Has cox-2 a prognostic role in non-small-cell lung cancer? A systematic review of the literature with meta-analysis of the survival results
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Cyclooxygenase-2 (COX-2) is overexpressed in lung cancer, especially in adenocarcinoma (ADC). Our aim was to determine the prognostic value of COX-2 on survival in patients with
lung cancer. Studies evaluating the survival impact of COX-2 in lung cancer, published until December 2005, were selected. Data for estimation of individual hazard ratios (HR) for survival
were extracted from the publications and combined in a pooled HR. Among 14 eligible papers, all dealing with non-small-cell lung cancer, 10 provided results for meta-analysis of survival
data (evaluable studies). Cyclooxygenase-2 positivity was associated with reduced survival, improved survival or no statistically significant impact in six, one and seven studies,
respectively. Combined HR for the 10 evaluable studies (1236 patients) was 1.39 (95% confidence intervals (CI): 0.97–1.99). In stage I lung cancer (six evaluable studies, 554 patients), it
was 1.64 (95% CI: 1.21–2.24). No significant impact was shown in ADC. A slight detrimental effect on survival in patients with lung cancer is associated with COX-2 expression, but the
statistical significance is not reached. This effect is statistically significant in stage I, suggesting that COX-2 expression could be useful at early stages to distinguish those with a
worse prognosis. SIMILAR CONTENT BEING VIEWED BY OTHERS PROGNOSIS OF RECURRENCE AFTER COMPLETE RESECTION IN EARLY-STAGE LUNG ADENOCARCINOMA BASED ON MOLECULAR ALTERATIONS: A SYSTEMATIC
REVIEW AND META-ANALYSIS Article Open access 31 October 2023 PROGNOSTIC AND PREDICTIVE VALUE OF THE NEWLY PROPOSED GRADING SYSTEM OF INVASIVE PULMONARY ADENOCARCINOMA IN CHINESE PATIENTS: A
RETROSPECTIVE MULTICOHORT STUDY Article 10 January 2022 PD-L1 EXPRESSION, _EGFR_ AND _KRAS_ MUTATIONS AND SURVIVAL AMONG STAGE III UNRESECTED NON-SMALL CELL LUNG CANCER PATIENTS: A DANISH
COHORT STUDY Article Open access 19 August 2021 MAIN Lung cancer is a major cause of death despite diagnostic and therapeutic improvements. The overall 5-year survival rate is around 10%
(Boyle and Ferlay, 2005). Some independent prognostic factors for survival have already been identified. They include, for small cell lung cancer (SCLC): disease extent and performance
status (PS) (Paesmans et al, 2000); for non-small-cell lung cancer (NSCLC): PS, stage and, with lower impact, age, sex and weight loss (Paesmans et al, 1995; Strauss, 1997). The biological
factors involved in carcinogenesis should also be considered as potential survival prognostic factors. Some of them, like angiogenesis and factors reflecting proliferative state, have
already been identified in patients with lung cancer (Kanters et al, 1995). In order to clarify the prognostic impact of other biological factors in lung cancer, our group has performed
systematic reviews of the literature with meta-analyses. It allowed us to show that VEGF (Delmotte et al, 2002), microvessel density (Meert et al, 2002b), EGFR (Meert et al, 2002a),
HER-2/Neu (Meert et al, 2003), Ki-67 (Martin et al, 2004), K-Ras (Mascaux et al, 2005a) and p53 (Steels et al, 2001) have a negative impact on survival, whereas Bcl-2 (Martin et al, 2003) is
associated with a favourable survival effect, at least when studying their impact in univariate analysis. Recent attention has been drawn to prostaglandins and cyclooxygenases (COX) with
the discovery that colonic polyps in patients with familial adenomatous polyposis (FAP) are decreased after the administration of non-steroidal anti-inflammatory drugs (NSAIDs) (Waddell and
Loughry, 1983). Cyclooxygenases are key enzymes in the conversion of arachidonic acid to prostaglandin and exist as two isoforms, COX-1 and COX-2 (Smith and Langenbach, 2001).
Cyclooxygenase-1 is constitutively expressed in nearly all cell types and plays a central role in many normal physiological processes, such as cytoprotection of gastric mucosa. _COX_-2 is a
highly inducible gene, activated by cytokines, growth factors, phorbol esters, oncogenes and chemical carcinogens (Smith and Langenbach, 2001). Overexpression of COX-2 has been reported in
many human malignancies including head and neck carcinomas (Gallo et al, 2002; Lin et al, 2002), oesophagus (Lagorce et al, 2003), colon (Sinicrope and Gill, 2004), breast (Ranger et al,
2004), pancreas (Kokawa et al, 2001) and prostatic cancer (Edwards et al, 2004). In NSCLC, an increase in COX-2 expression was detected both in adenocarcinomas (ADC) and in squamous cell
carcinomas (SQCC), but at a higher level in ADC than in SQCC (Hida et al, 1998; Wolff et al, 1998; Ochiai et al, 1999). Cyclooxygenase-2 expression was also increased in atypical adenomatous
hyperplasia, a possible precursor of ADC (Hida et al, 1998; Wolff et al, 1998; Hosomi et al, 2000; Hasturk et al, 2002) and in severe dysplasia and _in situ_ carcinoma, precursors of SQCC
(Mascaux et al, 2005b). However, the literature remains controversial about the prognostic value of COX-2 for survival in patients with lung cancer. In order to clarify this question, we
performed a systematic review of the literature with methodological assessment and meta-analysis. MATERIALS AND METHODS SELECTION OF THE PUBLICATIONS To be eligible for the systematic
review, a study had to fulfil the following criteria: to deal only with lung cancer (any stage or histology), to analyse the association between COX-2 and survival, to assess COX-2 on the
primary tumour (not on metastatic tissue or tissue adjacent to the tumour), to have been published as a full paper in English or French. Abstracts were excluded because they do not provide
sufficient data to evaluate the methodology of the trial and/or to perform meta-analysis. Studies were identified by an electronic search on Medline databank and using the following
keywords: ‘lung cancer’, ‘lung carcinoma’, ‘lung neoplasms’, ‘lung tumor’, ‘lung tumors’, ‘lung tumour’, ‘lung tumours’, ‘lung adenocarcinoma’, ‘lung squamous’, ‘NSCLC’, ‘non-small cell lung
cancer’, ‘non small cell lung cancer’, ‘non-small cell lung carcinoma’, ‘non small cell lung carcinoma’, ‘SCLC’, ‘small cell lung cancer’, ‘small cell lung carcinoma’, ‘cyclooxygenase’,
‘cyclooxygenase-2’, ‘COX-2’. The bibliographies reported in all the identified studies were used to complete this search, which ended on December 2005. METHODOLOGICAL ASSESSMENT To assess
the methodology, each study report was read independently by 10 investigators. The participation of many readers was a guarantee for the correct interpretation of the articles. The
methodological evaluation was scored according to the European Lung Cancer Working Party (ELCWP) scale previously published (Steels et al, 2001) and applied in other meta-analyses (Delmotte
et al, 2002; Meert et al, 2002a, 2002b, 2003; Martin et al4, 2003, 2004; Mascaux et al, 2005a). Each item was assessed using an ordinal scale (possible values: 2, 1, 0). A consensus was
reached in regular meetings where at least two-thirds of the investigators needed to be present. As the assessed items were objective ones, a consensus was always obtained. The overall score
evaluated several dimensions of the methodology, grouped in four main categories: the scientific design, the description of laboratory methods used to identify COX-2 expression, the
generalisability of the results and the analysis of the study data. Each category had a maximum score of 100 points, with a maximal theoretical score of 400 points. When an item was not
applicable to a study, its value was not taken into account in the total of the concerned category. The final scores were expressed as percentages, ranging from 0 to 100%, higher values
reflecting better methodological quality. Studies included in the systematic review were called ‘eligible’, those providing sufficient data for the meta-analysis ‘evaluable’. To be eligible,
studies had to provide univariate survival analysis according to COX-2. STATISTICAL METHODS A study was considered significant if the _P_-value for the statistical test, comparing survival
distributions between the groups with and without COX-2 increase, was <0.05. A study was called respectively, ‘positive’ or ‘negative’ when COX-2 increase was identified as a significant
favourable or unfavourable prognostic factor for survival. These studies were further called ‘significant’ ones. Finally, a study was called ‘not significant’ if no statistically significant
difference between the two groups was detected. The association between two continuous variables was measured by the Spearman rank correlation coefficient. Non-parametric tests were used to
compare the distribution of the quality scores according to the value of a discrete variable (Mann–Whitney tests for dichotomic variables and Kruskal–Wallis tests for multiple classes
variables). For the quantitative aggregation of the survival results, we measured the impact of COX-2 increase on survival by hazard ratio (HR) between the two survival distributions. For
each trial, this HR was estimated by a method depending on the data provided in the publication. The most accurate method consisted of extracting the estimated HR and its standard error
(s.e.) from the reported results using two of the following parameters: the HR and its confidence interval (CI) or the _O_−_E_ statistic (difference between numbers of observed and expected
events), and the log-rank statistic or its _P_-value. If these data were not available, the total number of events, the number of patients at risk in each group and the log-rank statistic or
its _P_-value were used to allow for an approximation of the HR estimate. Finally, if the only exploitable data were in the form of graphical representations of the survival distributions,
survival rates at some specified times were extracted in order to reconstruct the HR estimate and its variance, with the assumption that the rate of patients censored was constant during the
study follow-up (Parmar et al, 1998). If this last method was used, three independent persons read the curves to reduce inaccuracy in the extracted survival rates. The individual HR
estimates were combined into an overall HR using Peto's method (Yusuf et al, 1985), which consisted of using a fixed-effect model assuming homogeneity of the individual true HRs. This
assumption was tested by performing _χ_2 tests for heterogeneity. If the assumption of homogeneity had to be rejected, we used a random-effect model as a second analysis. By convention, an
observed HR<1 implied a better survival for the group with COX-2 increase. This impact of COX-2 on survival was considered statistically significant if the 95% CI for the overall HR did
not overlap 1. When data about global survival of the entire patients' population were available, survival was analysed globally. If authors only reported the results separately for
different subgroups, those results corresponding to different cohorts of patients were treated separately in the meta-analysis. RESULTS STUDY SELECTION AND CHARACTERISTICS Fourteen
publications, published between 1999 and 2005, were eligible for the systematic review (Achiwa et al, 1999; Hosomi et al, 2000; Khuri et al, 2001; Brabender et al, 2002; Kim et al, 2003;
Ab' Saber et al, 2004; Araki et al, 2004; Lu et al, 2004; Yamaguchi et al, 2004; Brattstrom et al, 2005; Laga et al, 2005; Marrogi et al, 2005; Richardson et al, 2005; Yuan et al,
2005). These publications concerned different cohorts of patients. The total number of included patients was 1543, ranging from 53 to 259 patients per study (median: 92). The main
characteristics of the 14 eligible publications are reported in Table 1. Nine were dealing with all types of NSCLC, four with ADC and one with large-cell carcinoma. Seven studies only
concerned locoregional diseases (two studies concerned stages I–IIIA and four, stages I–IIIB), four only stage I disease and four all stages (I–IV). Ten studies evaluated COX-2 expression by
immunohistochemistry (IHC), two studies assessed COX-2 mRNA overexpression by reverse transcription–polymerase chain reaction (RT–PCR) in real time and the last two studies determined COX-2
gene amplification by _in situ_ hybridisation. Among the 14 studies eligible for the systematic review, four (Hosomi et al, 2000; Ab' Saber et al, 2004; Brattstrom et al, 2005; Marrogi
et al, 2005) were inevaluable for the meta-analysis owing to a lack of data in the publication, not allowing to calculate the individual HR and its variance. STUDY RESULTS REPORT Six of the
14 studies identified COX-2 overexpression as a poor prognostic factor for survival (with five evaluable for the meta-analysis) whereas one reported that it was a good prognostic factor
(evaluable for the meta-analysis). The seven other studies showed no statistically significant impact of COX-2 overexpression on survival (four evaluable for meta-analysis). Overall, in
NSCLC, the rates of COX-2 protein overexpression (detected by IHC), COX-2 mRNA expression (RT–PCR) and COX-2 gene amplification (detected by _in_ s_itu_ hybridisation) were respectively,
62.4% (number of evaluable tumours _n_=833, 51.7% (_n_=149) and 59.8% (_n_=254). For ADC, IHC and ISH assessments reported were respectively, 69% of COX-2-positive tumours (_n_=368) and
41.2% (_n_=34). The rates of positive tumours by IHC, RT–PCR and _in situ_ hybridisation in stage I NSCLC were, respectively, 65% (_n_=240), 50% (_n_=60) and 59.8% (_n_=254). QUALITY
ASSESSMENT The overall quality score ranged from 36.3 to 66.0% with a median of 51.5%. No statistically significant quality difference was shown between significant and non-significant
studies for the global score (median: 55.4 _vs_ 48.9%, _P_=0.06). There was also no statistically significant difference between evaluable and non-evaluable studies for meta-analysis in
terms of global scores (51.5 _vs_ 53.4%, _P_=0.78). We performed the same analysis of the scores for the 10 studies evaluable for meta-analysis. Their overall quality score ranged between
41.8 and 66%, with a median of 51.5%. As previously observed among eligible publications, there was no statistically significant difference between significant and non-significant studies
evaluable for the meta-analysis according to the global score (median of 54.6 _vs_ 48.4%, _P_=0.09). META-ANALYSIS The meta-analysis was performed on 10 studies (1236 patients) dealing with
NSCLC, and were shown to have similar methodological scores. The individual HRs of the 10 evaluable studies were calculated by one of the three methods reported in the Materials and Methods
section according to available data. One study reported the data needed to directly calculate the estimated HR (95% CI). In two trials, HR was approximated by the total number of events and
the log-rank statistic. For the seven remaining studies, HR had to be extrapolated from the graphical representation of the survival distributions. The results of the meta-analysis are
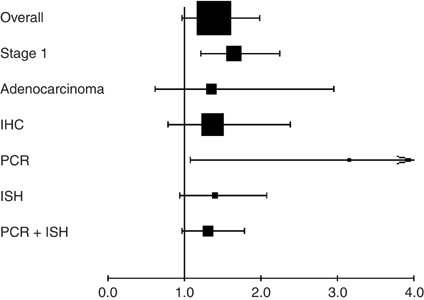
reported in Table 2 and in Figure 3. Overall, COX-2 overexpression was not associated with a significant impact on survival. As the test for heterogeneity was highly significant
(_P_<0.001), we also applied a random-effect model in calculating the HR, which was 1.39 (95% CI: 0.97–1.99) (Figure 1). Regarding subgroup analyses (Figure 3), we had the adequate data
to aggregate the studies dealing with stage I, with ADC and according to the technique used to detect Cox-2. We first performed an interaction test to assess whether there might be a
differential effect of COX-2 according to stage, histology or the technique. We found one significant interaction between COX-2 and stage (_P_<0.01). When we aggregated the six studies
(Achiwa et al, 1999; Khuri et al, 2001; Araki et al, 2004; Lu et al, 2004; Richardson et al, 2005; Yuan et al, 2005) giving separate results about stage I NSCLC, the combined HR was
statistically significant by using the random-effect model: HR 1.64, 95% CI (1.21–2.24) as there was indeed a significant heterogeneity (_P_=0.04) (Figure 2). We did not observe a
statistically significant effect of COX-2 on survival in ADC (five evaluable studies) (Achiwa et al, 1999; Araki et al, 2004; Yamaguchi et al, 2004; Richardson et al, 2005; Yuan et al, 2005)
with HR 1.35 (95% CI 0.62–2.95) (random effect; test of heterogeneity _P_<0.001). We also found one significant interaction between COX-2 and the technique used for its detection
(_P_=0.003). The test of heterogeneity was significant for the IHC studies (_P_=0.00001), but neither for RT–PCR studies (_P_=0.1), nor, for ISH studies (_P_=0.18). As the number of studies
in the subgroups was small, we only report the HR estimated by the random effect because of a lack of power of the test of heterogeneity. The HRs were the following: for the six studies with
IHC (833 patients) 1.06 (95% CI 0.64–1.77), for the two RT–PCR studies (149 patients) 3.15 (1.08–9.21), for the two ISH studies (254 patients) 1.40 (0.94–2.07) and for RT–PCR and ISH
studies together (403 patients) 1.31 (0.97–1.76). DISCUSSION The present systematic review of the literature about the impact of COX-2 overexpression on survival in lung cancer found a
slight role of COX-2 on overall survival in NSCLC, without not reaching statistical significance. When the analysis was restricted to stage I NSCLC, we observed a statistically significant
detrimental effect of COX-2 on survival, suggesting that this prognostic factor could be of importance in early-stage NSCLC. In subgroup analysis according to the different techniques used
to detect COX-2, results were only significant with RT–PCR. The search for a potential prognostic role of COX-2 in survival for patients with lung cancer is based on its frequent
overexpression in NSCLC and also on its potential interference with most pathways implicated in lung carcinogenesis. The role of COX-2 in oncogenesis has widely been studied by _in vitro_
experiments and by _in vivo_ analyses based on animal models. In lung cancer, COX-2 overexpression is associated with microvascular angiogenesis (Masferrer et al, 2000) and resistance to
apoptosis (Liu et al, 1998; Hida et al, 2000). Cyclooxygenase-2 overexpression also decreases host immunity (Huang et al, 1998) and alters cell adhesion with enhancement of invasion and
metastasis (Tsujii et al, 1997). Despite all these experimental observations, our meta-analysis failed to demonstrate in univariate analysis a statistically significant impact of COX-2
expression as a prognostic factor for overall survival in patients with NSCLC. In subgroup analysis, we observed a significant effect in stage I NSCLC. Cyclooxygenase-2 overexpression might
modify the prognosis of early-stage NSCLC: early lung cancer overexpressing COX-2 would be more aggressive and would have a worse prognosis than those without COX-2 abnormality. These data
could be helpful to determine among stage I diseases those who could benefit from a more aggressive treatment. But the present results concerning the prognostic role of COX-2 in stage I
NSCLC still need to be confirmed by adequately designed prospective studies with multivariate analysis before a potential clinical application. It should be noted that COX-2 appears early in
oncogenesis for SQCC (Mascaux et al, 2005b) as well as for ADC (Hida et al, 1998; Wolff et al, 1998; Hosomi et al, 2000; Hasturk et al, 2002). In a previous study (Mascaux et al, 2005b), we
observed that COX-2 expression increases in bronchial preneoplastic lesions at the stage of severe dysplasia and particularly in clones of cells showing atypia: this suggests an active role
of COX-2 in bronchial epithelial cells transformation to malignancy. These data could partially explain the prognostic role of COX-2 at stage I, its impact being lost at later steps because
of the potential interaction with many factors. Our analysis had to deal with heterogeneity problems. There was a highly significant heterogeneity among the 10 evaluable studies included in
the meta-analysis. This could be explained by the type of patients and the disease characteristics, or by the diversity in the techniques used to identify alteration of COX-2 status. Only
six evaluable studies used IHC, two ISH and two RT–PCR. The results of subgroup analysis according to the technique used to detect COX-2 support this hypothesis. Results for the six IHC
studies were not significant and a high heterogeneity was detected between the studies (_P_=0.003). This heterogeneity could be explained by the fact that the technique of IHC is not
comparable among the six studies. The primary antibodies were different and so was the revelation protocols, and different levels of positivity (0, 10, 50%, different scores combining
intensity and percentage, intensity only) were used. As another example, when ISH and RT–PCR (two different techniques assessing RNA) studies were aggregated together, the heterogeneity
increased (_P_=0.03) as compared with ISH alone (_P_=0.18) or RT–PCR alone (_P_=0.1), and with only a few studies, the results were statistically significant for the RT–PCR subgroup, which
is the most standardised technique. It is thus very important to use a well-defined and well-standardised technique to be reproducible for the evaluation of biological markers. Particularly,
the protocol of IHC should be the same between different laboratories (same antibody, same revelation protocol (pH and compounds of the solutions, heating method etc) and same criteria of
evaluation for the positivity of the marker) so that the results could be compared and eventually, aggregated. Some other biases could be due to the methodology used to perform our
systematic review. We performed a methodological assessment of the studies to avoid some selection biases (more detailed reports of significant trials), as we performed in prior studies
about biological prognostic factors in lung cancer (Steels et al, 2001). The absence of a detectable difference in quality score between significant and non-significant studies, and between
evaluable and non-evaluable studies, encourages us to perform a quantitative aggregation (meta-analysis) of the results of the individual trials. However, in the present review, numbers of
studies are small, preventing us to analyse any potential difference between significant and non-significant, or evaluable and non-evaluable studies. However, this approach does not prevent
all potential biases. Publication bias, choice of language, selection of fully published studies only, method of extrapolation of HR, validity of a meta-analysis based on systematic review
of the literature as compared with those based on individual data were already discussed in our previous papers (Steels et al, 2001). Some eligible trials had to be excluded from the
meta-analysis because they did not provide sufficient data on survival. Among the four excluded studies, only one (25%) was statistically significant, whereas a higher proportion of the
studies evaluable for the meta-analysis were significant (60%). It is known that negative studies are less frequently published or, if they are, with less detailed results, making them less
assessable. The methodological quality of trials, according to the global score, was not significantly different between evaluable and non-evaluable studies for the quantitative aggregation
of individual survival results. Nevertheless, such an approach does not fully protect a potential bias owing to the impossibility taking into account all the studies with negative or
non-significant results. Our meta-analysis is based on published data collected by a systematic review of the literature and can only be performed by univariate analysis. This is a limit to
this type of work, which appears thus as a preliminary step before performing multivariate studies. Many interesting data arise from multivariate analyses and particularly from proteomic and
genomic wide screen analysis, which is probably the way of the future. But if microarrays is an interesting technique providing very meaningful data, it should be kept in mind that it
remains a research screening technique and that it could not be applied in routine because of the high price. It should also be noted that COX-2 expression increases in patient treated by
taxanes (Subbaramaiah et al, 2003; Altorki et al, 2005), providing an argument to treat patients with lung cancer by an association of taxanes and anti-COX-2 drugs. The studies analysing
COX-2 expression after a specific treatment were not included in this meta-analysis because treated and untreated tumours do not have the same biological behaviour and should not be
aggregated together. This topic, COX-2 expression in pretreated lung tumours, should be the topic of another systematic review. In conclusion, when all stages and histologies are considered,
there is a trend for COX-2 overexpression as a prognostic factor for survival in patients with NSCLC, but there is a high heterogeneity between the studies and these results are not
statistically significant. Interestingly, our meta-analysis showed with more evidence that COX-2 has a detrimental effect on survival in stage I NSCLC. This prognostic role of COX-2 at
earliest stage of NSCLC could be of clinical interest in the selection of the patients eligible for induction or adjuvant chemotherapy. Hazard ratio was also significant for the studies
using RT–PCR and not for those using IHC, suggesting that a better standardisation of the technique to define and to detect COX-2 positivity is required to the generalisability of the
results. Our results need to be confirmed by an adequately designed prospective study and the exact role of COX-2 overexpression needs to be determined by an appropriate multivariate
analysis taking into account the classical well-defined (at the moment of the study) prognostic factors for lung cancer such as PS, stage, age, sex, weight loss. CHANGE HISTORY * _ 16
NOVEMBER 2011 This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication _ REFERENCES * Ab' Saber AM, Massoni Neto
LM, Bianchi CP, Ctenas BB, Parra ER, Eher EM, Pereira JC, Takagaki T, Yamaguchi NH, Capelozzi VL (2004) Neuroendocrine and biologic features of primary tumors and tissue in pulmonary large
cell carcinomas. _Ann Thorac Surg_ 77: 1883–1890 Article PubMed Google Scholar * Achiwa H, Yatabe Y, Hida T, Kuroishi T, Kozaki K, Nakamura S, Ogawa M, Sugiura T, Mitsudomi T, Takahashi T
(1999) Prognostic significance of elevated cyclooxygenase 2 expression in primary, resected lung adenocarcinomas. _Clin Cancer Res_ 5: 1001–1005 CAS PubMed Google Scholar * Altorki NK,
Port JL, Zhang F, Golijanin D, Thaler HT, Duffield-Lillico AJ, Subbaramaiah K, Dannenberg AJ (2005) Chemotherapy induces the expression of cyclooxygenase-2 in non-small cell lung cancer.
_Clin Cancer Res_ 11: 4191–4197 Article CAS PubMed Google Scholar * Araki K, Hashimoto K, Ardyanto TD, Osaki M, Shomori K, Nakamura H, Ito H (2004) Co-expression of Cox-2 and EGFR in
stage I human bronchial adenocarcinomas. _Lung Cancer_ 45: 161–169 Article PubMed Google Scholar * Boyle P, Ferlay J (2005) Cancer incidence and mortality in Europe, 2004. _Ann Oncol_ 16:
481–488 Article CAS PubMed Google Scholar * Brabender J, Park J, Metzger R, Schneider PM, Lord RV, Holscher AH, Danenberg KD, Danenberg PV (2002) Prognostic significance of
cyclooxygenase 2 mRNA expression in non-small cell lung cancer. _Ann Surg_ 235: 440–443 Article PubMed PubMed Central Google Scholar * Brattstrom D, Wester K, Bergqvist M, Hesselius P,
Malmstrom PE, Wagenius G, Brodin O (2005) HER-2, EGFR, COX-2 expression status correlated to microvessel density and survival in resected non-small cell Lung cancer. _Acta Oncol_ 43: 80–86
Google Scholar * Delmotte P, Martin B, Paesmans M, Berghmans T, Mascaux C, Meert AP, Steels E, Verdebout JM, Lafitte JJ, Sculier JP (2002) [VEGF and survival of patients with lung cancer: a
systematic literature review and meta-analysis]. _Rev Mal Respir_ 19: 577–584 CAS PubMed Google Scholar * Edwards J, Mukherjee R, Munro AF, Wells AC, Almushatat A, Bartlett JM (2004)
HER2 and COX2 expression in human prostate cancer. _Eur J Cancer_ 40: 50–55 Article CAS PubMed Google Scholar * Gallo O, Masini E, Bianchi B, Bruschini L, Paglierani M, Franchi A (2002)
Prognostic significance of cyclooxygenase-2 pathway and angiogenesis in head and neck squamous cell carcinoma. _Hum Pathol_ 33: 708–714 Article CAS PubMed Google Scholar * Hasturk S,
Kemp B, Kalapurakal SK, Kurie JM, Hong WK, Lee JS (2002) Expression of cyclooxygenase-1 and cyclooxygenase-2 in bronchial epithelium and nonsmall cell lung carcinoma. _Cancer_ 94: 1023–1031
Article CAS PubMed Google Scholar * Hida T, Kozaki K, Muramatsu H, Masuda A, Shimizu S, Mitsudomi T, Sugiura T, Ogawa M, Takahashi T (2000) Cyclooxygenase-2 inhibitor induces apoptosis
and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. _Clin Cancer Res_ 6: 2006–2011 CAS PubMed Google Scholar * Hida T, Yatabe Y, Achiwa H,
Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahashi T (1998) Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in
adenocarcinomas. _Cancer Res_ 58: 3761–3764 CAS PubMed Google Scholar * Hosomi Y, Yokose T, Hirose Y, Nakajima R, Nagai K, Nishiwaki Y, Ochiai A (2000) Increased cyclooxygenase 2 (COX-2)
expression occurs frequently in precursor lesions of human adenocarcinoma of the lung. _Lung Cancer_ 30: 73–81 Article CAS PubMed Google Scholar * Huang M, Stolina M, Sharma S, Mao JT,
Zhu L, Miller PW, Wollman J, Herschman H, Dubinett SM (1998) Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages:
up-regulation of interleukin 10 and down-regulation of interleukin 12 production. _Cancer Res_ 58: 1208–1216 CAS PubMed Google Scholar * Kanters SD, Lammers JW, Voest EE (1995) Molecular
and biological factores in the prognosis of non-small cell lung cancer. _Eur Respir J_ 8: 1389–1397 Article CAS PubMed Google Scholar * Khuri FR, Wu H, Lee JJ, Kemp BL, Lotan R, Lippman
SM, Feng L, Hong WK, Xu XC (2001) Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. _Clin Cancer Res_ 7: 861–867 CAS PubMed Google
Scholar * Kim HS, Youm HR, Lee JS, Min KW, Chung JH, Park CS (2003) Correlation between cyclooxygenase-2 and tumor angiogenesis in non-small cell lung cancer. _Lung Cancer_ 42: 163–170
Article PubMed Google Scholar * Kokawa A, Kondo H, Gotoda T, Ono H, Saito D, Nakadaira S, Kosuge T, Yoshida S (2001) Increased expression of cyclooxygenase-2 in human pancreatic neoplasms
and potential for chemoprevention by cyclooxygenase inhibitors. _Cancer_ 91: 333–338 Article CAS PubMed Google Scholar * Laga AC, Zander DS, Cagle PT (2005) Prognostic significance of
cyclooxygenase 2 expression in 259 cases of non-small cell lung cancer. _Arch Pathol Lab Med_ 129: 1113–1117 CAS PubMed Google Scholar * Lagorce C, Paraf F, Vidaud D, Couvelard A, Wendum
D, Martin A, Flejou JF (2003) Cyclooxygenase-2 is expressed frequently and early in Barrett's oesophagus and associated adenocarcinoma. _Histopathology_ 42: 457–465 Article CAS PubMed
Google Scholar * Lin DT, Subbaramaiah K, Shah JP, Dannenberg AJ, Boyle JO (2002) Cyclooxygenase-2: a novel molecular target for the prevention and treatment of head and neck cancer. _Head
Neck_ 24: 792–799 Article PubMed Google Scholar * Liu XH, Yao S, Kirschenbaum A, Levine AC (1998) NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates
bcl-2 expression in LNCaP cells. _Cancer Res_ 58: 4245–4249 CAS PubMed Google Scholar * Lu C, Soria JC, Tang X, Xu XC, Wang L, Mao L, Lotan R, Kemp B, Bekele BN, Feng L, Hong WK, Khuri FR
(2004) Prognostic factors in resected stage I non-small-cell lung cancer: a multivariate analysis of six molecular markers. _J Clin Oncol_ 22: 4575–4583 Article PubMed Google Scholar *
Marrogi AJ, Travis WD, Welsh JA, Khan MA, Rahim H, Tazelaar H, Pairolero P, Trastek V, Jett J, Caporaso NE, LIotta LA, Harris CC (2005) Nitric oxide synthase, cyclooxygenase 2, and vascular
endothelial growth factor in the angiogenesis of non-small cell lung carcinoma. _Clin Cancer Res_ 6: 4739–4744 Google Scholar * Martin B, Paesmans M, Berghmans T, Branle F, Ghisdal L,
Mascaux C, Meert AP, Steels E, Vallot F, Verdebout JM, Lafitte JJ, Sculier JP (2003) Role of Bcl-2 as a prognostic factor for survival in lung cancer: a systematic review of the literature
with meta-analysis. _Br J Cancer_ 89: 55–64 Article CAS PubMed PubMed Central Google Scholar * Martin B, Paesmans M, Mascaux C, Berghmans T, Lothaire P, Meert AP, Lafitte JJ, Sculier JP
(2004) Ki-67 expression and patients survival in lung cancer: systematic review of the literature with meta-analysis. _Br J Cancer_ 91: 2018–2025 Article CAS PubMed PubMed Central
Google Scholar * Mascaux C, Iannino N, Martin B, Paesmans M, Berghmans T, Dusart M, Haller A, Lothaire P, Meert AP, Noel S, Lafitte JJ, Sculier JP (2005a) The role of _RAS_ oncogene in
survival of patients with lung cancer: a systematic review of the literature with meta-analysis. _Br J Cancer_ 92: 131–139 Article CAS PubMed Google Scholar * Mascaux C, Martin B,
Verdebout JM, Ninane V, Sculier JP (2005b) COX-2 expression during early lung squamous cell carcinoma oncogenesis. _Eur Respir J_ 26: 198–203 Article CAS PubMed Google Scholar *
Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K (2000) Antiangiogenic and antitumor activities of cyclooxygenase-2
inhibitors. _Cancer Res_ 60: 1306–1311 CAS PubMed Google Scholar * Meert AP, Martin B, Delmotte P, Berghmans T, Lafitte JJ, Mascaux C, Paesmans M, Steels E, Verdebout JM, Sculier JP
(2002a) The role of EGF-R expression on patient survival in lung cancer: a systematic review with meta-analysis. _Eur Respir J_ 20: 975–981 Article CAS PubMed Google Scholar * Meert AP,
Martin B, Paesmans M, Berghmans T, Mascaux C, Verdebout JM, Delmotte P, Lafitte JJ, Sculier JP (2003) The role of HER-2/neu expression on the survival of patients with lung cancer: a
systematic review of the literature. _Br J Cancer_ 89: 959–965 Article CAS PubMed PubMed Central Google Scholar * Meert AP, Paesmans M, Martin B, Delmotte P, Berghmans T, Verdebout JM,
Lafitte JJ, Mascaux C, Sculier JP (2002b) The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta-analysis. _Br J
Cancer_ 87: 694–701 Article PubMed PubMed Central Google Scholar * Ochiai M, Oguri T, Isobe T, Ishioka S, Yamakido M (1999) Cyclooxygenase-2 (COX-2) mRNA expression levels in normal lung
tissues and non-small cell lung cancers. _Jpn J Cancer Res_ 90: 1338–1343 Article CAS PubMed PubMed Central Google Scholar * Paesmans M, Sculier J-P, Lecomtre J, Thiriaux J, Libert P,
Sergysels R, Bureau G, Dabouis G, Van Custem O, Mommen P, Ninane V, Klastersky J, for the European Lung Cancer Working Party (2000) Prognostic factors in patients with small cell lung
cancer: analysis of a series of 763 patients included in four consecutive prospective trials and with a minimal 5-year follow-up duration. _Cancer_ 89: 523–533 Article CAS PubMed Google
Scholar * Paesmans M, Sculier J-P, Libert P, Bureau G, Dabouis G, Thiriaux J, Michel J, Van Custem O, Sergysels R, Mommen P (1995) Prognostic factors for survival in advanced non-small cell
lung cancer: univariate and multivariate analyses including recursive partitioning and amalgamation algorithms in 1052 patients. _J Clin Oncol_ 13: 1221–1230 Article CAS PubMed Google
Scholar * Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. _Stat Med_ 17: 2815–2834 Article
CAS PubMed Google Scholar * Ranger GS, Thomas V, Jewell A, Mokbel K (2004) Elevated cyclooxygenase-2 expression correlates with distant metastases in breast cancer. _Anticancer Res_ 24:
2349–2351 CAS PubMed Google Scholar * Richardson CM, Richardson D, Swinson DE, Swain WA, Cox G, O'Byrne KJ (2005) Cyclooxygenase-2 protein levels are independent of epidermal growth
factor receptor expression or activation in operable non-small cell lung cancer. _Lung Cancer_ 48: 47–57 Article CAS PubMed Google Scholar * Sinicrope FA, Gill S (2004) Role of
cyclooxygenase-2 in colorectal cancer. _Cancer Metast Rev_ 23: 63–75 Article CAS Google Scholar * Smith WL, Langenbach R (2001) Why there are two cyclooxygenase isozymes. _J Clin Invest_
107: 1491–1495 Article CAS PubMed PubMed Central Google Scholar * Steels E, Paesmans M, Berghmans T, Branle F, Lemaitre F, Mascaux C, Meert AP, Vallot F, Lafitte JJ, Sculier JP (2001)
Role of p53 as a prognostic factor for survival in lung cancer: a systematic review of the literature with a meta-analysis. _Eur Respir J_ 18: 705–719 Article CAS PubMed Google Scholar *
Strauss GM (1997) Prognostic markers in resectable non-small cell lung cancer. _Hematol Oncol Clin N Am_ 11: 409–434 Article CAS Google Scholar * Subbaramaiah K, Marmo TP, Dixon DA,
Dannenberg AJ (2003) Regulation of cyclooxgenase-2 mRNA stability by taxanes: evidence for involvement of p38, MAPKAPK-2, and HuR. _J Biol Chem_ 278: 37637–37647 Article CAS PubMed Google
Scholar * Tsujii M, Kawano S, Dubois RN (1997) Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. _Proc Natl Acad Sci USA_ 94: 3336–3340 Article CAS
PubMed PubMed Central Google Scholar * Waddell WR, Loughry RW (1983) Sulindac for polyposis of the colon. _J Surg Oncol_ 24: 83–87 Article CAS PubMed Google Scholar * Wolff H,
Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimaki A (1998) Expression of cyclooxygenase-2 in human lung carcinoma. _Cancer Res_ 58: 4997–5001 CAS PubMed Google Scholar *
Yamaguchi NH, Lichtenfels AJ, Demarchi LM, da Silva AP, Garippo AL, Alves VF, Michelin C, Azevedo PM, Moya T, Takagaki T, Saldiva PH, Vollmer RT, Capelozzi VL (2004) COX-2, MMP-9, and
Noguchi classification provide additional prognostic information about adenocarcinoma of the lung. A study of 117 patients from Brazil. _Am J Clin Pathol_ 121: 78–86 Article PubMed Google
Scholar * Yuan A, Yu CJ, Shun CT, Luh KT, Kuo SH, Lee YC, Yang PC (2005) Total cyclooxygenase-2 mRNA levels correlate with vascular endothelial growth factor mRNA levels, tumor angiogenesis
and prognosis in non-small cell lung cancer patients. _Int J Cancer_ 115: 545–555 Article CAS PubMed Google Scholar * Yusuf S, Peto R, Lewis J, Collins R, Sleight P (1985) Blockade
during and after myocardial infarction: an overview of the randomized trials. _Prog Cardiovasc Dis_ 27: 335–371 Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS Dr
Céline Mascaux was supported by a fellowship from National Fund for Scientific Research. This study was also supported by a grant from the FNRS (FRSM; 3.4624.04), a grant from ‘Télévie-FNRS’
(7.4529.04) and a grant from the ASBL ‘Les Amis de l'Institut Jules Bordet’. AUTHOR INFORMATION Author notes * C Mascaux: Research fellow FNRS (National Fund of Scientific Research),
Belgium AUTHORS AND AFFILIATIONS * Department of Intensive Care and Thoracic Oncology, Institut Jules Bordet, Centre des Tumeurs de l'Université Libre de Bruxelles, Brussels, B-1000,
Belgium C Mascaux, B Martin, T Berghmans, A-P Meert & J-P Sculier * Data Centre, Institut Jules Bordet, Centre des Tumeurs de l'Université Libre de Bruxelles, Brussels, B-1000,
Belgium M Paesmans * Department of Nuclear Medicine, Institut Jules Bordet, Centre des Tumeurs de l'Université Libre de Bruxelles, Brussels, B-1000, Belgium M Dusart * Department of
Pathology, Institut Jules Bordet, Centre des Tumeurs de l'Université Libre de Bruxelles, Brussels, B-1000, Belgium A Haller * Department of Surgery, Institut Jules Bordet, Centre des
Tumeurs de l'Université Libre de Bruxelles, Brussels, B-1000, Belgium P Lothaire * Chest Department, CHU Calmette, Lille, F-59037, France J-J Lafitte Authors * C Mascaux View author
publications You can also search for this author inPubMed Google Scholar * B Martin View author publications You can also search for this author inPubMed Google Scholar * M Paesmans View
author publications You can also search for this author inPubMed Google Scholar * T Berghmans View author publications You can also search for this author inPubMed Google Scholar * M Dusart
View author publications You can also search for this author inPubMed Google Scholar * A Haller View author publications You can also search for this author inPubMed Google Scholar * P
Lothaire View author publications You can also search for this author inPubMed Google Scholar * A-P Meert View author publications You can also search for this author inPubMed Google Scholar
* J-J Lafitte View author publications You can also search for this author inPubMed Google Scholar * J-P Sculier View author publications You can also search for this author inPubMed Google
Scholar CORRESPONDING AUTHOR Correspondence to C Mascaux. RIGHTS AND PERMISSIONS From twelve months after its original publication, this work is licensed under the Creative Commons
Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Mascaux, C., Martin, B., Paesmans, M. _et al._ Has Cox-2 a prognostic role in non-small-cell lung cancer? A systematic review of the literature with meta-analysis
of the survival results. _Br J Cancer_ 95, 139–145 (2006). https://doi.org/10.1038/sj.bjc.6603226 Download citation * Received: 22 February 2006 * Revised: 17 May 2006 * Accepted: 19 May
2006 * Published: 20 June 2006 * Issue Date: 17 July 2006 * DOI: https://doi.org/10.1038/sj.bjc.6603226 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
KEYWORDS * COX-2 * lung cancer * meta-analysis * systematic review * survival * prognostic factor
