The glutathione transferase mu null genotype leads to lower 6-mmpr levels in patients treated with azathioprine but not with mercaptopurine
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

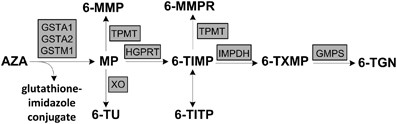
ABSTRACT The conversion of azathioprine (AZA) to mercaptopurine (MP) is mediated by glutathione transferase Mu1 (GSTM1), alpha1 (GSTA1) and alpha2 (GSTA2). We designed a case-control study
with data from the TOPIC trial to explore the effects of genetic variation on steady state 6-methylmercaptopurine ribonucleotide (6-MMPR) and 6-thioguanine nucleotide (6-TGN) metabolite
levels. We included 199 patients with inflammatory bowel disease (126 on AZA and 73 on MP). _GSTM1-null_ genotype carriers on AZA had two-fold lower 6-MMPR levels than AZA users carrying one
or two copies of _GSTM1_ (2239 (1006–4587) versus 4371 (1897–7369) pmol/8 × 108 RBCs; _P_<0.01). In patients on MP (control group) 6-MMPR levels were comparable (6195 (1551–10712) versus
6544 (1717–11600) pmol/8 × 108 RBCs; _P_=0.84). The 6-TGN levels were not affected by the GSTM1 genotype. The presence of genetic variants in _GSTA1_ and _GSTA2_ was not related to the
6-MMPR and 6-TGN levels. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Subscribe to this journal Receive 6 print issues and online access $259.00 per year only $43.17 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access
to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our
FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS GENETIC ASSOCIATION ANALYSIS AND FREQUENCY OF _NUDT15*3_ WITH THIOPURINE-INDUCED MYELOSUPPRESSION IN PATIENTS WITH
INFLAMMATORY BOWEL DISEASE IN A LARGE DUTCH COHORT Article 23 November 2024 ASSOCIATION OF _ITPA_ GENE POLYMORPHISMS WITH ADVERSE EFFECTS OF AZA/6-MP ADMINISTRATION: A SYSTEMATIC REVIEW AND
META-ANALYSIS Article 17 January 2022 PREDICTIVE ROLE OF _ITPA_ GENETIC VARIANTS IN THIOPURINE-RELATED MYELOTOXICITY IN CROHN’S DISEASE PATIENTS Article 21 June 2024 REFERENCES * Lamers MM,
van Oijen MG, Pronk M, Drenth JP . Treatment options for autoimmune hepatitis: a systematic review of randomized controlled trials. _J Hepatol_ 2010; 53: 191–198. Article CAS Google
Scholar * van Gelder T, van Schaik RH, Hesselink DA . Pharmacogenetics and immunosuppressive drugs in solid organ transplantation. _Nat Rev Nephrol_ 2014; 10: 725–731. Article CAS Google
Scholar * Amin J, Huang B, Yoon J, Shih DQ . Update 2014: advances to optimize 6-mercaptopurine and azathioprine to reduce toxicity and improve efficacy in the management of IBD. _Inflam
Bowel Dis_ 2015; 21: 445–452. Article Google Scholar * Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Theoret Y _et al_. Pharmacogenomics and metabolite measurement for
6-mercaptopurine therapy in inflammatory bowel disease. _Gastroenterology_ 2000; 118: 705–713. Article CAS Google Scholar * Eklund BI, Moberg M, Bergquist J, Mannervik B . Divergent
activities of human glutathione transferases in the bioactivation of azathioprine. _Mol Pharmacol_ 2006; 70: 747–754. Article CAS Google Scholar * Moden O, Mannervik B . Glutathione
transferases in the bioactivation of azathioprine. _Adv Cancer Res_ 2014; 122: 199–244. Article CAS Google Scholar * Hayes JD, Strange RC . Glutathione S-transferase polymorphisms and
their biological consequences. _Pharmacology_ 2000; 61: 154–166. Article CAS Google Scholar * Mannervik B, Board PG, Hayes JD, Listowsky I, Pearson WR . Nomenclature for mammalian soluble
glutathione transferases. _Methods Enzymol_ 2005; 401: 1–8. Article CAS Google Scholar * Pelin M, De Iudicibus S, Fusco L, Taboga E, Pellizzari G, Lagatolla C _et al_. Role of oxidative
stress mediated by glutathione-s-transferase in thiopurines' toxic effects. _Chem Res Toxicol_ 2015; 28: 1186–1195. Article CAS Google Scholar * Hayes JD, Pulford DJ . The
glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. _Crit Rev Biochem Mol Biol_ 1995; 30:
445–600. Article CAS Google Scholar * Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, Autrup JL _et al_. Metabolic gene polymorphism frequencies in control populations. _Cancer
Epidemiol Biomark Prev Publ Am Assoc Cancer Res Am Soc Prev Oncol_ 2001; 10: 1239–1248. CAS Google Scholar * Broekman MM, Bos C, Te Morsche RH, Hoentjen F, Roelofs HM, Peters WH _et al_.
GST Theta null genotype is associated with an increased risk for ulcerative colitis: a case-control study and meta-analysis of GST Mu and GST Theta polymorphisms in inflammatory bowel
disease. _J Hum Genet_ 2014; 59: 575–580. Article CAS Google Scholar * Stocco G, Martelossi S, Barabino A, Decorti G, Bartoli F, Montico M _et al_. Glutathione-S-transferase genotypes and
the adverse effects of azathioprine in young patients with inflammatory bowel disease. _Inflam Bowel Dis_ 2007; 13: 57–64. Article Google Scholar * Stocco G, Cuzzoni E, De Iudicibus S,
Franca R, Favretto D, Malusa N _et al_. Deletion of glutathione-s-transferase m1 reduces azathioprine metabolite concentrations in young patients with inflammatory bowel disease. _J Clin
Gastroenterol_ 2014; 48: 43–51. Article CAS Google Scholar * Moon W, Loftus EV Jr. . Review article: recent advances in pharmacogenetics and pharmacokinetics for safe and effective
thiopurine therapy in inflammatory bowel disease. _Alim Pharmacol Ther_ 2016 (e-pub ahead of print). * Morel F, Schulz WA, Sies H . Gene structure and regulation of expression of human
glutathione S-transferases alpha. _Biol Chem Hoppe-Seyler_ 1994; 375: 641–649. CAS PubMed Google Scholar * Coles BF, Morel F, Rauch C, Huber WW, Yang M, Teitel CH _et al_. Effect of
polymorphism in the human glutathione S-transferase A1 promoter on hepatic GSTA1 and GSTA2 expression. _Pharmacogenetics_ 2001; 11: 663–669. Article CAS Google Scholar * Tetlow N, Board
PG . Functional polymorphism of human glutathione transferase A2. _Pharmacogenetics_ 2004; 14: 111–116. Article CAS Google Scholar * Tetlow N, Liu D, Board P . Polymorphism of human Alpha
class glutathione transferases. _Pharmacogenetics_ 2001; 11: 609–617. Article CAS Google Scholar * Zhang W, Moden O, Mannervik B . Differences among allelic variants of human glutathione
transferase A2-2 in the activation of azathioprine. _Chemico-Biol Interactions_ 2010; 186: 110–117. Article CAS Google Scholar * Ning B, Wang C, Morel F, Nowell S, Ratnasinghe DL, Carter
W _et al_. Human glutathione S-transferase A2 polymorphisms: variant expression, distribution in prostate cancer cases/controls and a novel form. _Pharmacogenetics_ 2004; 14: 35–44. Article
CAS Google Scholar * Coenen MJ, de Jong DJ, van Marrewijk CJ, Derijks LJ, Vermeulen SH, Wong DR _et al_. Identification of patients with variants in TPMT and dose reduction reduces
hematologic events during thiopurine treatment of inflammatory bowel disease. _Gastroenterology_ 2015; 149: 907–917 e907. Article Google Scholar * Brockmoller J, Kerb R, Drakoulis N, Nitz
M, Roots I . Genotype and phenotype of glutathione S-transferase class mu isoenzymes mu and psi in lung cancer patients and controls. _Cancer Res_ 1993; 53: 1004–1011. CAS PubMed Google
Scholar * Dura P, Salomon J, Te Morsche RH, Roelofs HM, Kristinsson JO, Wobbes T _et al_. No role for glutathione S-transferase genotypes in Caucasian esophageal squamous cell or
adenocarcinoma etiology: an European case-control study. _BMC gastroenterology_ 2013; 13: 97. Article Google Scholar * Qin ZS, Niu T, Liu JS . Partition-ligation-expectation-maximization
algorithm for haplotype inference with single-nucleotide polymorphisms. _Am J Hum Genet_ 2002; 71: 1242–1247. Article CAS Google Scholar * Lennard L, Singleton HJ . High-performance
liquid chromatographic assay of the methyl and nucleotide metabolites of 6-mercaptopurine: quantitation of red blood cell 6-thioguanine nucleotide, 6-thioinosinic acid and
6-methylmercaptopurine metabolites in a single sample. _J Chromatogr_ 1992; 583: 83–90. Article CAS Google Scholar * Derijks LJ, Gilissen LP, Engels LG, Bos LP, Bus PJ, Lohman JJ _et al_.
Pharmacokinetics of 6-mercaptopurine in patients with inflammatory bowel disease: implications for therapy. _Therapeutic drug Monit_ 2004; 26: 311–318. Article CAS Google Scholar * Wong
DR, Coenen MJ, Vermeulen SH, Derijks LJ, van Marrewijk CJ, Klungel OH _et al_. Early assessment of thiopurine metabolites identifies patients at risk of thiopurine-induced leukopenia in
inflammatory bowel disease. _J Crohn's Colitis_ 2016 (e-pub ahead of print). * Nguyen TV, Vu DH, Nguyen TM, Lachaux A, Boulieu R . Relationship between azathioprine dosage and
thiopurine metabolites in pediatric IBD patients: identification of covariables using multilevel analysis. _Ther Drug Monit_ 2013; 35: 251–257. Article CAS Google Scholar * Lee MN, Kang
B, Choi SY, Kim MJ, Woo SY, Kim JW _et al_. Relationship between azathioprine dosage, 6-thioguanine nucleotide levels, and therapeutic response in pediatric patients with IBD treated with
azathioprine. _Inflam Bowel Dis_ 2015; 21: 1054–1062. Article CAS Google Scholar * Liu H, Ding L, Zhang F, Zhang Y, Gao X, Hu P _et al_. The impact of glutathione S-transferase genotype
and phenotype on the adverse drug reactions to azathioprine in patients with inflammatory bowel diseases. _J Pharmacol Sci_ 2015; 129: 95–100. Article CAS Google Scholar * Gilissen LP,
Wong DR, Engels LG, Bierau J, Bakker JA, Paulussen AD _et al_. Therapeutic drug monitoring of thiopurine metabolites in adult thiopurine tolerant IBD patients on maintenance therapy. _J
Crohn's Colitis_ 2012; 6: 698–707. Article Google Scholar * Stocco G, Pelin M, Franca R, De Iudicibus S, Cuzzoni E, Favretto D _et al_. Pharmacogenetics of azathioprine in
inflammatory bowel disease: a role for glutathione-S-transferase? _World J Gastroenterol_ 2014; 20: 3534–3541. Article Google Scholar * Weber G . Biochemical strategy of cancer cells and
the design of chemotherapy: G. H. A. Clowes Memorial Lecture. _Cancer Res_ 1983; 43: 3466–3492. CAS PubMed Google Scholar * Elion GB . The purine path to chemotherapy. _Science_ 1989;
244: 41–47. Article CAS Google Scholar * Derijks LJ, Wong DR . Pharmacogenetics of thiopurines in inflammatory bowel disease. _Curr Pharm Des_ 2010; 16: 145–154. Article CAS Google
Scholar * Goldberg R, Moore G, Cunningham G, Schulberg J, Marsh P, Brown S _et al_. Thiopurine metabolite testing in inflammatory bowel disease. _J Gastroenterol Hepatol_ 2016; 31: 553–560.
Article CAS Google Scholar * Weinshilboum RM, Sladek SL . Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. _Am J Hum Genet_
1980; 32: 651–662. CAS PubMed PubMed Central Google Scholar * Coskun M, Steenholdt C, de Boer NK, Nielsen OH . Pharmacology and optimization of thiopurines and methotrexate in
inflammatory bowel disease. _Clin Pharmacokinet_ 2016; 55: 257–274. Article CAS Google Scholar * Zur RM, Roy LM, Ito S, Beyene J, Carew C, Ungar WJ . Thiopurine S-methyltransferase
testing for averting drug toxicity: a meta-analysis of diagnostic test accuracy. _Pharmacogenom J_ 2016; 16: 305–311. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We
are deeply indebted to the participants of the TOPIC trial. We thank Rene H.M. te Morsche, J. Salomon and Wilbert H.M. Peters from the Department of Gastroenterology, Radboud University
Medical Center, Nijmegen, The Netherlands, for the measurement of TPMT activity, assistance with PCRs and interpretation of data. Furthermore, we thank Mariëlle Maas, Miet Fiddelaers,
Milevis Reitsma, Leonie Peters and Jean Cilissen from the Department of Clinical Pharmacy and Toxicology, Zuyderland Medical Center, Sittard-Geleen, The Netherlands for technical assistance
with metabolite measurements and Debbie Heinen, Marjolein M.J. van Donkelaar, Freshteh Golestani, Marlies E. de Vos, J.G. Angelien M. Heister, Doménique M.W. Nijsten, Mascha M.V.A.P.
Schijvenaars, and Martine E.C. Cranen from the Department of Human Genetics, Radboud University Medical Center, Nijmegen, The Netherlands for their support in data-management. We thank Dr
Sita H. Vermeulen, Department of Health Evidence, Radboud University Medical Center, Nijmegen, The Netherlands, and Prof. Dr Barbara Franke, Department of Human Genetics, Donders Institute
for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, The Netherlands, for their contribution to the design of the TOPIC trial. At last, we thank Prof. Dr Joost
P.H. Drenth from the Department of Gastroenterology, Radboud University Medical Center, Nijmegen, The Netherlands, for intellectual contributions to the content of the manuscript. AAM
Masclee8, M Pierik8, W Mares8, W Hameeteman8, PJ Wahab9, H Seinen9, MCM Rijk10, IM Harkema10, M de Bièvre11, L Oostenbrug11, CM Bakker11, M Aquarius11, C van Deursen11, AB van Nunen11, JG
Goedhard11, M Hamacher11, IAM Gisbertz12, BJ Brenninkmeijer12, ACITL Tan13, MN Aparicio-Pagés13, EM Witteman13, SAC van Tuyl14, R Breumelhof14, A Stronkhorst15, LPL Gilissen15, EJ Schoon15,
JWM Tjhie-Wensing16, A Temmerman16, JJ Nicolaï17, JD van Bergeijk18, DJ Bac18, BJM Witteman18, N Mahmmod18, JJ Uil18, H Akol18, RJTh Ouwendijk19, IP van Munster20, M Pennings20, AMP De
Schryver20, ThJM van Ditzhuijsen20, RCH Scheffer20, TEH Römkens20, DL Schipper20, PJ Bus21, JWA Straathof22, ML Verhulst22, PJ Boekema22, JTh Kamphuis22, HJ van Wijk22, JMJL Salemans22, JR
Vermeijden23, SDJ van der Werf24, RJ Verburg24, P Spoelstra25, JML de Vree25, K van der Linde25, HJA Jebbink25, M. Jansen25, H. Holwerda25, N van Bentem26, JJ Kolkman26, MGVM Russel26, GH
van Olffen26, MJ Kerbert-Dreteler26, M Bargeman26, JM Götz26, R Schröder26, JM Jansen27, LP Bos28, LGJB Engels28, MJL Romberg-Camps28, ETP Keulen28, AAJ van Esch29, JPH Drenth29, MCA van
Kouwen29, GJA Wanten29, TJ Bisseling29, TEH Römkens29, MWJ van Vugt29, PC van de Meeberg30, SJ van den Hazel30, WNHM Stuifbergen31, MJAL Grubben31, U de Wit31, GAH Dodemont31, RF Eichhorn31,
JMH van den Brande32, AHJ Naber32, EJ van Soest32, PJ Kingma32, NC Talstra33, KF Bruin33, FHJ Wolfhagen33, DW Hommes34, PPJ van der Veek34, JCA Hardwick34, RJ Stuyt34, HH Fidder34, B
Oldenburg35, TG Tan36 8Department of Gastroenterology, Academisch Ziekenhuis Maastricht, Maastricht, The Netherlands, 9Department of Gastroenterology, Rijnstate Ziekenhuis Arnhem, Arnhem,
The Netherlands, 10Department of Gastroenterology, Amphia Ziekenhuis, Breda, The Netherlands, 11Department of Gastroenterology, Atrium Medisch Centrum, Heerlen, The Netherlands, 12Department
of Gastroenterology, Bernhoven Hospital, Oss, The Netherlands, 13Department of Gastroenterology, Canisius Wilhelmina Ziekenhuis, Nijmegen, The Netherlands, 14Department of Gastroenterology,
Diakonessenhuis, Utrecht, The Netherlands, 15Department of Gastroenterology, Catharina Ziekenhuis, Eindhoven, The Netherlands, 16Department of Gastroenterology, Elkerliek Ziekenhuis,
Helmond, The Netherlands, 17HagaZiekenhuis, ‘s-Gravenhage, The Netherlands, 18Department of Gastroenterology, Gelderse Vallei Hospital, Ede, The Netherlands, 19Department of
Gastroenterology, Ikazia Hospital, Rotterdam, The Netherlands, 20Department of Gastroenterology, Jeroen Bosch Hospital, ‘s-Hertogenbosch, The Netherlands, 21Department of Gastroenterology,
Laurentius Hospital, Roermond, The Netherlands, 22Department of Gastroenterology, Máxima Medisch Centrum, Eindhoven-Veldhoven, The Netherlands, 23Department of Gastroenterology, Meander MC,
Amersfoort, The Netherlands, 24Department of Gastroenterology, MC Haaglanden, Den Haag, The Netherlands, 25Department of Gastroenterology, Medisch Centrum Leeuwarden, Leeuwarden, The
Netherlands, 26Department of Gastroenterology, Medisch Spectrum Twente, Enschede, The Netherlands, 27Department of Gastroenterology, Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands,
28Department of Gastroenterology, Orbis Medisch Centrum, Sittard-Geleen, The Netherlands, 29Department of Gastroenterology, Radboud university medical center, Nijmegen, The Netherlands,
30Department of Gastroenterology, Slingeland Hospital, Doetinchem, The Netherlands, 31Department of Gastroenterology, St Elisabeth Ziekenhuis, Tilburg, The Netherlands, 32Department of
Gastroenterology, Tergooiziekenhuizen, Blaricum-Hilversum, The Netherlands, 33Department of Gastroenterology, TweeSteden Ziekenhuis, Tilburg, The Netherlands, 34Department of
Gastroenterology, University Medical Centre Leiden, Leiden, The Netherlands, 35Department of Gastroenterology, University Medical Centre Utrecht, Utrecht, The Netherlands, 36Department of
Gastroenterology, Ziekenhuisgroep Twente, Hengelo, The Netherlands. AUTHOR CONTRIBUTIONS Study design: MB, GW, DW, MC, DJ. Performed experiments: MB, HR. Metabolite measurements: DW, PH.
Data collection: MB, CM, MC, DW. Data analysis: MB, HR, MC. Writing of the manuscript: MB, DW, GW, DJ, MC. Interpretation of data and critical revision of the manuscript for important
intellectual content: HG, AV, HS, LD, OK, HR. Study supervision: DJ, MC. AUTHOR INFORMATION Author notes * M M T J Broekman, D R Wong, G J A Wanten, H M Roelofs, C J van Marrewijk, O H
Klungel, A L M Verbeek, P M Hooymans, H-J Guchelaar, H Scheffer, L J J Derijks, M J H Coenen and D J de Jong: TOPIC recruitment team: The TOPIC recruitment team was responsible for patient
recruitment and collection of clinical data. Compensation was given to the members of the recruitment team for additional biochemical measurements and examinations that had to be performed
for the TOPIC study. TOPIC recruitment team members are listed in Acknowledgements section. AUTHORS AND AFFILIATIONS * Department of Gastroenterology, Radboud University Medical Center,
Radboud Institute for Molecular Life Sciences, Nijmegen, The Netherlands M M T J Broekman, G J A Wanten, H M Roelofs & D J de Jong * Department of Clinical Pharmacy, Pharmacology and
Toxicology, Zuyderland Medical Center, Sittard-Geleen, The Netherlands D R Wong & P M Hooymans * Department of Human Genetics, Radboud University Medical Center, Radboud Institute for
Health Sciences, Nijmegen, The Netherlands C J van Marrewijk, H Scheffer & M J H Coenen * Department of Pharmacoepidemiology and Pharmacotherapy, Utrecht University, Utrecht, The
Netherlands O H Klungel * Radboud University Medical Center, Radboud Institute for Health Sciences, Nijmegen, The Netherlands A L M Verbeek * Department of Clinical Pharmacy and Toxicology,
Leiden University Medical Center, Leiden, The Netherlands H-J Guchelaar * Department of Clinical Pharmacy, Máxima Medical Center, Veldhoven, The Netherlands L J J Derijks Authors * M M T J
Broekman View author publications You can also search for this author inPubMed Google Scholar * D R Wong View author publications You can also search for this author inPubMed Google Scholar
* G J A Wanten View author publications You can also search for this author inPubMed Google Scholar * H M Roelofs View author publications You can also search for this author inPubMed Google
Scholar * C J van Marrewijk View author publications You can also search for this author inPubMed Google Scholar * O H Klungel View author publications You can also search for this author
inPubMed Google Scholar * A L M Verbeek View author publications You can also search for this author inPubMed Google Scholar * P M Hooymans View author publications You can also search for
this author inPubMed Google Scholar * H-J Guchelaar View author publications You can also search for this author inPubMed Google Scholar * H Scheffer View author publications You can also
search for this author inPubMed Google Scholar * L J J Derijks View author publications You can also search for this author inPubMed Google Scholar * M J H Coenen View author publications
You can also search for this author inPubMed Google Scholar * D J de Jong View author publications You can also search for this author inPubMed Google Scholar CONSORTIA IN COLLABORATION WITH
TOPIC RECRUITMENT TEAM CORRESPONDING AUTHOR Correspondence to D J de Jong. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION
Supplementary Information accompanies the paper on the The Pharmacogenomics Journal website SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLE S1 (DOCX 36 KB) POWERPOINT SLIDES POWERPOINT SLIDE
FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Broekman, M., Wong, D., Wanten, G. _et al._ The glutathione
transferase Mu null genotype leads to lower 6-MMPR levels in patients treated with azathioprine but not with mercaptopurine. _Pharmacogenomics J_ 18, 160–166 (2018).
https://doi.org/10.1038/tpj.2016.87 Download citation * Received: 26 February 2016 * Revised: 07 November 2016 * Accepted: 14 November 2016 * Published: 03 January 2017 * Issue Date: January
2018 * DOI: https://doi.org/10.1038/tpj.2016.87 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
