Genetic characterization of two gain-of-function alleles of the effector caspase drice in drosophila
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Caspases are the executioners of apoptosis. Although much is known about their physiological roles and structures, detailed analyses of missense mutations of caspases are lacking.
As mutations within caspases are identified in various human diseases, the study of caspase mutants will help to elucidate how caspases interact with other components of the apoptosis
pathway and how they may contribute to disease. DrICE is the major effector caspase in _Drosophila_ required for developmental and stress-induced cell death. Here, we report the isolation
and characterization of six _de novo drICE_ mutants, all of which carry point mutations affecting amino acids conserved among caspases in various species. These six mutants behave as
recessive loss-of-function mutants in a homozygous condition. Surprisingly, however, two of the newly isolated _drICE_ alleles are gain-of-function mutants in a heterozygous condition,
although they are loss-of-function mutants homozygously. Interestingly, they only behave as gain-of-function mutants in the presence of an apoptotic signal. These two alleles carry missense
mutations affecting conserved amino acids in close proximity to the catalytic cysteine residue. This is the first time that viable gain-of-function alleles of caspases are described in any
intact organism and provides a significant exception to the expectation that mutations of conserved amino acids always abolish the pro-apoptotic activity of caspases. We discuss models about
how these mutations cause the gain-of-function character of these alleles. SIMILAR CONTENT BEING VIEWED BY OTHERS DNASE II MEDIATES A PARTHANATOS-LIKE DEVELOPMENTAL CELL DEATH PATHWAY IN
_DROSOPHILA_ PRIMORDIAL GERM CELLS Article Open access 16 April 2021 IONIZING RADIATION INDUCES CELLS WITH PAST CASPASE ACTIVITY THAT CONTRIBUTE TO THE ADULT ORGAN IN _DROSOPHILA_ AND SHOW
REDUCED LOSS OF HETEROZYGOSITY Article Open access 05 January 2024 AKT1 AND DCIZ1 PROMOTE CELL SURVIVAL FROM APOPTOTIC CASPASE ACTIVATION DURING REGENERATION AND ONCOGENIC OVERGROWTH Article
Open access 12 November 2020 MAIN Apoptosis is a major form of programmed cell death.1 The core apoptotic machinery is evolutionary conserved with caspases as the fundamental components.1,
2, 3 Caspases are specific cysteine proteases that are produced as inactive zymogens composed of an N-terminal pro-domain, a large subunit region with the catalytic cysteine residue, and a
small subunit region at the carboxyl end.2, 4 Depending on their structures and functions, caspases are grouped into initiator and effector caspases.2, 3 Initiator caspases possess long
pro-domains, which facilitate the recruitment of initiator caspases into cell death signaling complexes for activation.2, 3, 5 Effector caspases are activated by initiator caspase complexes
through proteolytic processing, cleaving off the pro-domain and separating the large and small subunits. The active effector caspase is a tetramer composed of two large and two small
subunits and contains two catalytic sites.2, 3 Activated effector caspases cleave many protein targets to trigger the physiological and morphological changes characteristic of apoptosis. In
mammals, apoptotic initiator caspases are Caspase-8, -9, -2 and -10, and effector caspases involved in apoptosis are Caspase-3, -7 and -6.2, 3 Of the seven _Drosophila_ caspases, only the
initiator caspase Dronc (Caspase-9-like) and the effector caspases DrICE and Dcp-1 (Caspase-3-like) have been implicated in apoptosis in imaginal discs.1, 2, 6 Caspase activation is tightly
regulated in surviving cells. Inhibitor of apoptosis proteins (IAPs) directly bind to and inhibit processed caspases.7, 8, 9 The best-characterized IAPs are mammalian XIAP and _Drosophila_
IAP1 (DIAP1).10, 11 In cells committed to apoptosis, IAP-mediated inhibition of caspases is counteracted by IAP antagonists. Specifically, the IAP antagonists encoded by _reaper_, _hid_ and
_grim_ (_RHG_)12, 13, 14, 15 in _Drosophila_ trigger proteolytic degradation of DIAP116, 17, 18, 19, 20 which releases caspases from DIAP1 inhibition and triggers apoptosis. The
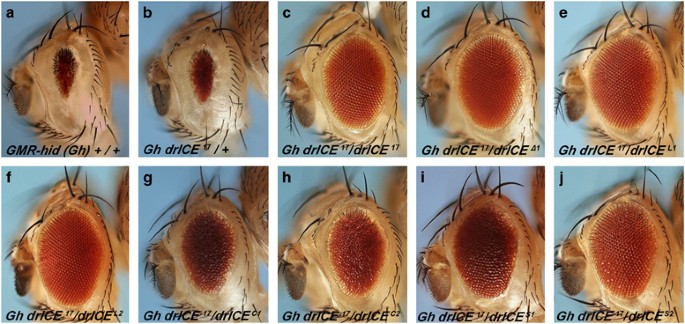
overexpression of the _RHG_ genes in the fly eye using the eye-specific _GMR_ promoter causes an eye ablation phenotype due to massive apoptosis (see, for example, _GMR_-_hid_ in Figure
1a).12, 13, 14 In fact, mutants of _diap1_, _dronc_ and _drICE_ genes were isolated in genetic screens searching for modifiers of the eye ablation phenotypes caused by _reaper_ or _hid_
overexpression.21, 22, 23, 24, 25, 26, 27 Mammalian IAP antagonists are Smac/Diablo and HtrA2/Omi, which function similarly to the RHG proteins.28, 29, 30, 31 Both IAPs and IAP antagonists
are under tight control by various mechanisms to ensure proper regulation of caspase activity.1, 6, 11, 32 Given the pivotal roles of apoptosis in development and tissue homeostasis, it is
not surprising that deregulation of caspases has been implicated in various pathological conditions, including neurodegeneration, autoimmune diseases and cancers.33 Mutations in _Caspase-10_
and _-8_ are found in autoimmune lymphoproliferative syndrome (ALPS) and ALPS-related disorders.34 Mutations and polymorphisms of _Caspase-8_, _-9_, _-3_ and _-7_ have been implicated in
various cancers.35, 36 Although some of these mutations disrupt the apoptotic activity of the affected caspase in cell culture studies,37, 38, 39, 40, 41 detailed understanding of how these
mutations affect the function and expression of the caspases is scarce. It is thus of great biological and clinical significance to isolate more caspase mutants, especially the ones carrying
point mutations, and to analyze their behaviors _in vivo_. The _Drosophila_ model system provides a great venue to answer such inquiries, given the conservation of caspase genes and the
well-established genetic techniques to isolate and characterize mutations in genes of interest. DrICE and Dcp-1 are the effector caspase orthologs of mammalian Caspase-3 and Caspase-7 (ref.
42,43; reviewed in ref. 2, 6). Although Dcp-1 has a crucial role in nurse cell death during mid-oogenesis, DrICE is required for apoptosis in most developmental and irradiation-induced cell
death.25, 44, 45 Four _drICE_ mutants have been reported so far, only one of which, _drICE__17_, is caused by a missense mutation.25, 44, 46 _drICE__Δ1_, _drICE__Δ1E4_ and _drICE__Δ2C8_ were
generated through imprecise P-element excisions, through which the entire _drICE_ open-reading frame was removed to produce null alleles.44, 46 _drICE__17_ carries an N to Y mutation at
residue 116 and was isolated in an EMS mutagenesis screen as a recessive suppressor of the small eye phenotype induced by _GMR_-_hid_. This missense mutation leads to decreased protein
stability and behaves as a strong hypomorphic allele.25 These alleles have improved our understanding of the essential roles of _drICE_ in developmental, stress-induced and _RHG_-induced
cell death;25, 44, 46 however, they have not provided information on how various amino-acid substitutions affect the behavior of the caspase protein. In this study, we describe the isolation
and characterization of six new EMS-induced recessive loss-of-function alleles of _drICE_, all of which affect highly conserved amino-acid residues. Four of these alleles produce unstable
DrICE proteins. However, the remaining two alleles behave genetically differently. Although they were isolated as loss-of-function alleles homozygously, they surprisingly display
gain-of-function characteristics in the presence of a wild-type copy of _drICE_, that is, in a heterozygous condition. Interestingly, they only behave as gain-of-function mutants in the
presence of an apoptotic signal. Molecularly, the mutations in these alleles change conserved residues in close proximity to the catalytic Cys residue. We discuss models about how these
mutations cause the gain-of-function character of these alleles. RESULTS ISOLATION OF _DE NOVO DRICE_ MUTANT ALLELES Ectopic expression of _hid_ under the control of the eye-specific _GMR_
promoter causes an eye ablation phenotype owing to excessive cell death (Figure 1a).14 This eye ablation phenotype has been used in EMS mutagenesis screens to isolate mutants of genes
involved in apoptosis. To isolate additional _drICE_ mutant alleles to broaden our understanding of DrICE function in cell death, we performed an allele screen. The existing _drICE_ allele,
_drICE__17_, does not suppress the _GMR-hid_-induced eye ablation phenotype in a dominant or heterozygous manner (Figure 1b). Only homozygously (Figure 1c), or in trans to a _drICE_
deficiency (_drICE__Δ1_) (Figure 1d), can suppression of _GMR_-_hid_ be recorded. Therefore, to isolate new alleles of _drICE_, we took advantage of the fact that loss-of-heterozygosity of
_drICE__17_ causes strong suppression of _GMR_-_hid_. In an EMS mutagenesis screen, we screened 40 000 F1 progeny and recovered six suppressors, namely _L1, L2, C1, C2, S1_ and _S2_, which
suppressed _GMR_-_hid_ only in trans to _drICE__17_ (Figure 1e–j) and are therefore good candidates for new _drICE_ alleles. In addition, the six mutants are homozygously viable with wing
clearance defects (data not shown) as observed in _drICE__17_ and _drICE__Δ1_ alleles.25, 44 MOLECULAR CHARACTERIZATION OF THE NOVEL _DRICE_ ALLELES Through DNA sequencing, we identified in
each of these six alleles point mutations affecting amino acids highly conserved among effector caspases in various species (Figure 2a). _drICE__L1_ and _drICE__L2_ contain missense
mutations of amino acids located in the large subunit, changing Y86 to N and G94 to D, respectively. _drICE__S1_ and _drICE__S2_ bear nonsense mutations in the small subunit at positions 258
and 266, respectively, and produce carboxyl-end truncated _drICE_ proteins. _drICE__C1_ and _drICE__C2_ carry missense mutations of amino acids located near the catalytic cysteine, C211,
changing G213 to D and G219 to E, respectively. We refer to the alleles affecting the large subunit as class L alleles (_L1_ and _L2_), except the ones located close to the catalytic
cysteine residue, which we refer to as class C alleles (_C1_ and _C2_). Mutants affecting the small subunit are class S alleles (_S1_ and _S2_) (Figure 2b). DRICE PROTEIN LEVELS ARE MARKEDLY
DECREASED IN CLASS L AND CLASS S MUTANTS, BUT NOT IN CLASS C ALLELES To assess how these novel _drICE_ mutations affect DrICE function, we examined the stability of the mutant DrICE
proteins. Mosaic eye imaginal discs of the _drICE_ alleles from third instar larvae were labeled with an anti-DrICE polyclonal antibody.47 Levels of DrICE protein decreased markedly in
mutant clones of class L and class S alleles (Figure 3a’–e’), similar to _drICE__17_, which was previously shown to encode an unstable DrICE protein (ref. 25). These results suggest that
class L and class S alleles produce little or unstable DrICE protein. In contrast, class C alleles behave differently. Although mutant clones of _drICE__C1_ contain slightly decreased
protein levels, _drICE__C2_ produces normal DrICE protein levels comparable to wild-type tissues (Figure 3f’ and g’). To verify that the class C alleles indeed encode recessive
loss-of-function alleles, we examined if they affect normal developmental cell death during eye development. At 28 h after puparium formation (APF), a wave of apoptosis removes all excess
interommatidial cells.48, 49, 50 This developmental cell death is lost in mutant clones of both class C alleles (Figure 4a’ and b’), suggesting that class C alleles are recessive
loss-of-function mutants. Therefore, because these two alleles produce normal DrICE protein levels, they affect DrICE activity independently of protein stability. Given that they have
mutations in highly conserved residues close to the catalytic cysteine residue (Figure 2), it is likely that these mutants have reduced catalytic activity. CLASS C ALLELES AFFECT SUBSTRATE
BINDING From a structural perspective, it is quite clear why these mutations (G213D and G219E) lead to inactivation of DrICE when they are homozygous. In caspases generally, the
substrate-binding groove loops 2, 3 and 4 from one half of the tetramer interact with loop 2′ from the opposite half of the tetramer to form an ordered substrate-binding groove (Figure 5a).
Loops 2 and 2′ form a lock that holds loops 3 and 4 into the proper conformation. If the loop 2/loop 2′ interaction is lost, loops 3 and 4 become disordered and the enzyme is unable to bind
substrate (Figure 5c). Based on examination of the DrICE structure (3SIP),51 residues G213 and G219 are both contained within loop 2 of DrICE. Neither of these positions can accommodate any
residue larger than a glycine (Figure 5b) and the G213D and G219E mutations are predicted to inhibit critical interactions between loops 2 and 2’. G213 forms an exceptionally tight contact
with the backbone of F256 on loop 3, the loop that forms the base of the substrate-binding groove (Figure 5b). Substitution of G213 with aspartate disrupts the contact with F256 and prevents
the tight association of loops 2 and 2′, which supports substrate binding. G219, also on loop 2, interacts with the side chain of V241, which is contained on loop 2′ (Figure 5b).
Substitution of G219 with any rotomers of glutamate results in steric clash with adjacent residues. This steric clash would be sufficient to prevent loop ordering and substrate binding
(Figure 5c), explaining the loss of enzymatic activity of these two mutants in a homozygous condition. CLASS C _DRICE_ ALLELES DOMINANTLY ENHANCE _GMR-REAPER_ The new _drICE_ alleles were
recovered as recessive suppressors of _GMR_-_hid_ (Figure 1). They are also recessive suppressors of _GMR_-_reaper_ (Supplementary Figure S1). Surprisingly, however, in the course of this
analysis, we noted that the two class C alleles—when heterozygous in trans to a wild-type (_drICE_+) allele—dominantly enhanced _GMR_-_reaper_ (Figure 6b), whereas class L and class S
alleles weakly suppress it (Figure 6a; Supplementary Figure S2). Class C alleles also appear to act as dominant enhancers of _GMR_-_hid_ (Supplementary Figure S3). One possibility by which
class C alleles dominantly enhance _GMR_-_reaper_ is through increased caspase activity. To examine this possibility, we performed fluorometric caspase assays with head extracts from
_GMR-reaper_ animals heterozygous for various _drICE_ alleles. Consistently, although the loss-of-function alleles _drICE__L2_ and _drICE__S1_ have lost significant caspase activity, the
class C allele _drICE__C1_ displayed increased caspase activity compared with _GMR-reaper_ alone (Figure 6e). Furthermore, more TUNEL-positive signals are observed in _GMR-reaper_ eye
imaginal discs heterozygous for class C alleles compared with _GMR-reaper_ in wild-type or heterozygous class L background (Figure 6f–j). Therefore, the dominant enhancement of the eye
ablation phenotype of _GMR_-_reaper_ by class C alleles is indeed due to increased cell death. To further characterize the new _drICE_ alleles, we examined DIAP1 degradation triggered by
reaper.16, 17, 18, 19, 20 In _GMR-reaper_ eye imaginal discs, DIAP1 degradation is very prominent in cells immediately posterior to the column of R8 photoreceptor neurons, which are very
resistant to apoptosis52 and where DIAP1 is not degraded (Figure 7a and b; R8 photoreceptor columns are marked by arrows; the zones of DIAP1 degradation by asterisks (*)). Interestingly,
reduced DrICE activity in heterozygous loss-of-function alleles partially protects DIAP1 from reaper-induced degradation (Figure 7c and d), which is consistent with the suppression of
_GMR-reaper_ by these alleles (Figure 6a). In contrast, the gain-of-function alleles _drICE__C1_ and _drICE__C2_ fail to protect DIAP1 from reaper-induced degradation (asterisks in Figure 6e
and f). These findings suggest that the complex interaction between reaper, DIAP1 and DrICE is affected by the class C alleles. We also examined if the class C alleles behaved as
gain-of-function alleles in the absence of an apoptotic signal such as _GMR_-_reaper_, and thus may cause inappropriate cell loss using the developing retina as a model. At 42 h APF, the
retina forms a highly regular lattice with a constant number of cells48, 49, 50 (Supplementary Figure S4). Inappropriate cell loss results in disruption of lattice symmetry and is easy to
score. However, for both class C alleles, we did not detect any irregularity in the appearance of the lattice that may indicate inappropriate cell loss (Supplementary Figure S4). This
analysis suggests that the class C mutations do not cause premature and inappropriate activation of DrICE in the absence of an apoptotic signal. Taken together, the class C alleles exert a
very complex genetic behavior. Homozygously mutant _drICE__C1_ and _drICE__C2_ are recessive loss-of-function alleles and produce mutant DrICE proteins with impaired substrate binding.
However, in the presence of functional DrICE protein encoded from the _drICE_+ allele, they trigger more cell death than normal and enhance the _GMR-reaper_ eye ablation phenotype. Thus, in
a heterozygous condition they behave as gain-of-function alleles. DISCUSSION In this study, we report the isolation and characterization of six new mutants of the _Drosophila_ effector
caspase _drICE_, the ortholog of mammalian caspase-3. According to the types and locations of the mutations in these six new _drICE_ alleles, the expression levels of the mutant DrICE
proteins and the genetic interactions with _GMR-hid_ or _GMR-reaper,_ we have grouped these mutants into three classes. Class L and S alleles carry point mutations in the large and small
subunits, respectively (Figure 2b). In addition, according to this classification criterion, _drICE__17_(N116Y) also belongs to the class L group. Both class L and class S alleles affect the
stability of their DrICE protein products and hence have reduced caspase activity. Overall, they are recessive loss-of-function _drICE_ alleles. The class C alleles _drICE__C1_ and
_drICE__C2_ carry missense mutations in conserved amino acids (G213D and G219E) in close proximity of the catalytic cysteine (C211) residue. In contrast to class L and class S alleles, these
mutations do not markedly impact DrICE protein stability. Most importantly, although these alleles act as loss-of-function alleles homozygously, in the presence of a wild-type _drICE_+
allele (i.e., heterozygously), they can enhance cell death induced by _reaper_ expression, suggesting that they can function as gain-of-function alleles. However, our analysis suggests that
class C mutants only behave as gain-of-function alleles in an apoptotic background such as _GMR_-_reaper_, whereas they do not cause spontaneous activation of DrICE in the absence of
apoptotic signals. Therefore, they can only act as gain-of-function mutants after proteolytic processing. The underlying mechanisms of the apoptotic enhancement by heterozygous class C
alleles are less clear. In the few cases where certain mutants behave as recessive loss-of-function and dominant gain-of-function alleles, dimerization is the underlying cause of this
different genetic behavior. For example, certain mutations of the _Toll_ receptor are recessive loss-of-function alleles, but are gain-of-function owing to physical interaction with the
wild-type allele.53, 54 Given that two processed DrICE molecules form an enzymatically active tetramer with two catalytic sites,2, 3 one can speculate that class C mutant subunits exert a
dominant effect via interaction with a wild-type DrICE molecule. Statistically, only 50% of the DrICE tetramers in the cell are composed of a wild-type and a class C subunit (referred to as
wt/class C heterotetramer; Figure 5d). The remaining 50% are either wild-type (Figure 5a) or class C tetramers (Figure 5c), the latter of which has no enzymatic activity. Thus, the wt/class
C heterotetramers actually have more activity as the data imply because they also compensate for the loss of activity of the class C tetramers. How can we explain the gain-of-function
activity of the wt/class C heterotetramers? Because of the loss of loop organization and substrate binding in the class C subunit (Figure 5), it is unlikely that the presence of the
wild-type subunit in the wt/class C heterotetramer 'rescues' the defect in the class C subunit. It is more likely that an upstream regulatory mechanism is deregulated by the
wt/class C heterotetramer. There are several possibilities. For example, because IAPs require effector caspases for inhibitory activity,55, 56 it may be possible that a wt/class C
heterotetramer does not produce as much active DIAP1 compared with a wild-type tetramer. Specifically, the N terminus of DIAP1 is an intramolecular inhibitor of DIAP1, keeping DIAP1 in an
auto-inhibited conformation, unable to bind and inhibit DrICE.51, 57, 58, 59 After caspase cleavage at D20 (likely by DrICE), the inhibitory N terminus is removed and now activated DIAP1 can
bind to DrICE and inhibit it. Because DIAP1 is a substrate of DrICE before it becomes an inhibitor, the wt/class C heterotetramer would produce less-active DIAP1 and thus would be
less-efficiently inhibited. Another possible model includes cooperativity of the binding of DIAP1 to the two active sites of the DrICE tetramer. Because the inhibitory region of DIAP1 (pink
rod in Figure 5d) binds to the substrate-binding domain of DrICE present in the 3SIP structure,51 DIAP1 does not bind to the mutant catalytic site of the class C subunit. If there is
cooperativity of the binding of DIAP1 to the two catalytic sites of DrICE, the failure of DIAP1 to bind to the class C catalytic side may affect the binding of DIAP1 to the catalytic site of
the wild-type subunit. Thus, the catalytic site of the wild-type subunit would be free from IAP inhibition, which should lead to increased activity only in the heterozygous condition.
Finally, we also take into account that class C alleles are only dominantly active in the presence of an apoptotic signal such as reaper (Figure 6,Supplementary Figure S4). Reaper and DrICE
compete for binding to the BIR1 domain of DIAP1.47 If reaper is in excess, it triggers degradation of DIAP1 (Figure 7b) and thus apoptosis.16, 17, 18, 19, 20 Therefore, owing to the failure
of DIAP1 to bind to the class C subunit, the wt/class C heterotetramer may shift the equilibrium towards binding of DIAP1 to reaper such that DIAP1 is more efficiently degraded by reaper,
resulting in higher enzymatic activity of the wt/class C heterotetramer. Consistent with this notion, reduced DrICE activity in heterozygous loss-of-function alleles partially protects DIAP1
from reaper-induced degradation (Figure 7c and d), whereas the class C alleles of DrICE fail to protect DIAP1 from degradation (Figure 7e and f), suggestive of decreased binding of the
wt/class C heterotetramer to DIAP1. These models to explain the gain-of-function behaviors of the class C alleles are not mutually exclusive and other models may be possible, too. To our
knowledge, this is the first time that viable gain-of-function alleles of caspases are described in any intact organism and provides a significant exception to the expectation that mutations
of conserved amino acids always abolish the pro-apoptotic activity of caspases. Because the affected residues of the class C alleles are conserved in other caspases (Figure 2a), it would be
of great interest to generate and test such mutations in other caspases to examine if it is a universal phenomenon. In _Caenorhabditis elegans_, a large collection of _ced-3_ mutants has
been characterized, but no gain-of-function alleles have been reported.60 Interestingly, one _ced-3_ allele, _n2433_, substitutes G360 with S. This glycine residue corresponds to G213 in
DrICE, which is changed to D in _drICE__C1_ (Figure 2). However, in contrast to _drICE__C1_, _n2433_ behaves as a dominant negative.60 It is unclear, if these different genetic behaviors are
due to the different amino-acid substitutions (S in _n2433_ vs D in _drICE__C1_) or to intrinsic differences between Ced-3 and DrICE. In mice, the _Melody_ mutation in Caspase-3 substitutes
the catalytic C with a S residue. This mutation also behaves as a dominant negative in a heterozygous condition.61 The only mutation in Caspase-3 that confers constitutively active
properties to Caspase-3 is the V266E substitution, which was characterized by _in vitro_ mutagenesis.62, 63 This mutation increases Caspase-3 activity 60-fold. V266 is located in the dimer
interface of the small subunit and the V266E mutation promotes dimerization of Caspase-3 without proteolytic processing.63 V266 is not conserved in DrICE or other caspases (Figure 2), but
even if it was, it is unlikely that such a mutation can be recovered _in vivo_ as it will cause dominant lethality due to excessive apoptosis. The allele-specific enhancement of apoptotic
activity by caspase mutants is also of clinical significance. For example, it might be of interest to determine whether such dominant mutations of caspases have a role in the pathogenesis
and development of neurodegenerative disorders, for which the underlying mechanism is apoptotic neuron loss. Sequence analysis of caspase genes in these patients will help in answering this
possibility. Conversely, it would also be of interest to design and screen for drugs that would slightly twist the conformation of caspase tetramers to increase their enzymatic activity and
induce cell death in tumors. MATERIALS AND METHODS ISOLATION AND IDENTIFICATION OF _DRICE_ MUTANT ALLELES An isogenized _FRT82B_ stock was used for EMS mutagenesis and also used as a control
for genetic analyses. _FRT82B_ males were treated with 25 mM EMS in 5% sucrose solution for 24 h. After recovery for 3 h, they were crossed to _GMR-hid drICE__17_ females, and incubated at
25 °C. In all, 40 000 F1 progeny were screened for suppression of the small eye phenotype of _GMR-hid drICE__17_. Six mutants were identified as _de novo drICE_ alleles by genetic analysis
and DNA sequencing as described in the Results section. FLY STOCKS AND GENETICS The following mutant and transgenic fly stocks were used: drICEL1; drICEL2; drICEC1; drICEC2; drICES1; drICES2
(this study); drICE17 (ref. 25); drICEΔ1 (ref. 44); GMR-hid;14 CyO,2xGMR-reaper;12 ey-FLP, FRT82B ubi-GFP;64 GMR-hid ey-FLP, FRT82B ubi-GFP.25 GMR-hid drICE17 is a recombinant chromosome
carrying a GMR-hid transgene and drICE17. Genetic mosaics for immunohistochemical analysis were obtained by crossing the FRT82B drICE alleles with ey-FLP; FRT82B drICE-/FRT82B ubi-GFP. DNA
SEQUENCING Genomic DNA from homozygous mutant flies or trans-heterozygous over _drICE__Δ1_flies was isolated and PCR-amplified using _drICE_-specific primers. PCR fragments were sequenced by
Sanger sequencing. The single amino-acid code was used. IMMUNOHISTOCHEMISTRY TUNEL and immunohistochemistry were carried out as described.65 Anti-DrICE antibody (a kind gift of Pascal
Meier) was raised in guinea pig47 and used at a dilution of 1 : 200. Anti-Dlg antibody (a kind gift of Georg Halder) was used at a dilution of 1 : 2000. Anti-DIAP1 (SK14) antibody (a kind
gift of Pascal Meier) was raised in guinea pig and used at a concentration of 1 : 400. Cy3- and Cy-5 fluorescent-conjugated secondary antibodies were obtained from Jackson ImmunoResearch
(West Grove, PA, USA). In general, 10–20 eye imaginal discs were analyzed, unless stated otherwise. Fluorescent Images were captured using a Zeiss Axio Imager Z1 with ApoTome technology or
an Olympus Optical FV500 confocal microscope. QUANTIFICATION OF _GMR-REAPER_-INDUCED EXCESSIVE CELL DEATH All TUNEL-positive cells in _GMR-reaper_ larval eye discs in various genotypes were
counted to indicate how much cell death was induced. For each genotype, 15–17 representative eye discs were counted. Significance was calculated by using unpaired two-tailed Student’s
_t_-test with a 95% confidence interval. FLUOROMETRIC CASPASE ASSAYS Fluorometric caspase assays were performed as described with modifications.66 Adult heads were dissected on ice and lysed
with a pestle in caspase assay buffer (50 mM HEPES, pH 7.5; 100 mM NaCl; 1 mM EDTA; 0.1% CHAPS; 10% sucrose; 5 mMDTT; 0.5% TritonX‐100; 4% glycerol; 1 × protease inhibitor cocktail
(Promega, Madison, WI, USA)) and then sonicated once for 7 s. Protein concentrations of the lysates were determined by Bradford Protein Assay. For each genotype, 40 μg of total protein
lysates were used, respectively. Protein lysates and 100 μM of the fluorometric substrate DEVD-AMC (MP Biomedicals, Santa Ana, CA, USA) were combined in a 96-well plate on ice. Reaction
volume was brought to 100 μl with caspase assay buffer. Spectrophotometer was used to measure fluorescence (excitation 385 nm and emission 460 nm) at 15 min intervals for 2 h at 37 °C.
ABBREVIATIONS * ALPS: autoimmune lymphoproliferative syndrome * APF: after puparium formation * Ced-3: cell death defective 3 * CyO: curly of Oster * Dcp-1: death caspase 1 * DIAP1:
death-associated inhibitor of apoptosis 1 * DNA: desoxyribonucleic acid * drICE: death-related ICE (interleukin converting enzyme) * dronc: death regulator Nedd2-like caspase * EMS: ethyl
methanesulfonate * Ey: eyeless * Flp: flippase * FRT82B: flippase recombination target at 82B * GFP: green fluorescent protein * Gh: GMR-hid * Hid: head involution defective * GMR: glass
multimer reporter * HtrA2: high-temperature-required protein A2 * IAP: inhibitor of apoptosis protein * PCR: polymerase chain reaction * R8: photoreceptor 8 * RHG: reaper, hid, grim *
Smac/Diablo: second mitochondria-derived activator of caspases/direct IAP binding protein with low pI * TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling * Ubi: ubiquitous
* wt: wild-type * XIAP: X-linked inhibitor of apoptosis REFERENCES * Fuchs Y, Steller H . Programmed cell death in animal development and disease. _Cell_ 2011; 147: 742–758. Article CAS
PubMed PubMed Central Google Scholar * Kumar S . Caspase function in programmed cell death. _Cell Death Differ_ 2007; 14: 32–43. Article CAS PubMed Google Scholar * Riedl SJ, Shi Y .
Molecular mechanisms of caspase regulation during apoptosis. _Nat Rev Mol Cell Biol_ 2004; 5: 897–907. Article CAS PubMed Google Scholar * Alnemri ES, Livingston DJ, Nicholson DW,
Salvesen G, Thornberry NA, Wong WW _et al_. Human ICE/CED-3 protease nomenclature. _Cell_ 1996; 87: 171. Article CAS PubMed Google Scholar * Bratton SB, Salvesen GS . Regulation of the
Apaf-1-caspase-9 apoptosome. _J Cell Sci_ 2010; 123 (Pt 19): 3209–3214. Article CAS PubMed PubMed Central Google Scholar * Xu D, Woodfield SE, Lee TV, Fan Y, Antonio C, Bergmann A .
Genetic control of programmed cell death (apoptosis) in Drosophila. _Fly_ 2009; 3: 78–90. Article CAS PubMed Google Scholar * Callus BA, Vaux DL . Caspase inhibitors: viral, cellular and
chemical. _Cell Death Differ_ 2007; 14: 73–78. Article CAS PubMed Google Scholar * Vaux DL, Silke J . IAPs, RINGs and ubiquitylation. _Nat Rev Mol Cell Biol_ 2005; 6: 287–297. Article
CAS PubMed Google Scholar * Gyrd-Hansen M, Meier P . IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. _Nat Rev Cancer_ 2010; 10: 561–574. Article CAS
PubMed Google Scholar * Galban S, Duckett CS . XIAP as a ubiquitin ligase in cellular signaling. _Cell Death Differ_ 2010; 17: 54–60. Article CAS PubMed Google Scholar * Orme M, Meier
P . Inhibitor of apoptosis proteins in Drosophila: gatekeepers of death. _Apoptosis_ 2009; 14: 950–960. Article PubMed Google Scholar * White K, Tahaoglu E, Steller H . Cell killing by
the Drosophila gene reaper. _Science_ 1996; 271: 805–807. Article CAS PubMed Google Scholar * Chen P, Nordstrom W, Gish B, Abrams JM . grim, a novel cell death gene in Drosophila. _Genes
Dev_ 1996; 10: 1773–1782. Article CAS PubMed Google Scholar * Grether ME, Abrams JM, Agapite J, White K, Steller H . The head involution defective gene of Drosophila melanogaster
functions in programmed cell death. _Genes Dev_ 1995; 9: 1694–1708. Article CAS PubMed Google Scholar * White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H . Genetic control of
programmed cell death in Drosophila. _Science_ 1994; 264: 677–683. Article CAS PubMed Google Scholar * Ryoo HD, Bergmann A, Gonen H, Ciechanover A, Steller H . Regulation of Drosophila
IAP1 degradation and apoptosis by reaper and ubcD1. _Nat Cell Biol_ 2002; 4: 432–438. Article CAS PubMed Google Scholar * Holley CL, Olson MR, Colon-Ramos DA, Kornbluth S . Reaper
eliminates IAP proteins through stimulated IAP degradation and generalized translational inhibition. _Nat Cell Biol_ 2002; 4: 439–444. Article CAS PubMed PubMed Central Google Scholar *
Yoo SJ, Huh JR, Muro I, Yu H, Wang L, Wang SL _et al_. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. _Nat Cell Biol_ 2002; 4: 416–424. Article CAS PubMed
Google Scholar * Hays R, Wickline L, Cagan R . Morgue mediates apoptosis in the Drosophila melanogaster retina by promoting degradation of DIAP1. _Nat Cell Biol_ 2002; 4: 425–431. Article
CAS PubMed Google Scholar * Wing JP, Schreader BA, Yokokura T, Wang Y, Andrews PS, Huseinovic N _et al_. Drosophila Morgue is an F box/ubiquitin conjugase domain protein important for
grim-reaper mediated apoptosis. _Nat Cell Biol_ 2002; 4: 451–456. Article CAS PubMed Google Scholar * Hay BA, Wassarman DA, Rubin GM . Drosophila homologs of baculovirus inhibitor of
apoptosis proteins function to block cell death. _Cell_ 1995; 83: 1253–1262. Article CAS PubMed Google Scholar * Goyal L, McCall K, Agapite J, Hartwieg E, Steller H . Induction of
apoptosis by Drosophila reaper, hid and grim through inhibition of IAP function. _EMBO J_ 2000; 19: 589–597. Article CAS PubMed PubMed Central Google Scholar * Lisi S, Mazzon I, White K
. Diverse domains of THREAD/DIAP1 are required to inhibit apoptosis induced by REAPER and HID in Drosophila. _Genetics_ 2000; 154: 669–678. CAS PubMed PubMed Central Google Scholar * Xu
D, Li Y, Arcaro M, Lackey M, Bergmann A . The CARD-carrying caspase Dronc is essential for most, but not all, developmental cell death in Drosophila. _Development_ 2005; 132: 2125–2134.
Article CAS PubMed Google Scholar * Xu D, Wang Y, Willecke R, Chen Z, Ding T, Bergmann A . The effector caspases drICE and dcp-1 have partially overlapping functions in the apoptotic
pathway in Drosophila. _Cell Death Differ_ 2006; 13: 1697–1706. Article CAS PubMed Google Scholar * Srivastava M, Scherr H, Lackey M, Xu D, Chen Z, Lu J _et al_. ARK, the Apaf-1 related
killer in Drosophila, requires diverse domains for its apoptotic activity. _Cell Death Differ_ 2007; 14: 92–102. Article CAS PubMed Google Scholar * Wilson R, Goyal L, Ditzel M,
Zachariou A, Baker DA, Agapite J _et al_. The DIAP1 RING finger mediates ubiquitination of Dronc and is indispensable for regulating apoptosis. _Nat Cell Biol_ 2002; 4: 445–450. Article CAS
PubMed Google Scholar * Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE _et al_. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and
antagonizing IAP proteins. _Cell_ 2000; 102: 43–53. Article CAS PubMed Google Scholar * Suzuki Y, Imai Y, Nakayama H, Takahashi K, Takio K, Takahashi R . A serine protease, HtrA2, is
released from the mitochondria and interacts with XIAP, inducing cell death. _Mol Cell_ 2001; 8: 613–621. Article CAS PubMed Google Scholar * Du C, Fang M, Li Y, Li L, Wang X . Smac, a
mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. _Cell_ 2000; 102: 33–42. Article CAS PubMed Google Scholar * Hegde R,
Srinivasula SM, Zhang Z, Wassell R, Mukattash R, Cilenti L _et al_. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis
protein-caspase interaction. _J Biol Chem_ 2002; 277: 432–438. Article CAS PubMed Google Scholar * Bergmann A . The role of ubiquitylation for the control of cell death in Drosophila.
_Cell Death Differ_ 2010; 17: 61–67. Article CAS PubMed Google Scholar * Agostini M, Tucci P, Melino G . Cell death pathology: perspective for human diseases. _Biochem Biophys Res
Commun_ 2011; 414: 451–455. Article CAS PubMed Google Scholar * Madkaikar M, Mhatre S, Gupta M, Ghosh K . Advances in autoimmune lymphoproliferative syndromes. _Eur J Haematol_ 2011; 87:
1–9. Article CAS PubMed Google Scholar * Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M _et al_. Apoptosis and cancer: mutations within caspase genes. _J Med Genet_ 2009;
46: 497–510. Article CAS PubMed Google Scholar * Olsson M, Zhivotovsky B . Caspases and cancer. _Cell Death Differ_ 2011; 18: 1441–1449. Article CAS PubMed PubMed Central Google
Scholar * Kim MS, Kim HS, Jeong EG, Soung YH, Yoo NJ, Lee SH . Somatic mutations of caspase-2 gene in gastric and colorectal cancers. _Pathol Res Pract_ 2011; 207: 640–644. Article CAS
PubMed Google Scholar * Park WS, Lee JH, Shin MS, Park JY, Kim HS, Kim YS _et al_. Inactivating mutations of the caspase-10 gene in gastric cancer. _Oncogene_ 2002; 21: 2919–2925. Article
CAS PubMed Google Scholar * Kim HS, Lee JW, Soung YH, Park WS, Kim SY, Lee JH _et al_. Inactivating mutations of caspase-8 gene in colorectal carcinomas. _Gastroenterology_ 2003; 125:
708–715. Article CAS PubMed Google Scholar * Soung YH, Lee JW, Kim HS, Park WS, Kim SY, Lee JH _et al_. Inactivating mutations of CASPASE-7 gene in human cancers. _Oncogene_ 2003; 22:
8048–8052. Article PubMed Google Scholar * Soung YH, Lee JW, Kim SY, Sung YJ, Park WS, Nam SW _et al_. Caspase-8 gene is frequently inactivated by the frameshift somatic mutation
1225_1226delTG in hepatocellular carcinomas. _Oncogene_ 2005; 24: 141–147. Article CAS PubMed Google Scholar * Fraser AG, Evan GI . Identification of a Drosophila melanogaster
ICE/CED-3-related protease, drICE. _EMBO J_ 1997; 16: 2805–2813. Article CAS PubMed PubMed Central Google Scholar * Song Z, McCall K, Steller H . DCP-1, a Drosophila cell death protease
essential for development. _Science_ 1997; 275: 536–540. Article CAS PubMed Google Scholar * Muro I, Berry DL, Huh JR, Chen CH, Huang H, Yoo SJ _et al_. The Drosophila caspase Ice is
important for many apoptotic cell deaths and for spermatid individualization, a nonapoptotic process. _Development_ 2006; 133: 3305–3315. Article CAS PubMed Google Scholar * Laundrie B,
Peterson JS, Baum JS, Chang JC, Fileppo D, Thompson SR _et al_. Germline cell death is inhibited by P-element insertions disrupting the dcp-1/pita nested gene pair in Drosophila. _Genetics_
2003; 165: 1881–1888. CAS PubMed PubMed Central Google Scholar * Kondo S, Senoo-Matsuda N, Hiromi Y, Miura M . DRONC coordinates cell death and compensatory proliferation. _Mol Cell
Biol_ 2006; 26: 7258–7268. Article CAS PubMed PubMed Central Google Scholar * Zachariou A, Tenev T, Goyal L, Agapite J, Steller H, Meier P . IAP-antagonists exhibit non-redundant modes
of action through differential DIAP1 binding. _EMBO J_ 2003; 22: 6642–6652. Article CAS PubMed PubMed Central Google Scholar * Baker NE . Cell proliferation, survival, and death in the
Drosophila eye. _Semin Cell Dev Biol_ 2001; 12: 499–507. Article CAS PubMed Google Scholar * Brachmann CB, Cagan RL . Patterning the fly eye: the role of apoptosis. _Trends Genet_ 2003;
19: 91–96. Article CAS PubMed Google Scholar * Roignant JY, Treisman JE . Pattern formation in the Drosophila eye disc. _Int J Dev Biol_ 2009; 53: 795–804. Article CAS PubMed PubMed
Central Google Scholar * Li X, Wang J, Shi Y . Structural mechanisms of DIAP1 auto-inhibition and DIAP1-mediated inhibition of drICE. _Na Commun_ 2011; 2: 408. Article Google Scholar *
Fan Y, Bergmann A . Multiple mechanisms modulate distinct cellular susceptibilities toward apoptosis in the developing Drosophila eye. _Dev Cell_ 2014; 30: 48–60. Article CAS PubMed
PubMed Central Google Scholar * Anderson KV, Jurgens G, Nusslein-Volhard C . Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene
product. _Cell_ 1985; 42: 779–789. Article CAS PubMed Google Scholar * Schneider DS, Hudson KL, Lin TY, Anderson KV . Dominant and recessive mutations define functional domains of Toll,
a transmembrane protein required for dorsal-ventral polarity in the Drosophila embryo. _Genes Dev_ 1991; 5: 797–807. Article CAS PubMed Google Scholar * Srinivasula SM, Hegde R, Saleh
A, Datta P, Shiozaki E, Chai J _et al_. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. _Nature_ 2001; 410: 112–116. Article CAS
PubMed Google Scholar * Tenev T, Zachariou A, Wilson R, Ditzel M, Meier P . IAPs are functionally non-equivalent and regulate effector caspases through distinct mechanisms. _Nat Cell Biol_
2005; 7: 70–77. Article CAS PubMed Google Scholar * Yan N, Wu JW, Chai J, Li W, Shi Y . Molecular mechanisms of DrICE inhibition by DIAP1 and removal of inhibition by Reaper, Hid and
Grim. _Nat Struct Mol Biol_ 2004; 11: 420–428. Article CAS PubMed Google Scholar * Ditzel M, Wilson R, Tenev T, Zachariou A, Paul A, Deas E _et al_. Degradation of DIAP1 by the N-end
rule pathway is essential for regulating apoptosis. _Nat Cell Biol_ 2003; 5: 467–473. Article CAS PubMed Google Scholar * Yokokura T, Dresnek D, Huseinovic N, Lisi S, Abdelwahid E, Bangs
P _et al_. Dissection of DIAP1 functional domains via a mutant replacement strategy. _J Biol Chem_ 2004; 279: 52603–52612. Article CAS PubMed Google Scholar * Shaham S, Reddien PW,
Davies B, Horvitz HR . Mutational analysis of the Caenorhabditis elegans cell-death gene ced-3. _Genetics_ 1999; 153: 1655–1671. CAS PubMed PubMed Central Google Scholar * Parker A,
Hardisty-Hughes RE, Wisby L, Joyce S, Brown SD . Melody, an ENU mutation in Caspase 3, alters the catalytic cysteine residue and causes sensorineural hearing loss in mice. _Mamm Genome_
2010; 21: 565–576. Article CAS PubMed PubMed Central Google Scholar * Pop C, Feeney B, Tripathy A, Clark AC . Mutations in the procaspase-3 dimer interface affect the activity of the
zymogen. _Biochemistry_ 2003; 42: 12311–12320. Article CAS PubMed Google Scholar * Walters J, Pop C, Scott FL, Drag M, Swartz P, Mattos C _et al_. A constitutively active and
uninhibitable caspase-3 zymogen efficiently induces apoptosis. _Biochem J_ 2009; 424: 335–345. Article CAS PubMed Google Scholar * Newsome TP, Asling B, Dickson BJ . Analysis of
Drosophila photoreceptor axon guidance in eye-specific mosaics. _Development_ 2000; 127: 851–860. CAS PubMed Google Scholar * McCall K, Peterson JS . Detection of apoptosis in Drosophila.
_Methods Mol Biol_ 2004; 282: 191–205. CAS PubMed Google Scholar * Denton D, Mills K, Kumar S . Methods and protocols for studying cell death in Drosophila. _Methods Enzymol_ 2008; 446:
17–37. Article CAS PubMed Google Scholar * Ganesan R, Jelakovic S, Campbell AJ, Li ZZ, Asgian JL, Powers JC _et al_. Exploring the S4 and S1 prime subsite specificities in caspase-3 with
aza-peptide epoxide inhibitors. _Biochemistry_ 2006; 45: 9059–9067. Article CAS PubMed Google Scholar * Ganesan R, Mittl PR, Jelakovic S, Grutter MG . Extended substrate recognition in
caspase-3 revealed by high resolution X-ray structure analysis. _J Mol Biol_ 2006; 359: 1378–1388. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We are thankful
to Georg Halder (Katholieke Universiteit Leuven, Belgium), Bruce Hay (Caltech, USA), and Pascal Meier (Institute of Cancer Research, UK) for fly stocks and antibodies. This work was
supported by the National Institute of General Medical Science (NIGMS) to AB. AUTHOR INFORMATION Author notes * D Xu Present address: Current address: Shodair Children’s Hospital, Helena,
MT, USA., AUTHORS AND AFFILIATIONS * The University of Texas MD Anderson Cancer Center, Houston, TX, USA Y Wu, J Garnett, D Xu, E R Flores & A Bergmann * Department of Molecular, Cell
and Cancer Biology, University of Massachusetts Medical School, Worcester, MA, USA J L Lindblad, H E Kamber Kaya & A Bergmann * University of Massachusetts Amherst, Amherst, MA, USA Y
Zhao & J Hardy Authors * Y Wu View author publications You can also search for this author inPubMed Google Scholar * J L Lindblad View author publications You can also search for this
author inPubMed Google Scholar * J Garnett View author publications You can also search for this author inPubMed Google Scholar * H E Kamber Kaya View author publications You can also search
for this author inPubMed Google Scholar * D Xu View author publications You can also search for this author inPubMed Google Scholar * Y Zhao View author publications You can also search for
this author inPubMed Google Scholar * E R Flores View author publications You can also search for this author inPubMed Google Scholar * J Hardy View author publications You can also search
for this author inPubMed Google Scholar * A Bergmann View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to A Bergmann.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Edited by DL Vaux Supplementary Information accompanies this paper on Cell Death
and Differentiation website SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE LEGENDS (DOC 30 KB) SUPPLEMENTARY FIGURE S1 (JPG 351 KB) SUPPLEMENTARY FIGURE S2 (JPG 196 KB) SUPPLEMENTARY FIGURE
S3 (JPG 377 KB) SUPPLEMENTARY FIGURE S4 (JPG 243 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wu, Y., Lindblad, J., Garnett, J. _et al._ Genetic
characterization of two gain-of-function alleles of the effector caspase DrICE in _Drosophila_. _Cell Death Differ_ 23, 723–732 (2016). https://doi.org/10.1038/cdd.2015.144 Download citation
* Received: 04 February 2013 * Revised: 14 September 2015 * Accepted: 29 September 2015 * Published: 06 November 2015 * Issue Date: April 2016 * DOI: https://doi.org/10.1038/cdd.2015.144
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative
