Phase i study of kw-2478, a novel hsp90 inhibitor, in patients with b-cell malignancies
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT BACKGROUND: KW-2478 is a novel, non-ansamycin, non-purine heat-shock protein 90 (Hsp90) inhibitor. METHODS: In this phase I, multicentre study, KW-2478 was administered
intravenously over 1 h at doses ranging from 14 to 176 mg m–2 once daily on days 1–5 of a 14-day cycle in a standard 3+3 design in 27 patients (22 with multiple myeloma and 5 with
non-Hodgkin lymphoma). Patients enrolled had relapsed/refractory disease previously treated with ⩾2 regimens. RESULTS: There were no dose-limiting toxicities, thus the maximum-tolerated dose
was not reached. KW-2478 was well tolerated and did not manifest significant retinal or ocular toxicity. The most common treatment-related adverse events were diarrhoea (33.3%), fatigue
(29.6%), headache (25.9%), hypertension (22.2%), nausea (14.8%), vomiting (7.4%), and dizziness (7.4%). Plasma concentrations peaked at the end of infusion and decayed in a biphasic manner
with a terminal half-life of ∼6 h. Target inhibition was inferred from the increase in Hsp70 levels in peripheral blood mononuclear cells at doses ⩾71 mg m–2. Twenty-four of 25 (96%)
evaluable patients showed stable disease, with five being free of disease progression for ⩾6 months. CONCLUSIONS: Preliminary clinical response data were encouraging and warrant further
investigation of KW-2478 in combination regimens for relapsed/refractory B-cell malignancies. SIMILAR CONTENT BEING VIEWED BY OTHERS A FIRST-IN-MAN PHASE 1 STUDY OF THE DNA-DEPENDENT PROTEIN
KINASE INHIBITOR PEPOSERTIB (FORMERLY M3814) IN PATIENTS WITH ADVANCED SOLID TUMOURS Article Open access 24 November 2020 PEVONEDISTAT, A NEDD8-ACTIVATING ENZYME INHIBITOR, IN COMBINATION
WITH IBRUTINIB IN PATIENTS WITH RELAPSED/REFRACTORY B-CELL NON-HODGKIN LYMPHOMA Article Open access 11 January 2023 POOLED ANALYSIS OF SAFETY DATA FROM CLINICAL TRIALS EVALUATING
ACALABRUTINIB MONOTHERAPY IN MATURE B-CELL MALIGNANCIES Article 27 April 2021 MAIN Tangible progress in improving clinical outcome for multiple myeloma (MM) has been primarily driven by the
successful introduction of the proteasome inhibitors (e.g. bortezomib) and immunomodulatory drugs (e.g. thalidomide, lenalidomide). However, nearly all patients eventually relapse or become
refractory following first- or second-line treatment (Moreau, 2012). These relapsed/refractory MM patients present a therapeutic challenge because of their demonstrated poor clinical outcome
(Sinha et al, 2012). As a result, novel agents that act in a mechanistically different manner to the proteasome inhibitors and thalidomide derivatives are needed so that they can be used
either successively or in combination with standard agents for this difficult-to-treat patient population. One emerging cellular target in malignancies is heat-shock protein 90 (Hsp90).
Heat-shock protein 90, a molecular chaperone required for the stability and function of many cellular proteins, is uniformly expressed in several human MM cell lines (Mitsiades et al, 2006;
Sharp and Workman, 2006). Heat-shock protein 90 inhibitors induce apoptosis in MM cell lines, disrupt the interaction between bone marrow stromal cells and MM cells, and upregulate other
Hsps, including Hsp70 (Banerji et al, 2005a; Mitsiades et al, 2006; Stühmer et al, 2008). A number of Hsp90 inhibitors have shown promise in early clinical trials of haematological and solid
tumours but some, such as AUY-922, SNX-5422, and alvespimycin, have been associated with visual disorders including blurred vision, flashes, delayed light/dark accommodation, and
photophobia, possibly due to photoreceptor degeneration and cell death (Zhou et al, 2012). Development of an effective Hsp90 inhibitor with improved overall, and specifically ocular, safety
could provide a therapeutic advance for patients with relapsed/refractory MM as well as other malignancies. KW-2478 is a novel, non-ansamycin, non-purine Hsp90 inhibitor that displays high
binding affinity to Hsp90 and potent antitumour activity in _in vitro_ and _in vivo_ models, including activity in primary MM patient samples (Nakashima et al, 2010; Ishii et al, 2012). The
activity of KW2478 against primary myeloma cells is retained in the presence of bone marrow stromal cells, suggesting that KW-2478 overcomes the protective effect of the bone marrow
microenvironment (Juliger et al, 2008). We designed a phase I study in patients with mature B-cell malignancies including MM, chronic lymphocytic leukaemia (CLL), and non-Hodgkin lymphoma
(NHL). The objectives of this study were to determine the maximum-tolerated dose (MTD), safety, and tolerability of KW-2478, characterise its pharmacokinetics and pharmacodynamics, and
provide preliminary information on clinical activity. MATERIALS AND METHODS STUDY DESIGN This phase I, open-label, multicentre, dose-escalation study was performed at six institutions in the
United Kingdom. The primary objectives were to determine the safety, tolerability, dose-limiting toxicities (DLTs), MTD, and recommended phase II dose (RP2D) of single-agent KW-2478
administered as five consecutive once-daily doses in a 14-day schedule. Secondary objectives were to characterise pharmacokinetics and pharmacodynamics, and report preliminary clinical
activity. The protocol, informed consent form, and amendments were reviewed and approved by an Independent Ethics Committee at each study centre. The study was conducted in accordance with
the Declaration of Helsinki, International Conference for Harmonisation Good Clinical Practice guidelines, and the Committee for Medicinal Products for Human Use guidelines. All patients
provided written informed consent. This study was registered at ClinicalTrials.Gov (NCT00457782). ELIGIBILITY CRITERIA Patients with confirmed relapsed/refractory MM, CLL, or NHL who had
failed ⩾2 prior standard treatment regimens and had no established therapeutic alternatives were eligible for this study. Patients had to be ⩾18 years of age, have an Eastern Cooperative
Oncology Group (ECOG) performance status of ⩽2 and a life expectancy ⩾3 months, and have adequate haematologic, renal, and hepatic function. STUDY ASSESSMENTS Demographics and medical
history were recorded at baseline. Safety was monitored through adverse event (AE) monitoring and laboratory assessments. Toxicities were graded using the National Cancer Institute Common
Terminology Criteria for Adverse Events v.3.0. Retinal toxicity was monitored by serial visual acuity and colour vision tests before each cycle, together with ophthalmic examination, retinal
photograph, electroretinogram (ERG), and autofluorescence imaging at least 21 days before the first dose of study drug, at any significant visual symptom/sign, and at the final study visit.
The ERGs incorporated the International Society for Clinical Electrophysiology of Vision standard recordings (Marmor et al, 2004). Dose-limiting toxicity was defined as any AE considered to
be related (possibly, probably, or definitely) to KW-2478, and meeting any of the following criteria during the first cycle of treatment: AE leading to a treatment delay or persisting
beyond day 14, grade ⩾4 non-haematological toxicity or grade 3 non-haematological toxicity deemed clinically significant, grade ⩾4 haematological toxicity (except those related to cytopenia
due to bone marrow infiltration by primary disease), and ⩾40% change from baseline in ERG or change of ERG deemed clinically significant. The MTD was defined as the highest dose at which at
least five of six patients in a cohort did not experience any DLTs. Clinical response was assessed after each or every other cycle, and at the final study visit using standard criteria for
MM (Bladé et al, 1998) and NHL (Cheson et al, 1996). TREATMENT KW-2478 (Kyowa Hakko Kirin Co. Ltd, Japan) was administered by intravenous infusion over 1 h at planned doses (14, 28, 47, 71,
99, or 132 mg m–2) on days 1–5 of a 14-day cycle in a standard 3+3 design. As the MTD was not reached at the 132 mg m–2 dose, an additional cohort (176 mg m–2) was subsequently added
following review of data by the Safety Monitoring Board. Patients could receive KW-2478 either on an outpatient basis or as an in-patient at the investigational site. If outpatient, patients
were discharged from the investigational site after the final pharmacokinetic sample (8 h after the fifth dose) and were asked to return to the investigational site on days 9–11. Patients
who were not evaluable because of early discontinuation during cycle 1 were replaced. An evaluable patient was defined as a patient who received all five doses of KW-2478 and completed all
study visits and procedures by day 14 of cycle 1, unless the patient had a treatment delay or interruption due to toxicity. Patients with a suboptimal response after having completed at
least two cycles of treatment and no evidence of progressive disease were given the option to escalate to the highest level for which safety had been established. Each patient was permitted
to continue receiving KW-2478 for up to 12 months or until disease progression, unacceptable toxicity, or withdrawal of consent. Haematopoietic growth factors were allowed after the end of
cycle 1, as per standard of care. Supportive care, including bisphosphonates and transfusion of packed red blood cells and/or platelets, was also allowed within accepted guidelines. Use of
any chemotherapy, radiotherapy, anticancer treatment, or corticosteroid therapy was not permitted during the study. PHARMACOKINETIC ANALYSES Blood samples were collected on days 1 and 5 at
the following times: predose, 0.833 h after start of infusion, and 0.167, 0.5, 1, 2, 4, and 8 h after the end of infusion, and predose for days 2 and 4. Heparinised plasma samples were
collected, stored at <–20 °C, and analysed at an independent central laboratory. Following solid-phase extraction, plasma KW-2478 concentrations were determined using a validated
reverse-phase, high-performance liquid chromatography with mass spectrometry/mass spectrometry detection with a lower limit of quantitation of 0.200 mg ml–1 The following pharmacokinetic
parameters were calculated using a standard non-compartmental model with WinNonlin v.5 (Pharsight Co., Princeton, NJ, USA): maximum observed plasma concentration (_C_max), areas under the
plasma concentration–time curve from time zero to last measurable time point (AUC0–_t_) and to infinity (AUC0–∞), terminal half-life (_t_½), elimination rate constant (_k_el), total plasma
clearance (CL), and accumulation ratio (_R_) comparing day 5 _vs_ day 1 for _C_max and AUC0–_t_. PHARMACODYNAMICS Levels of Hsp70 in peripheral blood mononuclear cells (PBMCs) were examined
as a biomarker for Hsp90 inhibition. Peripheral blood mononuclear cells were obtained from blood samples taken before treatment initiation and 8 h postdose on days 1 and 5 of cycle 1.
Samples were examined for change in Hsp70 expression using western blot analysis at an independent central laboratory. STATISTICAL ANALYSIS Adverse events were summarised per dose level and
were coded using the Medical Dictionary for Regulatory Activities v.10.0. If more than one AE was recorded for a patient within any system organ class or preferred term, the AE was counted
only once in the calculation of percentage of patients with an event. For patients who dose escalated to a higher dose level, all dose levels of KW-2478 received throughout the duration of
an AE were included. Pharmacokinetic and pharmacodynamic data, and clinical responses were summarised descriptively by dose level. RESULTS PATIENT CHARACTERISTICS A total of 27 patients (22
with MM and 5 with NHL) were recruited to seven different dose cohorts and were treated between 30 April 2007 and 9 September 2010. There were three patients in each of the first five
cohorts (14–99 mg m–2), while six patients were recruited in each of the final two cohorts (132 and 176 mg m–2). No patient with NHL was recruited to the first four cohorts. Patient
demographic and baseline clinical characteristics are shown in Table 1. Mean age was 63 years (range, 48–75 years), 26 (96.3%) were white, and 17 (63.0%) were male. The majority of patients
(88.9%; _n_=24) had an ECOG performance status of 0 or 1. Of the 22 MM patients, Durie–Salmon classification was stage I (_n_=2), stage II (_n_=7), stage III (_n_=9), and not recorded/not
done (_n_=4). Of the five NHL patients, Ann Arbor classification was stage III (_n_=1) and stage IV (_n_=4). All patients had received ⩾2 prior therapies, with 13 (48.1%) having received ⩾6
prior therapies. All patients with MM had achieved at least a partial response as best response to prior therapy; one NHL patient had achieved a complete or near-complete response. All 27
patients received at least one dose of study medication and were included in the safety population. A total of 25 patients (92.6%) were included in the efficacy evaluable population, as two
patients (1 each in the 71 and 176 mg m–2 cohorts) were excluded as they did not have response assessment. STUDY TREATMENT The mean number of treatment cycles completed was 1.5–3.7 (range,
1–7) at the 14–99 mg m–2 doses, 8.0 (range, 2–16) at 132 mg m–2, and 6.7 (range, 1–22) at 176 mg m–2. Eight patients received ⩾6 cycles, of which five were in the 132 or 176 mg m–2 cohorts.
No patients required KW-2478 dose reduction, although some patients required dose delays of 10 days or more, generally until AE resolution. Three patients had dose escalation (single step in
2 and two steps in 1). RECOMMENDED PHASE II DOSE There were no DLTs in any dose cohort. As a result, the MTD was not reached. The RP2D was therefore the highest dose tested: 176 mg m–2 once
daily on days 1–5 every 2 weeks. SAFETY Table 2 summarises AEs reported across all treatment cycles. A total of 282 AEs were reported by 27 patients (100%). The most common AEs were
diarrhoea (48.1%), followed by headache (40.7%), rhinitis (40.7%), and fatigue (33.3%). A total of 127 treatment-related AEs were reported by 21 patients (77.8%). The number of
treatment-related AEs per patient tended to increase with increasing dose of KW-2478 from an average of 1.0 at the 14 mg m–2 dose to 9.2 at the 176 mg m–2 dose: duration of exposure to
KW-2478 was longer in the higher dose cohorts (see previously), which might partially explain this result. The most common treatment-related AEs per patient were diarrhoea (33.3%), fatigue
(29.6%), headache (25.9%), hypertension (22.2%), nausea (14.8%), vomiting (7.4%), and dizziness (7.4%). Although hypertension was a common treatment-related AE, there was no apparent
dose-related increase in mean blood pressure for all patients during the study. Most AEs were grade 1 or 2. Ten patients (37.0%) experienced a total of 19 grade 3/4 AEs, of which five events
in three patients were considered possibly treatment-related (two episodes of lethargy and one of syncope; QT prolongation; and neutrophil decrease in respective patients). Two patients
died during the study (one after study completion), but both deaths were considered unrelated to study medication (bronchopneumonia and lower respiratory tract infection, respectively).
Overall, six patients (22.2%) experienced nine AEs classified as eye disorders, which were considered possibly related to KW-2478 in four patients: decreased visual acuity (grade 1 in one
patient on 28 mg m–2), blurred vision (grades 1 and 2 in two patients on 71 mg m–2), and dry eyes (grade 2 in one patient on 176 mg m–2) – all resolved except for dry eyes, which also
required treatment. There were no clinically significant abnormalities on routine ophthalmological examination nor fundus autofluorescence imaging. There were no notable effects on visual
acuity nor colour vision. In summary, there were no clinically meaningful changes in ophthalmic parameters. There were no clinically meaningful changes in haematological or biochemistry
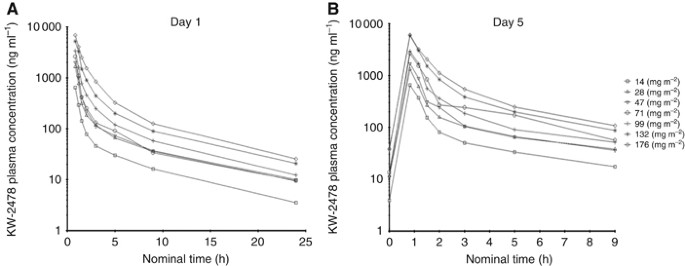
values, vital signs, body weight, nor ECG results. PHARMACOKINETICS The pharmacokinetics of KW-2478 are summarised in Table 3. Mean _C_max and AUC values increased linearly in relation to
dose on days 1 and 5, and accumulation ratios for these parameters indicated no relevant accumulation from days 1 to 5. Mean _t_½ (range, 5.4–6.6 h) and total CL (range, 23.1–43.0 l h–1)
appeared independent of dose. Plasma concentrations over time profiles for KW-2478 on days 1 and 5 are shown in Figure 1. Plasma concentration declined in a biphasic manner with a rapid
distribution phase and slower elimination phase. PHARMACODYNAMICS Induction of Hsp70 was measured in PBMCs of patients treated with KW-2478 as a surrogate marker of Hsp inhibition. A
representative western blot in a patient who received KW-2478 176 mg m–2 shows Hsp70 induction 8 h after the first dose on day 1, a return to normal on day 5 predose, and renewed induction 8
h after dosing on day 5 (Figure 2A). Heat-shock protein 70 induction of ⩾25% above baseline was consistently noted at doses ⩾71 mg m–2 at 8 h after dosing on days 1 and 5, with some
evidence of upregulation at lower doses, particularly on day 5 (Figure 2B). TUMOUR RESPONSE Best tumour response over all cycles was assessed among 21 MM patients and four NHL patients. No
patients achieved complete or partial responses. Among the 21 evaluable MM patients, 20 (95.2%) had stable disease (SD) and one (4.8%) had progressive disease. All four evaluable NHL
patients (100%) had SD. This gives an overall disease control rate of 96.0%. Eight patients had SD lasting ⩾3 months and five had SD lasting ⩾6 months, including one patient in each of the
132 and 176 mg m–2 cohorts with SD sustained for 12.9 and 11.9 months, respectively. All but one of these patients with a more durable (⩾6 months) SD received doses ⩾71 mg m–2. Details of
individual patient responses, duration of treatment, and reasons for discontinuation are given in Table 4. DISCUSSION Patients with refractory MM are inherently difficult to treat and have
considerable unmet need for novel treatment options. In this phase I study of the novel Hsp90 inhibitor, KW2478, as single-agent therapy, a disease stabilisation rate of 96% in a heavily
pretreated study population with resistant disease and no other therapeutic options is notable. In such patients with progressive MM, sustained SD could be considered clinically meaningful.
Eight patients had SD lasting for ⩾3 months and five patients had SD lasting for ⩾6 months: this included two patients, one in the 132 mg m–2 and one in the 176 mg m–2 groups who had SD
lasting for 12.9 and 11.9 months, respectively. Furthermore, all but one of these patients with a more durable SD occurred at doses ⩾71 mg m–2, that is, at doses showing demonstrable Hsp70
induction. Frequent and sustained SD is an encouraging finding for patients who had already failed ⩾2 prior regimens. The use of Hsp70 expression as a biomarker for inhibition of Hsp90 has
been validated by Banerji et al (2005b), who showed that induction of Hsp70 levels occurs with Hsp90 inhibitors at concentrations required for clinical efficacy. Heat-shock protein 70
induction was consistently demonstrated at KW-2478 doses ⩾71 mg m–2 in our current study. Proteasome inhibitors (bortezomib carfilzomib) represent a significant advance in the treatment of
MM, yet not all patients respond and disease responses are often short lived, especially in the relapsed/refractory setting. Overcoming disease resistance to proteasome inhibition is
therefore the focus of intense study. Among the cellular responses to proteasome inhibition is Hsp upregulation, indicating a state of cellular stress (Mitsiades et al, 2002). Thus, Hsp90
inhibitors may act to sensitise cells to the activity of agents such as bortezomib (Mitsiades et al, 2011). There are no apparent significant overlapping toxicities between KW-2478 and
bortezomib, as well as no apparent neurotoxicity nor significant thrombocytopenia with KW-2478. Unlike some other members of the Hsp90 inhibitor class (Rajan et al, 2011; Sessa et al, 2013),
there was no clinically significant ocular toxicity. Thus, there is a sound basis for the rational combination of KW-2478 with proteasome inhibition. In conclusion, the RP2D for
single-agent KW-2478 is 176 mg m–2 on days 1–5 every 14 days in patients with relapsed/refractory B-cell malignancies. KW-2478 was safe and well tolerated, with no DLTs or unexpected
toxicities being detected. Treatment-related AEs ⩾grade 3 were rare. Indication of clinical activity was suggested by sustained SD in some patients. Further clinical study of KW-2478 is
therefore warranted. The combination of KW-2478 and bortezomib is therefore being investigated in a phase I/II study in patients with refractory/relapsed MM (NCT01063907). REFERENCES *
Banerji U, O'Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, Simmons L, Maloney A, Raynaud F, Campbell M, Walton M, Lakhani S, Kaye S, Workman P, Judson I (2005a) Phase I
pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. _J Clin Oncol_ 23 (18): 4152–4161. Article CAS Google Scholar
* Banerji U, Walton M, Raynaud F, Grimshaw R, Kelland L, Valenti M, Judson I, Workman P (2005b) Pharmacokinetic-pharmacodynamic relationships for the heat shock protein 90 molecular
chaperone inhibitor 17-allylamino, 17-demethoxygeldanamycin in human ovarian cancer xenograft models. _Clin Cancer Res_ 11 (19): 7023–7032. Article CAS Google Scholar * Bladé J, Samson D,
Reece D, Apperley J, Björkstrand B, Gahrton G, Gertz M, Giralt S, Jagannath S, Vesole D (1998) Criteria for evaluating disease response and progression in patients with multiple myeloma
treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. _Br J Haematol_ 102 (5): 1115–1123.
Article Google Scholar * Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, Rai KR (1996) National Cancer Institute-sponsored Working Group guidelines for chronic
lymphocytic leukaemia: revised guidelines for diagnosis and treatment. _Blood_ 87 (12): 4990–4997. CAS PubMed Google Scholar * Ishii T, Seike T, Nakashima T, Juliger S, Maharaj L, Soga S,
Akinaga S, Cavenagh J, Joel S, Shiotsu Y (2012) Anti-tumor activity against multiple myeloma by combination of KW-2478, an Hsp90 inhibitor, with bortezomib. _Blood Cancer J_ 2 (4): e68.
Article CAS Google Scholar * Juliger S, Nakashima T, Maharaj L, Ishii T, Nakagawa H, Kanda Y, Oakerveel H, Cavenagh J, Akinaga S, Shiotsu Y, Joel S (2008) A novel heat shock protein (Hsp)
90 inhibitor KW-2478 shows activity in B-cell malignancies _in vitro_ and _in vivo_. _Blood_ 112 (11): 574 (abstract 1625). Google Scholar * Marmor MF, Holder GE, Seeliger MW, Yamamoto S
International Society for Clinical Electrophysiology of Vision (2004) Standard for clinical electroretinography (2004 update). _Doc Ophthalmol_ 108 (2): 107–114. Article Google Scholar *
Mitsiades CS, Davies FE, Laubach JP, Joshua D, San Miguel J, Anderson KC, Richardson PG (2011) Future directions of next-generation novel therapies, combination approaches, and the
development of personalized medicine in myeloma. _J Clin Oncol_ 29 (14): 1916–1923. Article Google Scholar * Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Kung AL, Davies FE, Morgan
G, Akiyama M, Shringarpure R, Munshi NC, Richardson PG, Hideshima T, Chauhan D, Gu X, Bailey C, Joseph M, Libermann TA, Rosen NS, Anderson KC (2006) Antimyeloma activity of heat shock
protein-90 inhibition. _Blood_ 107 (3): 1092–1100. Article CAS Google Scholar * Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph M, Libermann TA,
Treon SP, Munshi NC, Richardson PG, Hideshima T, Anderson KC (2002) Molecular sequelae of proteasome inhibition in human multiple myeloma cells. _Proc Natl Acad Sci USA_ 99 (22):
14374–14379. Article CAS Google Scholar * Moreau P (2012) The future of therapy for relapsed/refractory multiple myeloma: emerging agents and novel treatment strategies. _Semin Hematol_
49 (Suppl 1): S33–S46. Article Google Scholar * Nakashima T, Ishii T, Tagaya H, Seike T, Nakagawa H, Kanda Y, Akinaga S, Soga S, Shiotsu Y (2010) New molecular and biological mechanism of
antitumor activities of KW-2478, a novel nonansamycin heat shock protein 90 inhibitor, in multiple myeloma cells. _Clin Cancer Res_ 16 (10): 2792–2802. Article CAS Google Scholar * Rajan
A, Kelly RJ, Trepel JB, Kim YS, Alarcon SV, Kummar S, Gutierrez M, Crandon S, Zein WM, Jain L, Mannargudi B, Figg WD, Houk BE, Shnaidman M, Brega N, Giaccone G (2011) A phase I study of
PF-04929113 (SNX-5422), an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumor malignancies and lymphomas. _Clin Cancer Res_ 17 (21): 6831–6839.
Article CAS Google Scholar * Sessa C, Shapiro GI, Bhalla KN, Britten C, Jacks KS, Mita M, Papadimitrakopoulou V, Pluard T, Samuel TA, Akimov M, Quadt C, Fernandez-Ibarra C, Lu H, Bailey
S, Chica S, Banerji U (2013) First-in-human phase I dose-escalation study of the HSP90 inhibitor AUY922 in patients with advanced solid tumors. _Clin Cancer Res_ 19 (13): 3671–3680. Article
CAS Google Scholar * Sharp S, Workman P (2006) Inhibitors of the HSP90 molecular chaperone: current status. _Adv Cancer Res_ 95: 323–348. Article CAS Google Scholar * Sinha S, Lacy M,
Mikhael J, Hayman S, Buadi F, Detweiler-Short K, Dispenzieri A, Gertz M, Dingli D, Rajkumar SV, Kumar SK (2012) Response to salvage therapies and outcome in patients with multiple myeloma
relapsing after pomalidomide therapy. _Leukemia_ 26 (4): 839–841. Article CAS Google Scholar * Stühmer T, Zöllinger A, Siegmund D, Chatterjee M, Grella E, Knop S, Kortüm M, Unzicker C,
Jensen MR, Quadt C, Chène P, Schoepfer J, García-Echeverría C, Einsele H, Wajant H, Bargou RC (2008) Signalling profile and antitumor activity of the novel Hsp90 inhibitor NVP-AUY922 in
multiple myeloma. _Leukemia_ 22 (8): 1604–1612. Article Google Scholar * Zhou D, Teofilovici F, Liu Y, Ye J, Ying W, Ogawa LS, Inoue T, Lee W, Adjiri-Awere A, Kolodzieyski L, Tatsuta N,
Wada Y, Sonderfan AJ (2012) Associating retinal drug exposure and retention with the ocular toxicity profiles of Hsp90 inhibitors. _J Clin Oncol_ 30 (Suppl): abstract 3086. Google Scholar
Download references ACKNOWLEDGEMENTS Kyowa Hakko Kirin UK Ltd (KHK) funded the study. Before the start of the study, KHK agreed that the authors had full rights to submit the manuscript for
publication, KHK approval of the manuscript was not required, and publication of the manuscript was not contingent upon the approval of KHK. We also acknowledge Peter Todd, PhD (Tajut Ltd,
Kaiapoi, New Zealand), and Michael Kurman, MD (Kyowa Hakko Kirin Pharma Inc., Princeton, NJ, USA), for review and critical revisions for important intellectual content and editorial
assistance in the preparation of this manuscript, and Li Liu, PhD, and Rocco Ballerini, PhD (Kyowa Hakko Kirin Pharma Inc., Princeton, NJ, USA), for statistical support. We thank the study
investigators for their invaluable contribution to this study. AUTHOR CONTRIBUTIONS All authors contributed equally to data analysis and interpretation, figure creation, and writing of the
manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * UCL Cancer Institute, University College London, Huntley Street, London, WC1E 6DD, UK K Yong * Department of Haematology, Christie
Hospital/University of Manchester, Wilmslow Road, Manchester, M20 4BX, UK J Cavet * Cancer Research UK Clinical Centre, Southampton General Hospital, Tremona Road, Southampton, SO16 6YD, UK
P Johnson * Myeloma Institute for Research and Therapy, West Markham Street, Little Rock, 72205, AR, USA G Morgan * Centre for Clinical Haematology, Nottingham University Hospital, Hucknall
Road, Nottingham, NG5 1PB, UK C Williams * Kyowa Hakko Kirin Pharma Inc., Princeton, NJ, USA D Nakashima * Kyowa Hakko Kirin Co. Ltd, Tokyo Research Triangle Park, Tokyo, 194-8533, Japan S
Akinaga * Department of Haematology, St. Bartholomew's Hospital, West Smithfield, London, SE24 9LG, UK H Oakervee & J Cavenagh Authors * K Yong View author publications You can also
search for this author inPubMed Google Scholar * J Cavet View author publications You can also search for this author inPubMed Google Scholar * P Johnson View author publications You can
also search for this author inPubMed Google Scholar * G Morgan View author publications You can also search for this author inPubMed Google Scholar * C Williams View author publications You
can also search for this author inPubMed Google Scholar * D Nakashima View author publications You can also search for this author inPubMed Google Scholar * S Akinaga View author
publications You can also search for this author inPubMed Google Scholar * H Oakervee View author publications You can also search for this author inPubMed Google Scholar * J Cavenagh View
author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to K Yong. ETHICS DECLARATIONS COMPETING INTERESTS D Nakashima and S
Akinaga are employees of Kyowa Hakko Kirin Co. Ltd (Tokyo and USA). The other authors declare no conflict of interest. RIGHTS AND PERMISSIONS This work is licensed under the Creative Commons
Attribution-Non-Commercial-Share Alike 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/ Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Yong, K., Cavet, J., Johnson, P. _et al._ Phase I study of KW-2478, a novel Hsp90 inhibitor, in patients with B-cell malignancies. _Br J Cancer_ 114, 7–13 (2016).
https://doi.org/10.1038/bjc.2015.422 Download citation * Received: 06 July 2015 * Revised: 12 October 2015 * Accepted: 26 October 2015 * Published: 22 December 2015 * Issue Date: 12 January
2016 * DOI: https://doi.org/10.1038/bjc.2015.422 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * chaperone * Hsp90 * KW-2478 * multiple myeloma *
phase I
