Myofibroblast phenotype and apoptosis in keloid and palmar fibroblasts in vitro
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Keloid formation is a wound healing response, which fails to resolve and leads to formation of a raised collagen mass extending beyond the original wound margins. Keloids are
typically excluded from palms and soles. Therefore we compared keloid and palmar fibroblasts _IN VITRO_ using fibroblasts from nonaffected individuals as controls. Collagen I, α-smooth
muscle actin and thrombospondin-1 were found at higher levels in keloid than in palmar fibroblasts. These differences were ameliorated by addition of TGFβ1. The potential for resolution of
the wound healing response was estimated analyzing apoptosis during serum starvation. Annexin V and TUNEL assays showed that palmar fibroblasts underwent faster apoptosis, than did the
keloid fibroblasts, and started detaching. Addition of TGFβ1 counteracted this effect. The weak expression of the myofibroblast phenotype and the advanced apoptosis of palmar fibroblasts
suggest mechanisms for the exclusion of keloids from palmar sites. SIMILAR CONTENT BEING VIEWED BY OTHERS THE HEDGEHOG-GLI1 PATHWAY IS IMPORTANT FOR FIBROPROLIFERATIVE PROPERTIES IN KELOIDS
AND AS A CANDIDATE THERAPEUTIC TARGET Article Open access 07 December 2023 SINGLE CELL TRANSCRIPTOMICS REVEALS THE CELLULAR HETEROGENEITY OF KELOIDS AND THE MECHANISM OF THEIR AGGRESSIVENESS
Article Open access 19 December 2024 ESTABLISHMENT OF A HUMANIZED MOUSE MODEL OF KELOID DISEASES FOLLOWING THE MIGRATION OF PATIENT IMMUNE CELLS TO THE LESION: PATIENT-DERIVED KELOID
XENOGRAFT (PDKX) MODEL Article Open access 01 August 2023 INTRODUCTION Keloids occur due to deviations from the normal wound healing process. The defect is found predominantly in individuals
of African origin (about 10% incidence in the African-American population) and is excluded from the glabrous tissue of palmar and plantar surfaces. A raised scar forms, which extends beyond
the boundaries of the original wound. It contains thick and highly crosslinked collagen bundles and excessive proteoglycan and fibronectin (for recent reviews see1,2,3). Although there have
been many _in vitro_ and _in vivo_ studies on keloids, it is still not known what causes their formation. The particular protein expression pattern in keloids may be related to a specific
cytokine environment. Higher (IFN-β, TNF-α, IL-6) and lower (IFN-γ, IFN-α, IL-2, TNF-β) levels were found in the peripheral blood mononuclear-cells (PMNC) of black keloid formers.4 _In
vitro_ keloid fibroblasts demonstrate a variety of differences in behaviour compared to normal fibroblasts. Some, but not all cultures of keloid fibroblasts, show higher levels of collagen I
than do normal fibroblasts.5,6,7,8,9 In addition, TGFβ1, which is known to enhance matrix production, has been reported to have differential effects on proliferation rates and collagen
synthesis of keloid _vs_ normal fibroblasts.10,11,12 TGFβ1 mRNA was detected in fibroblasts and endothelial cells within the expanding borders of the active keloid,7 implicating TGFβ1 in the
development of this scar. Decorin, a natural inhibitor of TGFβ,13 was found unaltered in the keloid tissue.14,15 The progression through the different stages of normal wound healing leads
to granulation tissue formation and fibroblast differentiation into myofibroblasts. In normal wounds the myofibroblast marker, α-smooth muscle actin (α-SMA), peaks between 7–15 days, when
70% of the wound fibroblasts have the myofibroblast phenotype.16 _In vitro_, a negative dependence of the myofibroblast phenotype on the plating density has been documented.17 On the other
hand, TGβ1 has a strong positive effect on the expression of α-SMA.18 α2β1 integrins are necessary for the TGFβ1 induction of α-SMA.19,20 The presence of the EDA splicing variant of
fibronectin21,22,23 and the intracellular tension response to the deformability of the extracellular matrix (ECM)19,20 also appear to be crucial for the TGFβ1 effect on the myofibroblast
phenotype. As wounds close there is a decrease in cellularity due to apoptosis. This ensures the transition between granulation tissue and scar. The myofibroblasts gradually disappear and by
day 30 the fibroblast population is indistinguishable from that found in normal dermis.16,24,25 Myofibroblasts can undergo apoptosis. This is seen in Dupuytren fibromatosis, where it
coincides with an elevated expression of TGFβ1.26,27,28 However, recent findings in a model of regressing granulation tissue have suggested that differentiation into myofibroblasts might not
be a prerequisite for apoptosis.29 Keloids, when studied immunohistochemically show α-SMA only around microvessels. In contrast, cultured keloid fibroblasts have detectable levels of
α-SMA.30 When plated on collagen-coated plates or embedded in collagen gels normal and keloid fibroblasts down-regulate their α-SMA synthesis, while upregulating their α2β1 integrins.31 When
apoptosis was studied in keloids, the number of apoptotic cells were found to either decrease distally from the keloid lesion edges32 or to decrease near them.33 The delayed apoptosis has
been ascribed to the presence of somatic mutations in both p53 alleles of cells within the center of the keloid.34 Irrespective of these conflicting reports on the location of apoptotic
cells, delayed apoptosis in keloid fibroblasts could be responsible for the production of vast amounts of ECM prior to the eventual disappearance of the cells, leaving only the acellular
collagenous scar. In an effort to determine the mechanisms whereby keloids form and keloid prone individuals are spared keloid formation on glabrous tissues, an _in vitro_ assessment was
carried out. Comparisons were made among fibroblasts isolated from palmar surfaces, from nonpalmar sites and from keloids. Matrix production, myofibroblast expression and apoptosis were
monitored in the absence and presence of exogenously supplied TGFβ1. Under a variety of experimental conditions palmar fibroblasts demonstrated a weaker myofibroblast phenotype and higher
growth factor requirements. RESULTS Differences in the phenotype between palmar and nonpalmar fibroblasts were evaluated using cells isolated from keloid and nonaffected donors. Palmar
fibroblasts were smaller and reached confluence 1–2 days earlier than their nonpalmar or keloid counterparts (data not shown). In order to reveal differences between cultures from different
sources, cells were maintained with or without serum for 3 days after reaching confluence. Ascorbic acid was present during these 3 days to ensure collagen I secretion. COLLAGEN I, Α-SMA AND
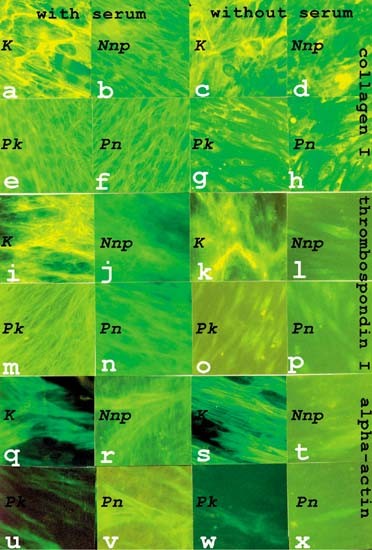
TSP-1 ARE UPREGULATED IN KELOID CULTURES The cellular distribution and organization of collagen I, α-SMA, and the TGFβ1-activator thrombospondin-1 (TSP-1) shown in Figure 1, were analyzed
in fibroblasts plated on chamber slides at 5 cells/mm2. Keloid fibroblast (K) cultures accumulated the most collagen I (Figure 1a). It was found to be organized into thicker fibers than the
rest of the cultures (Nnp, nonpalmar fibroblasts from a nonaffected donor; Pk, palmar fibroblasts from a keloid patient; Pn, palmar fibroblasts from a nonaffected donor) (Figure 1b,e,f). The
abundance and thickness of the collagen fibers were not significantly changed when K were cultured in serum-free medium for 72 h (Figure 1c). However, there was some reduction in the
collagen content in Nnp (Figure 1d) and much greater reductions in the collagen content in palmar fibroblasts (P) (Figure 1g,h). In the serum-free Pk and Pn cultures all remaining collagen
appeared to be intracellular, little or no extracellular collagen was detected. Similar to the results with collagen I, K cultures contained the most TSP-1 (Figure 1i). As TSP-1 colocalized
with collagen (data not shown), it was also found in thick fibers. Little difference in abundance was observed in the serum-starved K cultures (Figure 1k). This contrasted with the non-K. In
the serum-containing media there were lower levels of extracellular TSP-1 (thin and lower intensity fibers) (Figure 1j,m,n) and in the serum-starved cultures extracellular TSP-1 was no
longer detectable (Figure 1l,o,p). α-SMA was found in the greatest abundance and in stress fibers in K incubated in presence (Figure 1q) or absence (Figure 1s) of serum. This actin was also
detected in the non-K cultures, but to a lesser extent (Figure 1r,u,v), being especially low in Pk (Figure 1u). Barely detectable levels were seen in the serum-starved cultures of the non-K
strains (Figure 1t,w,x). α-SMA levels have been reported to depend on the plating density.17 Therefore, FACS analyses were used (Figure 2) to compare the distribution of α-SMA fluorescence
among individual fibroblasts in serum-starved cultures when seeded at a low plating density (lpd) of 5 cells/mm2 and at an intermediate density of 50 cells/mm2. As shown in Figure 2 at both
plating densities the highest intensities were detected in K cultures, compared to P cultures (Pk and Pn). Nnp had intermediate levels. PROTEIN EXPRESSION IN FIBROBLASTS FROM KELOID FORMERS
AND NONAFFECTED INDIVIDUALS Protein extracts from equal numbers of cells of twelve cell cultures (four K, four Pk, two Pk and two Pn) plated at 50 cells/mm2, were analyzed by immunoblotting
using antibodies specific to a larger set of proteins known to be modulated during wound healing and apoptosis (Figure 3A–F). Two of these markers, collagen and α-SMA, showed consistent
differences between K and to a lesser extent between Nnp and P (Figure 3G). After 72 h of serum-starvation P showed little accumulation of collagen I and α-SMA (Figure 3G). K had
consistently higher collagen I levels and variably higher α-SMA levels, perhaps reflecting strain-dependent differences.35,36 Pk grown in serum containing medium, showed similar lower levels
of α-SMA and collagen I compared to the other fibroblast cultures (not shown). Less pronounced changes were the 10–50% higher levels of fibronectin (_t_-test; _P_=0.2) and vimentin
(_P_=0.38) in K _vs_ Pk cultures. When the general actin antibody was used no significant difference was seen between P and non-P. The levels of integrin β1 and Bax showed no consistent
variation among the cultures from different sources. However, we were able to detect more p53 in two out of four Pk (Palm D, Palm H in Figure 3D) compared to their paired K. When
normalization of the immunoblot results was done per protein (e.g. actin) the results of higher collagen I and higher α-SMA in K remained valid. The fibronectin levels became similar amongst
cultures and three out of four Pk showed higher p53 than did K. The fourth donor had barely detectable levels of p53 in either K or Pk (data not shown). As shown in Figure 4, the addition
of TGFβ1 to the serum-starved confluent cultures blunted the differences in collagen I and α-SMA content between K and non-K fibroblasts. All cultures (palmar and nonpalmar) showed
upregulation of collagen I (Figure 4A) and α-SMA (Figure 4C), when TGFβ1 was present in the media (lanes 2, 4, 6, 8). At the same time little effect was seen on the vimentin levels. Similar
conclusions were drawn from immunohistochemical analyses (not shown). APOPTOSIS IN SERUM STARVED KELOID FIBROBLASTS IS LOW After 72 h of serum withdrawal there was a striking difference
between cultures. Those of Pk (Figure 5c) and Pn (data not shown) contained many loosely bound and detaching round vesicles (cells). Few were found in K (Figure 5a) and Nnp (data not shown).
The addition of TGFβ1 alleviated this effect of serum withdrawal (Figure 5d). As lactate dehydrogenase (LDH) activity was not detected in the media of any cultures, there was not a
significant degree of lysis during starvation (not shown). Pk and Pn maintained in a serum-containing medium did not demonstrate detaching cells (not shown). The TUNEL assay for DNA
fragmentation was used to ascertain whether the appearance of the palmar cultures following serum withdrawal reflected an enhanced apoptosis. As shown in Figure 6, nuclei were differently
stained, with the darkest color (an indication of advanced apoptosis) within the picnotic nuclei (Figure 6). These appear to correspond to cells in the process of detachment (Figure 5).
Normally shaped TUNEL-positive cells correspond to a preapoptotic stage.37,38 The larger keloid fibroblasts had a regular shape, weak coloration and few picnotic nuclei (Figure 6a). This was
not influenced by TGFβ1 (Figure 6b). Both Pk and Pn had fuzzy nuclear membranes and many picnotic nuclei (Figure 6e,g). The addition of TGFβ1 caused improvements in nuclear definition and a
decrease in the number of dark picnotic nuclei (Figure 6f,h). Pk had a pronounced granular intranuclear structure (Figure 6f), which was not present in K (Figure 6b). The Nnp nuclei had
more staining than did K nuclei (compare Figure 6a and c). Some of the heavily stained cells appeared shrunken and smaller in size than the rest. TGFβ1 showed an effect on Nnp (Figure 6d).
Cells were bigger but still stained more than K (Figure 6b). In the presence of serum the preapoptotic state of K and non-K fibroblasts seemed comparable (not shown). Analysis of
TUNEL-stained fibroblasts by FACS revealed distinct differences between K, Nnp and P (Figure 7a). In these experiments, the level of apoptosis was measured by the cell count at corresponding
fluorescence intensities. The lowest level of apoptosis was seen in K fibroblasts (e.g. Keloid T) while intermediate (e.g. Nonpalm w) and high levels (e.g. Palm T, Palm l) were found in the
rest of the cultures. When membrane alteration and integrity were evaluated by Annexin V binding and Propidium iodide (PI) uptake (Figure 7b), results were similar to those obtained by
TUNEL. K and Nnp had the most live cells; 91±2% of the cells showed low levels of both FITC-Annexin V- and PI-fluorescence. This is significantly different from P, which contained 79±9% live
cells (_t_-test: _P_=0.075). Pk showed higher early and late apoptosis levels than K. Pn and Nnp were in intermediate states. Pn had the highest amount of necrotic cells. [The terminology
and definition for apoptosis and necrosis are as previously described.]39 It is possible that the Pk had already lost necrotic cells by detachment into the media (cf. Figure 5c). DISCUSSION
KELOID FIBROBLASTS DIFFER IN PHENOTYPE FROM PALMAR FIBROBLASTS Individuals prone to keloid formation are spared aberrant wound healing on their palmar and plantar surfaces, suggesting either
that the resident fibroblasts differ in phenotype or in microenvironment. Fibroblasts grown on plastic in submerged culture are under conditions resembling a fresh wound, thus revealing
features that may not be detectable in an already formed keloid. The experiments that are presented herein revealed intrinsic differences in the phenotypes of Pn/Pk and K fibroblasts. The
Nnp showed characteristics intermediate between Pk/Pn and K. The differences between palmar and nonpalmar fibroblasts may serve as clues about the development of keloids and about the reason
for their exclusion from glabrous tissue. Fibroblasts from keloids accumulated the most α-SMA, collagen I and TSP-1 under all culture conditions (Figures 1,2,3). Their α-SMA stress-fibers
were more prominent–thicker and abundant. There were also thicker collagen I and TSP-1 positive fibers in the keloid cultures (Figure 1a,c,i,k). This correlates with reports demonstrating a
higher lysyl oxidase (the enzyme catalyzing collagen crosslinking) expression in myofibroblasts.40 The differences between K and non-K were more pronounced when cells were deprived of serum
for 3 days. The keloid cultures were the least influenced by the serum withdrawal (Figure 1c _vs_ g; k _vs_ o; s _vs_ w, etc). Palmar fibroblasts, especially Pk, had low levels of α-SMA,
collagen and TSP-1 (Figures 1,2,3). The predominance of the myofibroblast phenotype (i.e. expression of α-SMA) in the K cultures remained even when we took into account the effect of plating
density17 (Figure 2). This suggests that this phenotype is an intrinsic feature of keloid fibroblasts. However, addition of TGFβ1 during serum starvation blunted the differences between
fibroblast cultures by causing an increase in the expression of α-SMA, collagen I (Figure 4) and TSP-1 (not shown) in all cultures. This cytokine has previously been demonstrated _in vivo_
and _in vitro_ to determine the expression of collagen I7,9,41 and α-SMA.42 The transient appearance of myofibroblasts in a healing wound is a prerequisite for proper wound healing and
correlates with TGFβ1 activity.43 Although the amount of TGFβ1 present in the conditioned media of normal skin fibroblasts was found equal to that in keloid fibroblasts embedded in a fibrin
maxtrix gel,44 our data (Figure 4) suggest that requirements for and levels of TGFβ1 might still determine the difference between palmar and nonpalmar fibroblasts (see ‘Note added in proof’
at the end). In addition, TGFβ1 activity may be greater in keloids as K expressed higher levels of the TGFβ1-activator TSP-1 (Figure 1i, k _vs_ j, l–p).45 Alternatively, in keloid formers,
the higher level of TNFα and lower level of γ-interferon, secreted by activated PMNs at the growing margins of the keloids,4 may also lead to increased collagen I and α-SMA expression.42
These cytokine differences may be sufficient to explain the occurrence of keloids. DELAY OF APOPTOSIS IN KELOID CULTURES The effect of serum withdrawal on confluent fibroblasts in submerged
culture demonstrated yet another aspect of palmar fibroblast behavior. Their behavior correlated with previous reports of confluence and starvation as factors that may lead to
apoptosis.46,47,48,49 In contrast to Nnp and K, the serum starved Pk and Pn became increasingly dysadherent (Figure 5)–a landmark of late stage apoptosis.47 Using TUNEL (Figure 6) and
Annexin V (Figure 7) apoptosis assays to further specify the apoptotic stage, it was found that K were delayed in apoptosis compared to Pk, Pn, and Nnp, especially Pk from the corresponding
keloid patients (Figures 5,6,7). This correlated with the predominance of myofibroblast stress-fibers in K cultures (Figures 1,2,3) and is consistent with the observed reduction of
myofibroblast markers in wounds prior to apoptosis16 and with other reports33 showing delayed apoptosis in subconfluent keloid fibroblasts. Although there have been reports of p53 in keloids
(_in vivo_ and _in vitro_),33 our immunoblots (Figure 3D,G) showed higher levels of p53 in non-keloid cultures. In the two cases (Palm D and Palm H, Figure 3D) this correlated with a higher
apoptosis (actual FACS scans not shown). Similar to our results, a negative correlation between α-SMA levels and apoptosis has been found by treatment of fibroblasts with IL-1β. This
cytokine causes induction of apoptosis mainly in fibroblasts with higher α-SMA expression.50 Other data51 have shown that the basic fibroblast growth factor (bFGF) leads to decrease in α-SMA
and induction of apoptosis. Using model systems for different stages of wound healing it has been found that a stressed ECM delays apoptosis.29,52,53 At nonpalmar, keloid-prone sites,
continued expression of α-SMA and collagen I could generate such a stressed ECM. The delay in keloid fibroblast apoptosis as demonstrated in our experiments (Figures 5,6,7) would give time
for excess fibroblast proliferation and further ECM synthesis. After this one might imagine fibroblasts being discarded by apoptosis leaving a large acellular collagenous mass. Not
surprisingly, at the time biopsies are typically taken, the keloid has usually been formed for more than several weeks and we should not expect to detect any α-SMA30 (and data not shown).
Although it may not be the myofibroblasts that undergo apoptosis in normal wound healing,29 a pronounced myofibroblast phenotype may lead to a delay in apoptosis, as the one observed in our
experiments. In summary, in the absence of TGFβ1, we found a stronger myofibroblast phenotype and lower apoptosis level in K compared to Pk, Pn and Nnp. Differences between Nnp and Pk, Pn
were also observed especially in response to serum withdrawal. Thus the absence of keloids from the palmar surface of keloid formers may be due to particular exogenous/endogenous TGFβ1
availability and specific palmar fibroblast requirements for higher TGFβ1 levels to promote growth and protection against apoptosis. As TGFβ1 levels are transient, collagen and α-SMA
production could be down-regulated at palmar sites where a quicker resolution of fibrosis by apoptosis might occur. Further _in vivo_ and _in vitro_ studies on the levels of vitamins,
cytokines and other growth factors, as well as their receptors, in keloids and nonkeloid (P, Nnp) tissues will be necessary to advance our knowledge of the etiology of keloids and provide
clues for improved therapies. MATERIALS AND METHODS KELOID PATIENTS Fibroblasts were isolated from biopsies of keloids and palms of four keloid patients. In three keloid cases (marked KD,
KH, KL) fibroblasts were grown from keloid subjacent to the epidermis, and in one keloid case (KT) fibroblasts were grown from the keloid deeper in the dermis. In both cases the biopsies
were taken away from the lesion boundaries in order to avoid mixing of fibroblast populations from the keloid and the adjacent tissue. As control, fibroblasts were isolated from biopsies of
the nonpalmar regions of two nonaffected individuals (African-American and Caucasian) and from the palms of two other nonaffected individuals (African-American and Hispanic). For
experimentation cells were used between the second and fifth passage. No consistent differences were found with passage. CELL CULTURES Cells were plated either at 105 cells per p100 plate
(50 cells/mm2) or 104 cells per p100 (5 cells/mm2, low plating density (lpd)). Cultures were grown to confluence at 37°C and 7.5% CO2 in low glucose DMEM (Gibco, BRL) supplemented with 10%
fetal bovine serum (HyClone), 100 units/ml penicillin and streptomycin. The serum-starved samples were grown in the same medium without serum for 72 h. (The 10% serum-containing medium has
14 μg/ml ascorbic acid and less than 0.5 ng/ml TGFβ). At confluence L-ascorbic acid-sodium salt (Sigma) was added to 50 μg/ml. When indicated the medium was supplemented with recombinant
TFGβ1 (R&D Systems) to 5 ng/ml. For protein analysis the cells were harvested in phosphate-buffered saline (PBS) by scraping with a Teflon scraper. Aliquots were taken for measurements
of protein and DNA. The cocktail of proteinase inhibitors included 1 mM EDTA, 5 mM benzimide, 5 ng/μl leupeptin, 1 μg/ml pepstatin, 0.5 μg/ml aprotinin and 0.1 mM PMSF. PROTEIN
ELECTROPHORESIS 1.5 mm Precast NOVEX (San Diego, CA) gradient 4–12% Tris-Glycine polyacrylamide SDS gels were used according to the conditions specified by the manufacturer. Samples were
sonicated, appropriate volumes of 10× sample buffer (0.6 M Tris-HCL, pH 6.8, 1.4(v/v) β-mercaptoethanol, 0.1% bromphenol-blue, 50%(w/v) SDS) were added and the samples were heated to 95°C
for 5 min before loading. Equal numbers of cells, according to nucleic acid content were loaded (approximately 2–4 μg nucleic acids per lane). SeeBlue™ prestained protein molecular weight
standards (NOVEX) were used together with 2 μg of rat-tail collagen type I (Upstate Biotechnology, NY, USA) as markers. NUCLEIC ACID AND PROTEIN CONCENTRATION Protein contents were
determined using the Bradford reagent with BSA as standard. Nucleic acid contents were estimated by measuring the absorption at 260 nm. PRIMARY ANTIBODIES Primary antibodies were as set out
in Table 1. SECONDARY ANTIBODIES Anti-[mouse/rabbit IgG (H+L)]-peroxidase conjugates (Boehringer, Mannheim) were used for the Western blots at 1 : 2500–5000. R-Phycoerythrin (PE) conjugated
anti-mouse IgG (whole molecule) (Sigma) were used at 1 : 100; affinity purified fluorescein (FITC)-conjugated goat anti-mouse IgG (H+L) (Jackson Immunoresearch Labs) and anti-rabbit F9(ab’)2
(G+L) IgG (TAGO, Burlingame, CA, USA) were diluted 1 : 100 prior to application. IMMUNOHISTOCHEMISTRY Acetone-fixed cells on chamber slides were treated with universal blocking solution
Power Block (Biogenex) or FBS 10% in PBS for 10 min. Primary antibodies were used at dilutions specified above. Incubations with secondary FITC- or PE-conjugated mouse/rabbit IgGs were for
60 min at room temperature. After washes with PBS the chamber slides were mounted with Vectashield plus DAPI (Vector Laboratories, Burlingame, CA, USA), visualized and photographed using a
Nikon Optiphot microscope with automatic exposure adjustment at 50× magnification on Kodak Gold Max 800 film. WESTERN BLOTTING Gels were electroblotted at 4 V DC overnight in a Semiphor
apparatus TE70 (Hoefer/Pharmacia). The blots were blocked in 5% nonfat dry milk, 1% BSA in PBS with 0.05% Tween (PBS/Tween). Primary antibodies were diluted in 5% nonfat milk-PBS and blots
were incubated for 1 h at room temperature or overnight at 4°C. After three PBS/Tween washes, the blots were incubated with peroxidase-conjugated secondary antibody (diluted in PBS/Tween
with 5% nonfat milk) for 30 min to 1 h. Detection was with the SuperSignal CL-HRP Substrate System (Pierce) for 5 min followed by exposure on X-ray film for seconds to minutes. Scanning of
the X-ray films was performed using ScanMaker Et (Mikrotek) and Adobe Photoshop 4.0 software. Densitometry was carried out with the automatic digitizing system Un-SCAN-IT gel version 4.1 for
Windows (Silk Sci. Corp., 1996) and Microsoft Excel 97 SR-1. To reprobe blots, antibodies were removed with a 30 min incubation at 60°C in 2% SDS, 62.5 mM Tris-HCL, pH 7, 100 mM
β-mercaptoethanol. Blots were then blocked for 15 min. APOPTOSIS (CHAMBER SLIDES) Cells grown on chamber slides were acetone fixed and microwave pretreated for 5 min. This approach proved to
be very efficient in revealing most cells in a preapoptotic or apoptotic state.38 After fixation the In Situ Cell Death Detection Kit, POD (Boehringer, Mannheim) was used according to the
manufacturer's protocol, with peroxidase as the detecting reagent. The antibody conjugate was diluted 1 : 2. The enzyme was omitted in the negative controls. FACS ANALYSIS Cells were
harvested by incubation with trypsin (0.05%) and EDTA (0.02%). About 1×106 cells were used per sample. When needed cells were fixed for 5 min with ice-cold acetone at −20°C, and washed twice
in PBS afterwards. Alternatively, fixation was performed using a 20 min incubation in HistoChoice (Amresco, Solon, Ohio) at room temperature, followed by a 5 min permeabilization on ice in
0.3% Triton in PBS with two subsequent washes in PBS. The samples were filtered through 35 μm cell strainer caps of Falcon polystryrene tubes before being applied to the FACS machine.
Α-SMOOTH MUSCLE ACTIN ANALYSES The cells were resuspended in 100 μl PBS and incubated for 1 h at room temperature with the 1 : 100 diluted antibody. After two PBS washes, the secondary
antibody (FITC conjugated mouse IgG, or, alternatively PE conjugated mouse IgG) was applied for 1 h at room temperature and later removed by PBS washes. Control cells for each fibroblast
culture, incubated only with the secondary antibody were used to define the background. TUNEL ASSAY (BOEHRINGER, IN SITU CELL DEATH DETECTION KIT, FLUORESCEIN) The samples were resuspended
in 50 μl TUNEL reaction mix and incubated for 1 h at 37°C with agitation. A control sample incubated without the enzyme was prepared for each fibroblast culture. A PBS wash was performed at
the end of the reaction. ANNEXIN V-FITC/PROPIDIUM IODIDE (PI) ANALYSES The ApoAlert Annexin V Apoptosis kit (Clontech) was used according to the manufacturer's procedure.54 Cells
incubated only in the binding buffer (without Annexin V and Propidium iodide) served as control for each fibroblast culture. After 15 min of dark incubation the samples were analyzed by
FACS. Cells with high Annexin V-FITC fluorescence (FL1) and low PI-fluorescence (FL2) (Figure 7b, lower right quadrant of the histogram) are considered in early apoptosis (or preapoptotic);
the ones with high FL2 and low FL1 (Figure 7b, upper left quadrant) as necrotic (the PI is able to enter the nuclei), the ones with high FL1 and FL2 (Figure 7b, upper right quadrant) as late
apoptotic (kit's manual). The position of the lines defining these four regions is determined using control cells incubated without Annexin V and PI. FLOW CYTOMETRY Flow cytometry was
performed on a Becton Dickinson FACScan with CellQuest software Version 3.2. LIGHT MICROSCOPY An Olympus VANOX-T microscope with an LBT filter model AHBT on 160T Kodak slide film was used
together with an inverted microscope Carl Zeiss IM 35. STATISTICAL ANALYSIS The effects reported were found in at least three independent experiments with several keloid patient cultures, as
well as with cultures of cells from several non-affected individuals. The Student _t_-test was used for comparison of the means. ABBREVIATIONS * ECM: extracellular matrix * FACS:
fluorescence aided cell sorting * K: keloid fibroblasts * lpd: low plating density * Nnp: nonaffected nonpalmar fibroblasts * P: palmar fibroblasts * _P_ : the probability that you are
incorrect in stating that the two means are different according to Student's _t_-test * PBS: phosphate buffered saline * PI: propidium iodide * Pk: palmar fibroblasts from a keloid
patient * Pn: palmar fibroblasts from a nonaffected individual * TSP-1: thrombospondin-1 * TUNEL: terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labeling REFERENCES *
Berman B and Bieley HC . (1995) Keloids. _J. Am. Acad. Derm._ 33: 117–123 Article CAS PubMed Google Scholar * Tredget EE, Nedelec B, Scott PG and Ghahary A . (1997) Hypertrophic scars,
keloids and contractures. _Surg. Clin. North Am._ 77: 701–730 Article CAS PubMed Google Scholar * Tuan T-l and Nichter LS . (1998) The molecular basis of keloid and hypertrophic scar
formation. _Molec. Med. Today_ 4: 19–23 Article CAS Google Scholar * McCauley RL, Chopra V, Li Y-Y, Herndon DN and Robson MC . (1992) Altered cytokine production in black patients with
keloids. _J. Clin. Immunol._ 12: 300–308 Article CAS PubMed Google Scholar * Abergel RP, Pizzuro D, Meeker CA, Lask G, Matsuoka LY, Minor RR, Chu M-L and Uitto J . (1985) Biochemical
composition of the connective tissue in keloids and analysis of collagen metabolism in keloid fibroblast cultures. _J. Invest. Dermatol._ 84: 384–390 Article CAS PubMed Google Scholar *
Ala-Kokko L, Rintala A and Savolainen E-R . (1987) Collagen gene expression in keloids: Analysis of collagen metabolism and type I, II, IV and V procollagen mRNA in keloid tissue and keloid
fibroblast cultures. _J. Invest. Dermatol._ 89: 238–244 Article CAS PubMed Google Scholar * Peltonen J, Hsiao LL, Jaakkola S, Sollberg S, Aumailley M, Timpl R, Chu M-L and Uitto J .
(1991) Activation of collagen gene expression in keloids: Co-localization of type I and VI collagen and transforming growth factor-β1 mRNA. _J. Invest. Dermatol._ 97: 240–248 Article CAS
PubMed Google Scholar * Friedman DW, Boyd CD, Mackenzie JW, Norton P, Olson RM and Deak SB . (1993) Regulation of collagen gene expression in keloids and hypertrophic scars. _J. Surg.
res._ 55: 214–222 Article CAS PubMed Google Scholar * Bettinger DA, Yager DR, Diegelman RF and Cohen IK . (1996) The effect of TGF-β on keloid fibroblasts proliferation and collagen
synthesis. _Plastic Reconstr. Surg._ 98: 827–833 Article CAS Google Scholar * Sato H, Suzuki A, Funahashi M, Takezawa T, Ogawa Y and Yoshizato K . (1996) Characteristics of growth,
morphology, contractility and protein expression of fibroblasts derived from keloid. _Wound Repair Regener._ 4: 103–114 Article CAS Google Scholar * Younai S, Nichter LS, Wellisz T,
Reinisch J, Nimni ME and Tuan T-L . (1994) Modulation of collagen synthesis by transforming growth factor-β in keloid and hypertrophic scar fibroblasts. _Ann. Plast. Surg._ 33: 148–154
Article CAS PubMed Google Scholar * Kikuchi K, Kadono T and Takehara K . (1995) Effects of various growth factors and histamine on cultured keloid fibroblasts. _Dermatol._ 190: 4–8
Article CAS Google Scholar * Yamaguchi Y, Mann DM and Ruoslahti E . (1990) Negative regulation of the transforming growth factor-β by the proteoglycan decorin. _Nature_ 346: 281–284
Article CAS PubMed Google Scholar * Tan EML, Hoffren J, Rouda S, Greenbaum S, Fox JW, Moore JH and Dodge GR . (1993) Decorin, versican and biglycan gene expression by keloid and normal
dermal fibroblasts: Differential regulation by basic fibroblast growth factor. _Exp. Cell Res._ 209: 200–207 Article CAS PubMed Google Scholar * Hunzelmann N, Anders S, Sollberg S,
Schonherr E and Krieg T . (1996) Coordinate induction of collagen type I and biglycan expression in keloids. _Br. J. Dermatol._ 135: 94–399 Article Google Scholar * Darby I, Skalli O and
Gabbiani G . (1990) α-Smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. _Lab. Investig._ 63: 21–29 CAS PubMed Google Scholar * Masur SK,
Dewal HS, Dinh TT, Ehrenburg I and Petridou S . (1996) Myofibroblasts differentiate from fibroblasts when plated at low density. _Proc. Natl. Acad. Sci._ 93: 4219–4223 Article CAS PubMed
PubMed Central Google Scholar * Desmoulière A, Geinoz A, Gabbiani F and Gabbiani G . (1993) Transforming factor-β1 induces α-smooth muscle actin expression in granulation tissue
myofibroblasts and in quiescent and growing cultured fibroblasts. _J. Cell Biol._ 122: 103–111 Article PubMed Google Scholar * Narani N, Arora PD, Lew A, Luo M, Glogauer M, Ganss B and
McCuloch CAG . (1997) Transforming growth factor-β induction of α-smooth muscle actin is dependent on the deformability of the collagen matrix. _Curr. Top. Pathol._ 93: 47–60 Article Google
Scholar * Arora PD, Narani N and McCulloch CAG . (1999) The compliance of collagen gels regulates transforming growth factor-β induction of α-smooth muscle action in fibroblasts. _Am. J.
Pathol._ 154: 871–882 Article CAS PubMed PubMed Central Google Scholar * Serini G, Bochaton-Piallat M-L, Ropraz P, Geinoz A, Borsi L, Zardi L and Gabbiani G . (1998) The fibronectin
domain ED-A is crucial for myofibroblastic phenotype induction by transforming factor-β1. _J. Cell Biol._ 142: 873–881 Article CAS PubMed PubMed Central Google Scholar * Balza E, Borsi
L, Allemanni G and Zardi L . (1988) Transforming growth factor β regulates the level of different fibronectin isoforms in normal human cultured fibroblasts. _FEBS Lett._ 228: 42–44 Article
CAS PubMed Google Scholar * Bürger A, Wagner C, Viedt C, Reis B, Hug F and Hänsch GM . (1998) Fibronectin synthesis by human tubular epithelial cells in culture: Effects of PDGF and TGF-β
on synthesis and splicing. _Kidney Int._ 54: 407–415 Article PubMed Google Scholar * Clark RAF . (1993) Regulation of fibroplasia in cutaneous wound repair. _Am. J. Med. Sci._ 306: 42–48
Article CAS PubMed Google Scholar * Desmoulière A, Redard M, Darby I and Gabbiani . (1995) Apoptosis mediates the decrease in cellularity during the transition between granulation
tissue and scar. _Am. J. Pathol._ 146: 56–66 PubMed PubMed Central Google Scholar * Berndt A, Koshmehl H, Mandel U, Gabler U, Luo X, Celeda D, Zardi L and Katenkamp D . (1995) TGFβ and
bFGF synthesis and localization in Dupuytren's disease (nodular palmar fibromatosis) relative to cellular activity, myofibroblast phenotype and oncofetal variants of fibronectin.
_Histochem. J._ 27: 1014–1020 Article CAS PubMed Google Scholar * Wilutzky B, Berndt A, Katenkamp D and Koshmehl H . (1998) Programmed cell death in nodular palmar fibromatosis (Morbus
Dupuytren). _Histol. Histopathol._ 13: 67–72 CAS PubMed Google Scholar * Desmoulière A, Geinoz A, Gabbiani F and Gabbiani G . (1993) Transforming factor-β1 induces α-smooth muscle actin
expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. _J. Cell Biol._ 122: 103–111 Article PubMed Google Scholar * Grinnell F, Zhu M, Carlson
MA and Abrams JM . (1999) Release of mechanical tension triggers apoptosis of human fibroblasts in a model of regressing granulation tissue. _Exp. Cell Res._ 248: 608–619 Article CAS
PubMed Google Scholar * Ehrlich HP, Desmoulière A, Diegelman RF, Cohen IK, Compton CC, Garner WL, Kapanci Y and Gabbiani G . (1994) Morphological and immunochemical differences between
keloid and hypertrophic scar. _Am. J. Pathol._ 145: 105–113 CAS PubMed PubMed Central Google Scholar * Ehrlich HP, Cremona O and Gabianni G . (1998) The expression of alpha 2 beta 1
integrin and alpha smooth muscle actin in fibroblasts grown on collagen. _Cell Biochem. Funct._ 16: 129–137 Article CAS PubMed Google Scholar * Appleton I, Brown NJ and Willoughby DA .
(1996) Apoptosis, necrosis and proliferation. Possible implications in the etiology of keloids. _Am. J. Pathol._ 149: 1441–1447 CAS PubMed PubMed Central Google Scholar * Ladin DA, Hou
Z, Patel D, McPhail M, Olson JC, Saed GM and Fivenson DP . (1998) p53 and apoptosis alterations in keloids and keloid fibroblasts. _Wound Repair Regen._ 6: 28–37 Article CAS PubMed Google
Scholar * Saed GM, Ladin D, Olson J, Han X, Hou Z and Fivenson D . (1998) Analysis of p53 gene mutations in keloids using polymerase chain reaction-based single strand conformational
polymorphism and DNA sequencing. _Arch. Dermatol._ 134: 963–967 Article CAS PubMed Google Scholar * Moulin V, Castilloux G, Jean A, Garrel DR, Auger FA and Germain L . (1996) In vitro
models to study wound healing fibroblasts. _Burns_ 22: 359–362 Article CAS PubMed Google Scholar * Moulin V, Castilloux G, Auger FA, Garrel D, O'Connor-McCourt MD and Germain L .
(1998) Modulated response to cytokines of human wound healing myofibroblasts compared to dermal fibroblasts. _Exp. Cell Res._ 238: 283–293 Article CAS PubMed Google Scholar * Gorczyca W,
Gong J and Darzynkiewicz Z . (1993) Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. _Cancer
Res._ 53: 1945–1951 CAS PubMed Google Scholar * Negoiescu A, Lorimier P, Labat-Moleur F, Drouet C, Robert C, Guillermet C, Brambilla C and Brambilla E . (1996) In situ apoptotic cell
labeling by the TUNEL method: improvement and evaluation of cell preparations. _J. Histochem. Cytochem._ 44: 959–968 Article Google Scholar * Trump BF and Berezesky IK . (1998) The
reaction of cells to lethal injury: Oncosis and necrosis–the role of calcium. In: When Cells Die. Lockshin RA, Zakeri Z and Tilly JL eds. (New York: J Wiley & Sons) pp. 57–97 * Peyrol S,
Raccurt M, Gerard F, Gleyzal C, Grimaud JA and Sommer P . (1997) Lysyl oxidase gene expression in the stromal reaction to in situ and invasive ductal breast carcinoma. _Am. J. Pathol._ 150:
497–507 CAS PubMed PubMed Central Google Scholar * Babu M, Diegelman R and Oliver N . (1992) Keloid fibroblasts exhibit an altered response to TGF-β. _J. Invest. Dermatol._ 99: 650–655
Article CAS PubMed Google Scholar * Desmoulière A . (1995) Factors influencing myofibroblast differentiation during wound healing and fibrosis. _Cell. Biol. Intern._ 19: 471–476 Article
Google Scholar * Frank S, Madlener M and Werner S . (1996) Transforming growth factors β1, β2, β3 and their receptors are differentially regulated during normal and impaired wound
healing. _J. Biol. Chem._ 271: 10188–10193 Article CAS PubMed Google Scholar * Younai S, Venters G, Vu S, Nichter L, Nimni ME and Tuan T-L . (1996) Role of growth factors in scar
contraction: An in vitro analysis. _Ann. Plast. Surg._ 36: 495–502 Article CAS PubMed Google Scholar * Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SMF, Lawler JH, Hynes RO,
Boivin GP and Bouck N . (1998) Thrombospondin-1 is the major activator of TGFβ-1 in vivo. _Cell_ 93: 1159–1170 Article CAS PubMed Google Scholar * Mymric J, Shire K and Bayley ST .
(1994) Induction of apoptosis by adenovirus type 5 E1A in rat cells requires a proliferation block. _Oncogene_ 9: 1187–1193 Google Scholar * Desjardins LM and MacManus JP . (1995) An
adherent cell model to study different stages of apoptosis. _Exp. Cell Res._ 216: 380–387 Article CAS PubMed Google Scholar * Ishizaki Y, Cheng L, Mudge AW and Raff MC . (1995)
Programmed cell death by default in embryonic cells, fibroblasts, and cancer cells. _Exp. Cell Res._ 216: 380–387 Article Google Scholar * Brezden CB and Rauth AM . (1996) Differential
cell death in immortalized and nonimmortalized cells at confluency. _Oncogene_ 12: 201–206 CAS PubMed Google Scholar * Zhang H-Y, Gharaee-Kermani M and Phan SH . (1997) Regulation of lung
fibroblast α-smooth muscle actin expression, contractile phenotype and apoptosis by IL-1β. _J. Immunol._ 158: 1392–1399 CAS PubMed Google Scholar * Funato N, Moriyama K, Shimokawa H and
Kuroda T . (1997) Basic fibroblast growth factor induces apoptosis in myofibroblastic cells isolated from rat palatal mucosa. _Biochem. Biophys. Res.Commun._ 240: 21–26 Article CAS PubMed
Google Scholar * Grinnell F . (1994) Fibroblasts, myofibroblasts and wound contraction. _J. Cell Biol._ 124: 401–404 Article CAS PubMed Google Scholar * Fluck J, Querfeld C, Cremer A,
Niland S, Krieg T and Solverg S . (1998) Normal human primary fibroblasts undergo apoptosis in three-dimensional contractile gels. _J. Invest. Dermatol._ 110: 153–157 Article CAS PubMed
Google Scholar * Martin SJ, Reutelingsperger CPM, McGahon AJ, Rader JA, van Schie RCAA, LaFace DM and Green DR . (1995) Early distribution of plasma membrane phosphatidylserine is a general
feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Abl. _J. Exp. Med._ 182: 1545–1556 Article CAS PubMed Google Scholar Download
references ACKNOWLEDGEMENTS We thank Dr. C Pullis for the FACS analysis, Dr. N Ramamurthy and the members of the Simon group for helpful discussions and advice. This work was partially
funded by a grant to AE Katz from the American Association of Plastic and Reconstructive Surgery. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Living Skin Bank, SUNY at Stony Brook, 11794,
NY, USA C C Chipev, R Simman, G Hatch & M Simon * Burn Center, University Hospital, SUNY at Stony Brook, 11794, NY, USA R Simman * Department of Surgery, Health Sciences Center, SUNY at
Stony Brook, 11794, NY, USA R Simman & A E Katz * Department of Otolaryngology, Health Sciences Center, SUNY at Stony Brook, 11794, NY, USA A E Katz * Department of Dermatology, Health
Sciences Center, SUNY at Stony Brook, 11794, NY, USA D M Siegel & M Simon * Department of Oral Biology and Pathology, Health Sciences Center, SUNY at Stony Brook, 11794, NY, USA M Simon
Authors * C C Chipev View author publications You can also search for this author inPubMed Google Scholar * R Simman View author publications You can also search for this author inPubMed
Google Scholar * G Hatch View author publications You can also search for this author inPubMed Google Scholar * A E Katz View author publications You can also search for this author inPubMed
Google Scholar * D M Siegel View author publications You can also search for this author inPubMed Google Scholar * M Simon View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to C C Chipev or M Simon. ADDITIONAL INFORMATION Edited by P Cohen RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Chipev, C., Simman, R., Hatch, G. _et al._ Myofibroblast phenotype and apoptosis in keloid and palmar fibroblasts _IN VITRO_. _Cell Death Differ_ 7, 166–176 (2000).
https://doi.org/10.1038/sj.cdd.4400605 Download citation * Received: 03 September 1999 * Accepted: 30 September 1999 * Published: 07 March 2000 * Issue Date: 01 February 2000 * DOI:
https://doi.org/10.1038/sj.cdd.4400605 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * alpha smooth muscle actin * collagen I *
thrombospondin-1 * serum starvation
