Reversal in the direction of movement of a molecular motor
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Access through your institution Buy or subscribe Kinesin and non-claret disjunctional (ncd) are molecular motors of the kinesin superfamily that move in opposite directions along
microtubules. The molecular basis underlying the direction of movement is unclear, although it is thought to be an intrinsic property of the motor domain, a conserved region about 330 amino
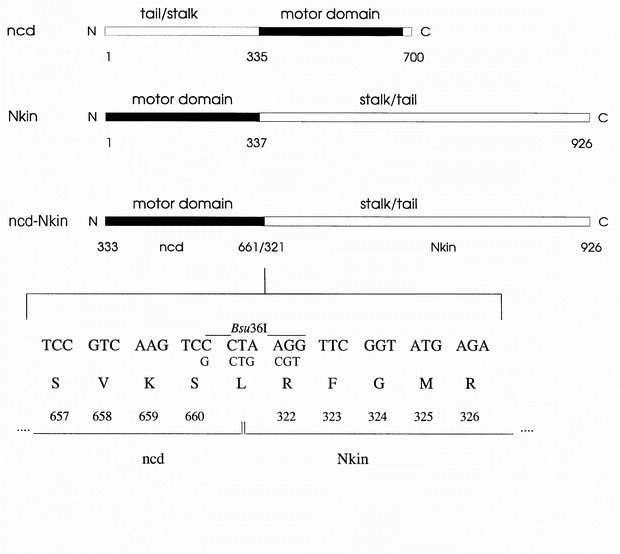
acids in length1,2,3. The motor domain is found at the amino terminus in conventional kinesins, but at the carboxy terminus in ncd4,5. Here we report on a chimaera composed of the motor
domain of the minus-end-directed kinesin of _Neurospora crassa_6. The bacterially expressed fusion protein was tested in motility assays using polarity-marked microtubules7. Surprisingly,
the chimaera moved towards the plus end, demonstrating that the polarity of force generation of the ncd motor domain has been reversed. This finding indicates that the domain organization,
particularly the position of the motor domain, is of fundamental importance for the polarity of force production. It also demonstrates that the direction of microtubule movement is not
controlled solely by the motor domain8. The identity of this polypeptide was investigated in immunoblots using a polyclonal antibody against the Nkin stalk/tail domain6 and a peptide
antibody (MMR48) against a consensus sequence 24 amino acids in length in the HIPYR region of animal kinesins13. The Nkin antibody recognized Nkin as well as the chimaera, but not GST–MC5
(ncd). Antibody MMR48 reacted strongly with Nkin, which was to be expected given the high degree of sequence conservation of the HIPYR peptide between animal kinesins and Nkin. Its reaction
with GST–MC5, however, was much weaker, because of considerable sequence divergence in the HIPYR peptide between ncd and animal kinesins. The chimaera showed the same weak reaction with
MMR48 as GST–MC5. Thus the chimaera combines the immunological characteristics of the Nkin stalk/tail domain and the ncd motor domain. This is a preview of subscription content, access via
your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this
article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in
* Learn about institutional subscriptions * Read our FAQs * Contact customer support REFERENCES * Bloom, G. & Endow, S. Motor proteins 1: kinesin. _Prot. Profiles_ 1, 1059–1116 (1994).
Google Scholar * Goldstein, L. S. B. With apologies to Scheherazade: Tails of 1001 kinesin motors. _Annu. Rev. Genet._ 27, 319–351 (1993). Google Scholar * Moore, J. D. & Endow, S. A.
Kinesin proteins: a phylum of motors for microtubule-based motility. _BioEssays_ 18, 207–219 (1996). Google Scholar * Walker, R. A., Salmon, E. D. & Endow, S. A. The _Drosophila_ claret
segretation protein is a minus-end directed motor molecule. _Nature_ 347, 780–782 (1990). Article ADS CAS Google Scholar * McDonald, H. B., Stewart, R. J. & Goldstein, L. S. B. The
kinesin-like _ncd_ protein of _Drosophila_ is a minus end-directed microtubule motor. _Cell_ 63, 1159–1165 (1990). Google Scholar * Steinberg, G. & Schliwa, M. The _Neurospora_
organelle motor: A distant relative of conventional kinesin with unconventional properties. _Mol. Biol. Cell_ 6, 1605–1618 (1995). Google Scholar * 7. Hyman, A. A. Preparation of marked
microtubules for the assay of the polarity of microtubule-based motors by fluorescence. _J. Cell Sci. (suppl.)_ 14, 125–127 (1991). Google Scholar * Stewart, R. J., Thaler, J. P. &
Goldstein, L. S. B. Direction of microtubule movement is an intrinsic property of the motor domains of kinesin heavy chain and _Drosophila_ ncd protein. _Proc. Natl Acad. Sci. USA_ 90,
5209–5213 (1993). Google Scholar * Endow, S. A., Henikoff, S. & Soler-Niedziela, L. Mediation of meiotic and early mitotic chromosome segregation in _Drosophila_ by a protein related to
kinesin. _Nature_ 345, 81–83 (1990). Article ADS CAS Google Scholar * Tabor, S. in _Current Protocols in Molecular Biology_(eds Ausubel, F. A. et al.)16.2.1–16.2.11 (Green/Wiley, New
York, (1990)). Google Scholar * Chandra, R., Salmon, E. D., Erickson, H. P., Lockhart, A. & Endow, S. A. Structural and functional domains of the _Drosophila_ ncd microtubule motor
protein. _J. Biol. Chem._ 268, 9005–9013 (1993). Google Scholar * Sablin, E. P., Kull, F. J., Cooke, R., Vale, R. D. & Fletterick, R. J. Crystal structure of the motor domain of the
kinesin-related motor ncd. _Nature_ 380, 555–559 (1996). Article ADS CAS Google Scholar * Marks, D. L., Larkin, J. M. & McNiven, M. A. Association of kinesin with the Golgi apparatus
in rat hepatocytes. _J. Cell Sci._ 107, 2417–2426 (1994). Google Scholar * Kull, J. Sabli, E. P., J. Sabli Lau, R., Fletterick, R. J. & Vale, R. D. Crystal structure of the kinesin
motor domain reveals a structural similarity to myosin. _Nature_ 380, 550–555 (1996). Article ADS CAS Google Scholar * Crevel, I. M. -T. C., Lockhart, A. & Cross, R. A. Weak and
strong states of kinesin and ncd. _J. Mol. Biol._ 257, 66–76 (1996). Google Scholar * Lockhart, A. & Cross, R. A. Origins of reversed directionality in the ncd molecular motor. _EMBO
J._ 13, 751–757 (1994). Google Scholar * Lockhart, A., Crevel, I. M. -T. C. & Cross, R. A. Kinesin and ncd bind through a single head to microtubules and compete for a shared MT binding
site. _J. Mol. Biol._ 249, 763–771 (1995). Google Scholar * Arnal, I., Metoz, F., DeBonis, S. & Wade, R. H. Three-dimensional structure of functional motor proteins on microtubules.
_Curr. Biol._ 6, 1265–1270 (1996). Google Scholar * Hirose, K., Lockhart, A., Cross, R. A. & Amos, L. A. Three-dimensional cryoelectron microscopy of dimeric kinesin and ncd motor
domains on microtubules. _Proc. Natl Acad. Sci. USA_ 93, 9539–9544 (1996). Google Scholar * Amos, L. A. & Hirose, K. The structure of microtubule–motor complexes. _Curr. Opin. Cell
Biol._ 9, 4–11 (1997). Google Scholar * Berliner, E., Young, E. C., Anderson, K., Mahtani, H. K. & Gelles, J. Failure of a single-headed kinesin to track parallel to microtubule
protofilaments. _Nature_ 373, 718–721 (1995). Article ADS CAS Google Scholar * Vale, R. D._et al_. Direct observation of single kinesin molecules moving along microtubules. _Nature_ 380,
451–453 (1996). Article ADS CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank S. Endow for the GST–MC5 clone; M. McNiven for the MMR48 antibody; M. Fritz for the
rhodamine-labelled tubulin; U. Euteneuer, H. Felgner, R. Gräf and S. Linder for advice; and S. Fuchs for technical assistance. This work was supported by a Graduiertenkolleg fellowship to
U.H. and a grant from the DFG to M.S. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Adolf-Butenandt-Institut, Zellbiologie, University of Munich, Schillerstrasse 42, Munich, 80336, Germany
Ulrike Henningsen & Manfred Schliwa Authors * Ulrike Henningsen View author publications You can also search for this author inPubMed Google Scholar * Manfred Schliwa View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Manfred Schliwa. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Henningsen, U., Schliwa, M. Reversal in the direction of movement of a molecular motor. _Nature_ 389, 93–96 (1997). https://doi.org/10.1038/38022 Download citation
* Received: 11 June 1997 * Accepted: 16 July 1997 * Issue Date: 04 September 1997 * DOI: https://doi.org/10.1038/38022 SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
