclusions presented here, have to be
considered as being preliminary. Another limitation in this study is partial unblinding, as blinded patients and psychiatrists were often able to correctly guess whether stimulation was ON
or OFF. There was, however, less confidence about the target that was being stimulated. The second crossover did not give a decisive answer regarding the superiority of either IC/BST
stimulation or ITP stimulation, but the last follow-up data might provide some clues, as six out of seven patients preferred IC/BST stimulation. After completion of both crossovers, only one
patient (C2) preferred further ITP over IC/BST stimulation. At last follow-up, that is, 8 years after implantation, she continued to experience a substantial decrease in depressive symptoms
as compared to the preoperative situation (HAM-D reduction from 31 to 6). Note that the charge densities for both targets were similar and can therefore not account for potential
differences in outcome per target. This finding also refutes our initial hypothesis that ITP stimulation might require lower charge densities than IC/BST. It has been suggested that
differentiation among symptom clusters of depression might guide the choice between stimulation targets.39 In our study, we could not detect differences in the phenomenology of MDD in the
only patient that responded better to ITP stimulation. She experienced a traumatic childhood and had a secondary diagnosis of an anxiety disorder, but was not the only patient with these
characteristics. Her anxiety diminished markedly under ITP stimulation and less under IC/BST stimulation. The other two patients with a secondary diagnosis of an anxiety disorder responded
better to IC/BST stimulation, with a substantial reduction in HAM-A scores, consistent with our findings in OCD patients.22 Careful analysis of the neuroanatomical position of the active
electrode contacts indicated that all patients were stimulated bilaterally in or bordering the targeted structure (note that the stimulated volume extends further that physical boundaries of
the contact22), sometimes with additional contacts outside this region. Variability in clinical outcome of DBS in this target can therefore not be explained solely by the anatomical
position of the cathodes. This is in line with previous research in another DBS target for TRD (subcallosal cingulate), showing that the contact location was not the only factor
discriminating responders from non-responders,40 and with our own findings with DBS for OCD in BST.22 Despite the absence of statistical significance in the crossovers, at the time of
writing, four patients continue to receive IC/BST stimulation and experience substantial alleviation of depressive symptoms. IC/BST and ITP stimulation were accompanied by similar adverse
events, and after the crossovers, the IC/BST target was generally chosen on account of better clinical effects, not because of fewer (or less severe) adverse events. Neuropsychological test
scores did not reveal any acute worsening of cognitive function during either IC/BST or ITP stimulation, which is in line with previous DBS studies.22, 41 As most severe adverse events, we
unfortunately report that two patients committed suicide during the course of this study. Patients suffering from severe depression are already at high risk for committing suicide (up to 15%
[ref. 42]). A patient not responding to DBS might lose any hope of recovery, as DBS is often regarded as a last resort. Nevertheless, even patients who do respond to DBS may continue to be
at risk for suicide, for example, in case of (temporary) relapses. Moreover, suicidal ideation might persist even when treatment reduces depressive symptoms in general.42 Other DBS for TRD
trials have also reported completed suicides,12, 43 although suicide rates remained below those of severe MDD patients without DBS.44 The intense follow-up provided after DBS implantation
might protect against suicide. However, it has been put forward that DBS might increase impulsivity,45 which could lower the threshold for suicide attempts in DBS patients.46 In our study,
the suicides did not seem to be related to DBS, nor were there any signs of increased impulsivity in these patients. Although we did not include new patients after the second suicide, these
unfortunate occurrences were not the deciding factor for the premature end of this study. Rather, recruitment of patients was much slower than expected (only seven patients in 5 years) and
implantation in ITP did not seem to have added beneficial effects in most patients, questioning the justification of continuing with a more complicated intervention, with four electrodes
instead of two. Although our within-subject approach allows for the most scientifically sound comparison of different DBS targets, the lengthy study design might discourage patients, as it
requires even more commitment than a ‘regular’ DBS crossover study with only one target. Taken together, a within-subject evaluation of different DBS targets remains very challenging.
Open-label trials in SCC, NAc and VC/VS generally found that about half of the TRD patients responded to the treatment,12, 13, 14, 15, 16, 17, 18 similar to our findings at last follow-up,
but response rates might be even higher for DBS in the supero-lateral MFB, with six out of seven responders in a pilot study.20 As mentioned above, different targets might influence
different symptom clusters, while resulting in comparable overall scores on depression rating scales, explaining the comparability of results in open-label studies. Electrical stimulation of
the NAc, for example, reduces anhedonia.47 Stimulation in SCC appears to first improve mood and only later are sleep disturbances and anxiety symptoms observed.48 In a rat model for
depression, DBS in the MFB and SCC analog seemed to alleviate different symptoms.49 A randomized controlled trial of DBS in VC/VS did not find a significant difference between an active and
sham-stimulation group.19 A recent multicenter crossover study of DBS in the anterior limb of IC had more promising results with a statistically significant reduction of depressive symptoms
during active stimulation as compared to no stimulation.50 This seems to be in line with our preliminary results of stimulation in IC/BST. In conclusion, we found a clinically relevant
effect of DBS on MDD symptoms in severe treatment-refractory patients for both IC/BST and ITP stimulation. These observations could not be statistically confirmed during the crossover phases
of this study, probably due to the limited sample size. We do find a statistically significant reduction in depressive symptoms when comparing baseline with 3-year follow-up and with last
follow-up (3–8 months). All patients, except one, preferred IC/BST stimulation. REFERENCES * Sobocki P, Jönsson B, Angst J, Rehnberg C . Cost of depression in Europe. _J Ment Health Policy
Econ_ 2006; 9: 87–98. PubMed Google Scholar * Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C _et al_. Disability-adjusted life years (DALYs) for 291 diseases and injuries in
21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. _Lancet_ 2012; 380: 2197–2223. Article Google Scholar * Kessler RC, Berglund P, Demler O, Jin R,
Merikangas KR, Walters EE . Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. _Arch Gen Psychiatry_ 2005; 62: 593–602.
Article Google Scholar * Angst J, Paksarian D, Cui L, Merikangas KR, Hengartner MP, Ajdacic-Gross V _et al_. The epidemiology of common mental disorders from age 20 to 50: results from the
prospective Zurich cohort Study. _Epidemiol Psychiatr Sci_ 2015; 25: 24–32. Article Google Scholar * Rush J, Trivedi MH, Wisniewski SR, Nierenberg A, Stewart JW, Warden D _et al_. Acute
and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. _Am J Psychiatry_ 2006; 163: 1905–1917. Article Google Scholar * Cuijpers P,
Karyotaki E, Weitz E, Andersson G, Hollon SD, van Straten A . The effects of psychotherapies for major depression in adults on remission, recovery and improvement: a meta-analysis. _J Affect
Disord_ 2014; 159: 118–126. Article Google Scholar * Slotema CW, Blom JD, Hoek HW, Sommer IEC . Should we expand the toolbox of psychiatric treatment methods to include Repetitive
Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. _J Clin Psychiatry_ 2010; 71: 873–884. Article Google Scholar * Pagnin D, de
Queiroz V, Pini S, Cassano GB . Efficacy of ECT in depression: a meta-analytic review. _J ECT_ 2004; 20: 13–20. Article Google Scholar * Solomon D, Keller MB, Leon C, Mueller TI, Lavori
PW, Shea MT _et al_. Multiple recurrences of major depressive disorder. _Am J Psychiatry_ 2000; 157: 229–233. Article CAS Google Scholar * Eaton WW, Shao H, Nestadt G, Lee HB, Bienvenu
OJ, Zandi P . Population-based study of first onset and chronicity in major depressive disorder. _Arch Gen Psychiatry_ 2008; 65: 513–520. Article Google Scholar * Drevets WC, Price JL,
Furey ML . Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. _Brain Struct Funct_ 2008; 213: 93–118. Article Google
Scholar * Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS _et al_. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. _Am J
Psychiatry_ 2011; 168: 502–510. Article Google Scholar * Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Kozarsky J, Chismar R _et al_. Subcallosal cingulate deep brain stimulation for
treatment-resistant unipolar and bipolar depression. _Arch Gen Psychiatry_ 2014; 69: 150–158. Article Google Scholar * Ramasubbu R, Anderson S, Haffenden A, Chavda S, Kiss ZHT .
Double-blind optimization of subcallosal cingulate deep brain stimulation for treatment-resistant depression: a pilot study. _J Psychiatry Neurosci_ 2013; 38: 325–332. Article Google
Scholar * Puigdemont D, Pérez-Egea R, Portella MJ, Molet J, de Diego-Adeliño J, Gironell A _et al_. Deep brain stimulation of the subcallosal cingulate gyrus: further evidence in
treatment-resistant major depression. _Int J Neuropsychopharmacol_ 2012; 15: 121–133. Article Google Scholar * Lozano AM, Giacobbe P, Hamani C, Rizvi SJ, Kennedy SH, Kolivakis TT _et al_.
A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. _J Neurosurg_ 2012; 116: 315–322. Article Google Scholar * Bewernick BH,
Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B _et al_. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression.
_Biol Psychiatry_ 2010; 67: 110–116. Article Google Scholar * Malone D, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN _et al_. Deep brain stimulation of the ventral
capsule/ventral striatum for treatment-resistant depression. _Biol Psychiatry_ 2009; 65: 267–275. Article Google Scholar * Dougherty DD, Rezai AR, Carpenter LL, Howland RH, Bhati MT,
O’Reardon JP _et al_. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. _Biol Psychiatry_ 2015;
78: 240–248. Article Google Scholar * Schlaepfer TE, Bewernick BH, Kayser S, Burkhard M, Coenen VA . Rapid effects of deep brain stimulation for treatment-resistant major depression.
_Biol Psychiatry_ 2013; 73: 1204–1212. Article Google Scholar * Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B . Electrical stimulation in anterior limbs of internal capsules
in patients with obsessive-compulsive disorder. _Lancet_ 1999; 354: 1526. Article CAS Google Scholar * Luyten L, Hendrickx S, Raymaekers S, Gabriëls L, Nuttin B . Electrical stimulation
in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. _Mol Psychiatry_ 2016; 21: 1272–1280. Article CAS Google Scholar * Gabriëls L, Cosyns P, Nuttin
B, Demeulemeester H, Gybels J . Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: psychopathological and neuropsychological outcome in three cases. _Acta
Psychiatr Scand_ 2003; 107: 275–282. Article Google Scholar * Nuttin BJ, Gabriëls L, Cosyns PR, Meyerson B, Andréewitch S, Sunaert SG _et al_. Long-term electrical capsular stimulation in
patients with obsessive-compulsive disorder. _Neurosurgery_ 2003; 52: 1263–1274. Article Google Scholar * Luyck K, Luyten L . Can electrical stimulation of the human bed nucleus of the
stria terminalis reduce contextual anxiety? An unanswered question. _Front Behav Neurosci_ 2015; 9: 69. Article Google Scholar * Luyten L, Casteels C, Vansteenwegen D, van Kuyck K, Koole
M, Van Laere K _et al_. Micro-positron emission tomography imaging of rat brain metabolism during expression of contextual conditioning. _J Neurosci_ 2012; 32: 254–263. Article CAS Google
Scholar * Davis M, Walker DL, Miles L, Grillon C . Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. _Neuropsychopharmacology_ 2010; 35:
105–135. Article Google Scholar * Dong H-W, Swanson LW . Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine,
autonomic, and behavioral aspects of energy balance. _J Comp Neurol_ 2006; 494: 142–178. Article Google Scholar * Crestani C, Alves F, Gomes F, Resstel L, Correa F, Herman J . Mechanisms
in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review. _Curr Neuropharmacol_ 2013; 11: 141–159. Article CAS Google Scholar *
Neumann W, Huebl J, Brücke C, Gabriëls L, Bajbouj M, Merkl A _et al_. Different patterns of local field potentials from limbic DBS targets in patients with major depressive and obsessive
compulsive disorder. _Mol Psychiatry_ 2014; 19: 1186–1192. Article Google Scholar * Fakhar K, Hastings E, Butson CR, Foote KD, Zeilman P, Okun MS . Management of deep brain stimulator
battery failure: battery estimators, charge density, and importance of clinical symptoms. _PLoS ONE_ 2013; 8: e58665. Article CAS Google Scholar * Jiménez F, Velasco F, Salin-Pascual R,
Hernandez J, Velasco M, Criales J _et al_. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. _Neurosurgery_ 2005;
57: 585–593. Article Google Scholar * Velasco F, Velasco M, Jiménez F, Velasco AL, Salin-Pascual R . Neurobiological background for performing surgical intervention in the inferior
thalamic peduncle for treatment of major depression disorders. _Neurosurgery_ 2005; 57: 439–448, discussion 439–48. Article Google Scholar * First M, Spitzer R, Gibbon M, Williams J
_Structured Clinical Interview for DSM-IV Axis I Disorders_, Patient Edition. Biometrics Research, New York State Psychiatric Institut: New York, 1995. * Nuttin B, Gielen F, van Kuyck K, Wu
H, Luyten L, Welkenhuysen M _et al_. Targeting bed nucleus of the stria terminalis for severe obsessive-compulsive disorder: more unexpected lead placement in obsessive-compulsive disorder
than in surgery for movement disorders. _World Neurosurg_ 2013; 80 (S30): e11–e16. Google Scholar * Mai JK, Paxinos G, Voss T . _Atlas of the Human Brain_. Third Edition, Academic Press:
San Diego, 2007. Google Scholar * Hamilton M . Rating depressive patients. _J Clin Psychiatry_ 1980; 41: 21–24. CAS PubMed Google Scholar * Zimmerman M, Chelminski I, Posternak M . A
review of studies of the Hamilton depression rating scale in healthy controls: implications for the definition of remission in treatment studies of depression. _J Nerv Ment Dis_ 2004; 192:
595–601. Article Google Scholar * Williams NR, Okun MS . Deep brain stimulation (DBS) at the interface of neurology and psychiatry. _J Clin Invest_ 2013; 123: 4546–4556. Article CAS
Google Scholar * Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A _et al_. Defining critical white matter pathways mediating successful subcallosal cingulate deep
brain stimulation for treatment-resistant depression. _Biol Psychiatry_ 2014; 76: 963–969. Article Google Scholar * Kubu C, Malone D, Chelune G, Malloy P, Rezai A, Frazier T _et al_.
Neuropsychological outcome after deep brain stimulation in the ventral capsule/ventral striatum for highly refractory obsessive-compulsive disorder or major depression. _Stereotact Funct
Neurosurg_ 2013; 9: 374–378. Article Google Scholar * Seo H-J, Jung Y-E, Jeong S, Kim J-B, Lee M-S, Kim J-M _et al_. Persistence and resolution of suicidal ideation during treatment of
depression in patients with significant suicidality at the beginning of treatment: the CRESCEND study. _J Affect Disord_ 2014; 155: 208–215. Article Google Scholar * Bewernick BH, Kayser
S, Sturm V, Schlaepfer TE . Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. _Neuropsychopharmacology_ 2012;
37: 1975–1985. Article CAS Google Scholar * Wulsin L, Vaillant G, Wells VE . A systematic review of the mortality of depression. _Psychosom Med_ 1999; 61: 6–17. Article CAS Google
Scholar * Luigjes J, Mantione M, van den Brink W, Schuurman PR, van den Munckhof P, Denys D . Deep brain stimulation increases impulsivity in two patients with obsessive-compulsive
disorder. _Int Clin Psychopharmacol_ 2011; 26: 338–340. PubMed Google Scholar * Turecki G . Dissecting the suicide phenotype: the role of impulsive-aggressive behaviours. _J Psychiatry
Neurosci_ 2005; 30: 398–408. PubMed PubMed Central Google Scholar * Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N _et al_. Deep brain stimulation to reward circuitry
alleviates anhedonia in refractory major depression. _Neuropsychopharmacology_ 2008; 33: 368–377. Article Google Scholar * Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy
SH . Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. _Biol Psychiatry_ 2008; 64: 461–467. Article Google Scholar * Edemann-Callesen H, Voget M, Empl
L, Vogel M, Wieske F, Rummel J _et al_. Medial forebrain bundle deep brain stimulation has symptom-specific anti-depressant effects in rats and as opposed to ventromedial prefrontal cortex
stimulation interacts with the reward system. _Brain Stimul_ 2015; 8: 714–723. Article Google Scholar * Bergfeld IO, Mantione M, Hoogendoorn MLC, Ruhé HG, Notten P, van Laarhoven J _et
al_. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression. _JAMA Psychiatry_ 2016; 73: 456. Article Google Scholar Download
references ACKNOWLEDGEMENTS We thank Professor Dr Fransisco Velasco for the help in targeting the ITP; Professor Dr Paul Cosyns for his contribution to the development of the protocol; and
Els Brunfaut and Laurean Matthijs for administering the neuropsychological test battery in our patients. We thank Professor Dr Jürgen K. Mai and Dr Milan Majtanik for providing
high-resolution images of the human brain atlas. We are grateful to the members of the Committee for Neurosurgery for Psychiatric Disorders, and the hospital staff for taking excellent care
of the patients. Finally, we would like to thank all participating patients and their families. The family members of the two patients who died by suicide provided written informed consent
for publication of this paper. This work was supported by the Research Foundation—Flanders (FWO) Project G072909N, FWO Research Grant (to LL) 1504614N, and by the Agency for Innovation by
Science and Technology (IWT-SBO090054). LL is a postdoctoral fellow of the FWO. AUTHOR INFORMATION Author notes * S Raymaekers and L Luyten: These authors contributed equally to this work. *
L Gabriëls and B Nuttin: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * KU Leuven Research Group Psychiatry, Leuven, Belgium S Raymaekers * Z.ORG UPC KU Leuven
Campus Gasthuisberg, Leuven, Belgium S Raymaekers, C Bervoets & L Gabriëls * KU Leuven Research Group Experimental Neurosurgery and Neuroanatomy, Leuven, Belgium L Luyten & B Nuttin
* KU Leuven Research Group Psychology of Learning and Experimental Psychopathology, Leuven, Belgium L Luyten * Department of Neurosurgery, UZ Leuven, Leuven, Belgium B Nuttin Authors * S
Raymaekers View author publications You can also search for this author inPubMed Google Scholar * L Luyten View author publications You can also search for this author inPubMed Google
Scholar * C Bervoets View author publications You can also search for this author inPubMed Google Scholar * L Gabriëls View author publications You can also search for this author inPubMed
Google Scholar * B Nuttin View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to S Raymaekers. ETHICS DECLARATIONS
COMPETING INTERESTS All devices were generously provided by Medtronic. They also provided grants for research, education and traveling to BN and LG, who hold the Medtronic Chair for
Stereotactic Neurosurgery in Psychiatric Disorders at KU Leuven. SR is supported by this Chair. BN co-owns a patent on DBS in OCD. CB reported no biomedical financial interests or potential
conflicts of interest. ADDITIONAL INFORMATION Supplementary Information accompanies the paper on the Translational Psychiatry website SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION
(DOCX 74 KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to
obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Raymaekers, S., Luyten, L., Bervoets, C. _et al._ Deep brain stimulation for treatment-resistant major depressive disorder: a comparison of two targets and
long-term follow-up. _Transl Psychiatry_ 7, e1251 (2017). https://doi.org/10.1038/tp.2017.66 Download citation * Published: 31 October 2017 * Issue Date: October 2017 * DOI:
https://doi.org/10.1038/tp.2017.66 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
