Landscape of the regulatory elements for lysine 2-hydroxyisobutyrylation pathway
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Short-chain fatty acids and their corresponding acyl-CoAs sit at the crossroads of metabolic pathways and play important roles in diverse cellular processes. They are also
precursors for protein post-translational lysine acylation modifications. A noteworthy example is the newly identified lysine 2-hydroxyisobutyrylation (Khib) that is derived from
2-hydroxyisobutyrate and 2-hydroxyisobutyryl-CoA. Histone Khib has been shown to be associated with active gene expression in spermatogenic cells. However, the key elements that regulate
this post-translational lysine acylation pathway remain unknown. This has hindered characterization of the mechanisms by which this modification exerts its biological functions. Here we show
that Esa1p in budding yeast and its homologue Tip60 in human could add Khib to substrate proteins both _in vitro_ and _in vivo_. In addition, we have identified HDAC2 and HDAC3 as the major
enzymes to remove Khib. Moreover, we report the first global profiling of Khib proteome in mammalian cells, identifying 6 548 Khib sites on 1 725 substrate proteins. Our study has thus
discovered both the “writers” and “erasers” for histone Khib marks, and major Khib protein substrates. These results not only illustrate the landscape of this new lysine acylation pathway,
but also open new avenues for studying diverse functions of cellular metabolites associated with this pathway. SIMILAR CONTENT BEING VIEWED BY OTHERS REVERSIBLE HISTONE DEACETYLASE ACTIVITY
CATALYZES LYSINE ACYLATION Article 26 March 2025 HISTONE ACYLATION MARKS RESPOND TO METABOLIC PERTURBATIONS AND ENABLE CELLULAR ADAPTATION Article Open access 11 December 2020 GLOBAL
PROFILING OF REGULATORY ELEMENTS IN THE HISTONE BENZOYLATION PATHWAY Article Open access 16 March 2022 INTRODUCTION About 17 000 cellular metabolites are now annotated in the Kyoto
Encyclopedia of Genes and Genomes (KEGG) database1. Previous studies demonstrated a few major mechanisms by which cellular metabolites exert their functions, such as reversible binding to a
protein to activate or inhibit its function, and serving as a precursor for protein post-translational modifications (PTMs)2,3,4. Nevertheless, the scope and broad functions of most cellular
metabolites remain unknown, representing one of major black boxes in modern biology5. Short-chain fatty acids are a family of metabolites that are generated by either metabolism of host
mammalian cells or microbial fermentation in the gut6. Emerging evidence suggests that the microbial-derived metabolites can be either harmful or beneficial to human health7,8. They have
broad functions in signaling, host metabolism and immunity9. Despite much progress, the molecular mechanisms through which these molecules exert their function remain elusive. It was
recently shown that short-chain fatty acids could serve as precursors for synthesis of their corresponding acyl-CoAs that in turn regulate histone modifications and gene expression10. This
result leads to an intriguing hypothesis that metabolites from microbial fermentation can regulate epigenetic programs and control gene expression11. 2-Hydroxyisobutyrate, a cellular
short-chain fatty acid, has been detected at high levels in the urine of obese people and is associated with the presence of some gut microbiota12,13. The dynamics of symbiotic gut
microbiota-associated metabolites, including 2-hydroxyisobutyrate, have been associated with diverse host metabolic phenotypes13,14. Remarkably, 2-hydroxyisobutyrate is a precursor for the
synthesis of 2-hydroxyisobutyryl-CoA and moreover, lysine 2-hydroxyisobutyrylation (Khib), a new type of histone PTM15. This histone mark poses unique features that differ from the widely
studied histone lysine acetylation (Kac) and methylation (Kme) marks. It has a unique chemical structure, specific genomic distributions and exhibits varied dynamics among diverse model
systems. ChIP-seq, gene expression analysis and immunodetection have indicated that in male germ cells H4K8hib is associated with regions of active gene transcription, in both meiotic and
post-meiotic cells. These lines of evidence suggest that Khib is mechanistically and functionally different from histone Kac and Kme15. Importantly, we have identified a unique regulatory
function of two cellular metabolites, 2-hydroxyisobutyrate and 2-hydroxyisobutyryl-CoA. Nevertheless, the key elements regulating this PTM pathway remain unknown, hindering functional
studies of this modification in diverse biological systems and disease settings. The history of PTM biology clearly shows that exploration of crucial regulatory proteins for a PTM pathway is
key for studying its biology. In this respect, Kac provides an excellent example. Kac was originally discovered in core histones in the 1960s, findings that led to diverse correlative
studies between histone Kac and chromatin structure and transcriptional activity16,17. The identification of histone acetyltransferases was a turning point for Kac biology18,19.
Demonstration of the involvement of Kac in the regulation of p53 function inspired the research community to investigate the regulatory roles of Kac in transcription factors20. The
identification of Kac substrates in cytosolic and mitochondrial proteins using proteomic approaches stimulated studies on the non-nuclear functions of this interesting PTM21,22,23,24.
Likewise, discovery of the building blocks for the Khib pathway will undoubtedly lay a concrete foundation for studying its diverse cellular functions. In this study, by screening a yeast
knock-out (YKO) library and through mutational studies, we found that the Esa1-containing _Saccharomyces cerevisiae_ piccolo NuA4 (picNuA4) histone acetyltransferase complex has Khib
transferase activity. Esa1p's human homolog Tip60, a well-known MYST family acetyltransferase member25, is also identified as a “writer” able to catalyze Khib in mammalian cells both
_in vitro_ and _in vivo_. In addition, we demonstrated that histone deacetylases2 (HDAC2) and HDAC3 in mammalian cells can both serve as “erasers” to remove Khib _in vitro_ and _in vivo_.
Moreover, we report the first global identification of Khib substrates in human cells. Our screen identified 6 548 unique Khib sites across 1 725 proteins in HeLa cells. Analysis of the
substrate proteins reveals that Khib is closely associated with the processes of transcription, translation, protein degradation and energy metabolism. Together, our study reveals key
building blocks of the Khib pathway, and therefore offers a rich source for studying the role of Khib in diverse cellular process and disease development. RESULTS ESA1P IN YEAST CATALYZES
THE KHIB MODIFICATION _IN VIVO_ AND _IN VITRO_ Identification of the regulatory enzymes of histone marks is instrumental in studying their functions. Previous studies suggested that p300, a
member of lysine acetyltransferases (KATs), can have enzymatic activity not only for lysine acetylation but also for lysine propionylation, butyrylation and crotonylation10,26. We therefore
hypothesized that some KATs may also have activity toward Khib. To test this hypothesis, we took advantage of the haploid YKO collection and performed western blot analysis in each mutant
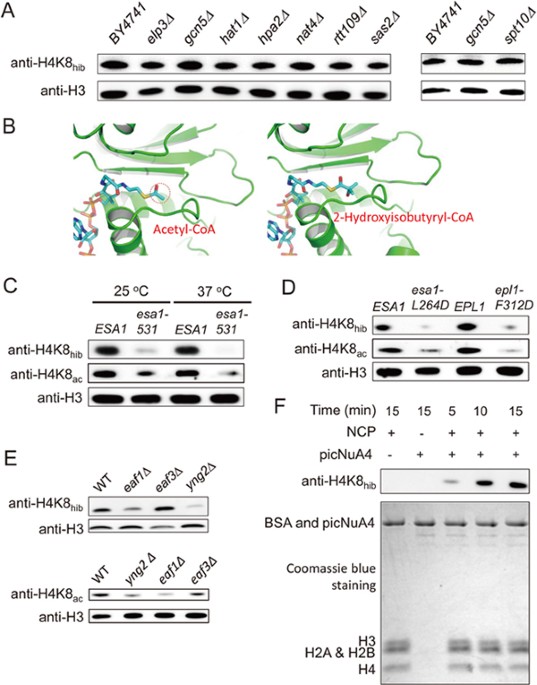
strain in which a non-essential KAT was deleted. We found, unfortunately, that none of these enzymes was required for maintaining the H4K8hib level (Figure 1A). In addition to these
non-essential KATs, the yeast genome encodes another KAT, Esa1p, the catalytic subunit of the nucleosome acetyltransferase of the H4 (NuA4) complex, which is required for cell viability27.
Esa1p associates with Yng2p, Eaf6p and Epl1p to form the core machinery termed picNuA4, which is joined by Eaf1p and other components to form the large complex28. We first tested if Esa1p
could bind 2-hydroxyisobutyryl-CoA using structural modeling. Recently, we solved the crystal structure of the NuA4 core complex, which revealed a space-sequence double recognition mechanism
for targeting the N-terminal tail of histone H429. When we examined the structure of the catalytic pocket of Esa1p bound to acetyl-CoA (PDB 5J9W), we found that the catalytic pocket of
Esa1p is large enough to accommodate other alkylated-CoAs with bigger acyl tails (Figure 1B). To test if 2-hydroxyisobutyryl-CoA would fit into the catalytic pocket of Esa1p, we modeled an
Esa1p/2-hydroxyisobutyryl-CoA structure based on the structure of acetyl-CoA-bound Esa1p (PDB 5J9W) with the terminal acetyl group replaced with a 2-hydroxyisobutyryl group (from PDB 4R3U).
As anticipated, the manner in which 2-hydroxyisobutyryl-CoA can bind to Esa1p is similar to that seen for the acetyl-CoA/Esa1p complex and allows a good fit of the 2-hydroxyisobutyryl group
into the catalytic pocket (Figure 1B). Therefore, it is possible that multiple acylations, including Khib, could be catalyzed by the same enzyme. We then carried out a series of experiments
to test whether Esa1p could catalyze H4K8hib acylation _in vivo_. First, we compared the levels of H4K8hib between an _ESA1_ temperature-sensitive mutant _esa1-531_ and its wild-type
counterpart. We found that H4K8hib persisted in the mutant at permissive temperature (25 °C), despite the reduced level of modification compared to the wild type. In contrast, at the
non-permissive temperature (37 °C), the modification was completely abolished (Figure 1C). Second, we constructed a strain carrying an amino-acid substitution (L264D) within the ER motif to
diminish the enzymatic activity of Esa1p29. As shown in Figure 1D, H4K8hib was dramatically decreased in this mutant. Therefore, a functional Esa1p is required for H4K8hib. Third, we asked
if Esa1p-mediated H4K8hib requires the intact picNuA4 complex, as known for acetylation. We then performed western blot analysis in two strains: one carrying a point mutation in Epl1p,
F312D, which disrupts its interaction with Esa1p; and the other with a deletion of _YNG2_. We found the amount of H4K8hib was drastically decreased in both strains (Figure 1D and 1E).
Finally, we tested if the NuA4 complex is also required for H4K8hib by knocking out _EAF1_, which maintains the picNuA4 complex but disrupts NuA4 complex30, or _EAF3_, another component of
the NuA4 complex. The _EAF1_ deletion strain showed moderate effect on H4K8hib whereas the _EAF3_ deletion strain did not (Figure 1E). Taken together, these results indicate that the picNuA4
complex is responsible for histone H4K8hib in _S. cerevisiae_. In order to demonstrate that Esa1p catalyzes H4K8hib directly, we performed an _in vitro_ assay using the purified picNuA4
complex and nucleosome core particles (NCPs)29. The 2-hydroxyisobutyryl-CoA was synthesized chemically (Supplementary information, Figure S1) and supplied as the donor. The reaction was
carried out at room temperature and the production of H4K8hib was visualized by western blot. We found that picNuA4 can efficiently catalyze 2-hydroxyisobutyrylation on H4K8 _in vitro_
(Figure 1F). Together, the results of these experiments carried out both _in vivo_ and _in vitro_ demonstrate that Esa1p is the “writer” of H4K8hib in yeast. Given the broad acetylation
substrate spectrum of Esa1p31, H4K8 may serve one of the histone Khib substrate sites for Esa1p. TIP60 HAS KHIB TRANSFERASE ACTIVITY _IN VITRO_ AND _IN VIVO_ Given the Khib transferase
activity of Esa1p in yeast, we next hypothesized that Tip60, the human homolog of Esa1p, might also be able to catalyze the Khib reaction. To test this hypothesis, we first carried out Khib
reactions _in vitro_ using recombinant histone H4 protein and 2-hydroxyisobutyryl-CoA as substrate and cofactor, respectively. Acetyl-CoA was used as a positive control for the reactions.
Western blot results showed that Tip60 could increase the global level of the histone H4Khib modification (Figure 2A), although it had a lower intrinsic preference toward the
2-hydroxyisobutyryl-CoA (Supplementary information, Figure S2). Mass spectrometry analysis identified H4K8, H4K12, H4K16 and H4K31 as Khib substrates of Tip60 (Supplementary information,
Figure S3), indicating that Tip60 is a potential mammalian Khib transferase _in vitro_. To confirm this result, a Tip60-specific inhibitor, TH183432, was used to determine whether it could
prevent H4 from undergoing 2-hydroxyisobutyrylation. Upon treatment of TH1834, the Khib level was significantly decreased in a similar fashion as Kac (Figure 2B), further suggesting that
Tip60 has Khib transferase activity _in vitro_. We next examined whether Tip60 regulates Khib _in vivo_. Knockdown of Tip60 by short interfering RNA reduced the global levels of Khib and Kac
on histones, while the Khib level on a specific residue, H4K8, was decreased slightly (Figure 2C). In support of this observation, we found that treatment of cells with the Tip60 inhibitor,
TH1834, clearly decreased H4K8hib signals (Figure 2D). By contrast, overexpression of full-length Tip60 by transient transfection increased both global histone Khib and H4K8hib levels and
enhanced the Kac positive control as expected (Figure 2E). Quantitative mass spectrometry showed that the levels of histone H4K8hib, H4K12hib and H4K16hib were increased 35%-46% in response
to overexpression of Tip60, indicating that these sites are Tip60-targeted Khib substrates (Supplementary information, Figure S4). Taken together, these results demonstrate that Tip60 can
regulate Khib both _in vitro_ and _in vivo_. HDAC2 AND HDAC3 ARE KHIB DEACYLASES _IN VITRO_ AND _IN VIVO_ HDACs, which are also called lysine deacetylases (KDACs), are a family of enzymes
that can remove acetyl groups from the amine at the epsilon position of lysine side chain. Given the fact that some HDACs, such as Sirt5 and Sirt6, can also catalyze removal of other forms
of lysine acylation33,34,35, we hypothesized that some HDAC member might also have enzymatic activity toward Khib. To test this possibility, we first carried out an _in vitro_ screen of the
11 class I, II and IV deacetylases (HDAC1-HDAC11) using core histones as the substrates (Figure 3A). This revealed that HDAC2 and HDAC3 have the highest activity for
de-2-hydroxyisobutyrylation, whereas HDAC1 only showed marginal activity _in vitro_. The _in vitro_ de-2-hydroxyisobutyrylation activity of HDAC3 could be further confirmed by the inhibition
of NaBu and TSA; two inhibitors for class I and II HDACs, but not NAM, an inhibitor for class III HDAC15. Together, these data suggested that HDAC2 and HDAC3 could remove Khib _in vitro_.
Next, we sought to determine whether HDACs1-3 could remove Khib _in vivo_. Consistent with the _in vitro_ assay, knockdown and overexpression of HDAC1 slightly affected the global level of
histone Khib (Figure 3B and 3C), while knockdown of HDAC3 or knockout of HDAC2 increased the global level of histone Khib more obviously (Figure 3D and 3E). Compared with HDAC2 knockout, the
double depletion of HDAC2 and HDAC3 led to a similar increase of the global histone Khib level. In contrast, overexpression of HDAC2 or HDAC3 reduced the global level of histone Khib
(Figure 3F). SILAC quantification of the histone Khib dynamics indicated that the levels of Khib decreased by 30% or more after HDAC3 overexpression (Supplementary information, Table S1).
Moreover, overexpression of both HDAC2 and HDAC3 enhanced such a decrease. Together, these data support the conclusion that HDAC2 and HDAC3 are Khib deacylases both _in vitro_ and _in vivo_.
PROTEOMICS SCREENING OF KHIB PEPTIDES IN HELA CELLS To globally identify Khib substrate proteins and their modification sites, we carried out proteomic screening in HeLa cells involving
peptide fractionation, affinity enrichment of Khib-peptides and HPLC-MS/MS analysis (Figure 4A). The specificity of the pan anti-Khib antibody used for immunoaffinity purification was
verified with a dot blot assay; the antibody could only detect the peptide library bearing a fixed 2-hydroxyisobutyrylated lysine but not the peptide library bearing a fixed unmodified
lysine, acetylation lysine, butyrylated lysine, crotonylated lysine or β-hydroxybutyrylated lysine (Supplementary information, Figure S5). The acquired raw MS data were analyzed by Maxquant
software with a false discovery rate of < 1% at protein, peptide and site levels. We further removed those hits with Andromeda scores lower than 40. The experiments were performed as
biological triplicates. Using this procedure, we identified 7 937 Khib sites on 1 901 proteins. To improve the reliability of the identified peptides, we eliminated 1 213 Khib sites with
localization probability scores < 0.75, and eliminated redundant sites. Using these criteria, we identified 6 548 unique Khib sites on 1 725 proteins in triplicate analysis (Supplementary
information, Data S1A). Of the 6 548 sites, 74% (4 867) were identified in at least two biological replicates (Figure 4B; Supplementary information, Data S1B), demonstrating the high
reproducibility of our procedure. These Khib sites were used as high-confidence data set in subsequent analysis. SEQUENCE PREFERENCE AND SUBCELLULAR LOCALIZATION OF THE KHIB PROTEOME The
identified Khib substrates have varied numbers of modification sites. We found that 617 proteins were modified at only one Khib site, while 80 proteins had more than 10 Khib sites, including
Plectin (PLEC) with 58 sites and Myosin-9 (MYH9) with 44 sites (Figure 4C; Supplementary information, Data S1B). To determine whether there are common sequence motifs in Khib peptides, we
aligned the amino-acid sequences surrounding Khib sites against all human background sequences. We found that the negatively-charged amino acids (aspartic acid and glutamic acid) were
enriched at both −1 and +1 positions, whereas the positively-charged amino acid lysine was enriched at −6, −5, +5 and +6 positions (Figure 5A). In addition, arginine and lysine residues were
under-represented at the −1 and +1 positions, respectively (Figure 5A). Interestingly, proline was largely depleted at most of the positions, making the sites distinct from the reported
flanking sequence preference studies of Kac, lysine malonylation (Kmal) and lysine succinylation (Ksucc) (Figure 5A)36,37,38,39. To explore the subcellular distribution of Khib substrates in
cells, we performed a cellular compartment analysis of the Khib proteome. Recently, some PTMs, such as Kmal and Ksucc, were reported being significantly enriched in mitochondria37,40. In
contrast, only ∼15% of Khib proteins were annotated in mitochondria, whereas 61% and 86% of Khib proteins localized exclusively or partially in nucleus and cytosol, respectively (Figure 5B).
This suggests that the Khib modification has a very different regulatory mechanism from Kmal and Ksucc. In contrast to Kmal and Ksucc, the subcellular distribution of Kac substrates is
similar to that of Khib substrates. The majority of Kac substrates often reside in either the cytoplasm or the nucleus, while mitochondria account for ∼5% or less of lysine-acetylated
proteins22. Given that cytoplasmic, mitochondrial and nuclear Kac can regulate various cellular processes, the Khib pathway's major functions are likely widespread in diverse
subcellular compartments. FUNCTIONAL ANNOTATION OF THE KHIB PROTEOME In order to explore the possible pathways affected by Khib, we performed a KEGG pathway enrichment analysis of the Khib
proteins1. Ribosome (adjusted _P_ = 5.65 × 10−35), spliceosome (adjusted _P_ = 6.60 × 10−25) and proteasome (adjusted _P_ = 4.94 × 10−13) pathways were most significantly enriched
(Supplementary information, Data S2A). Notably, 66%, 48% and 57% of proteins in these pathways were 2-hydroxyisobutyrylated, respectively (Supplementary information, Data S2A). In addition,
macromolecule transport-related pathways, such as RNA transport (adjusted _P_ = 2.65 × 10−10) and protein export (adjusted _P_ = 2.28 × 10−6) pathways, were also enriched (Supplementary
information, Data S2A). Moreover, our data showed that Khib proteins were enriched in energy metabolic networks, such as the citric cycle (adjusted _P_ = 2.28 × 10−11), fatty acid metabolism
(adjusted _P_ = 1.24 × 10−5) and pyruvate metabolism (adjusted _P_ = 2.08 × 10−5) (Supplementary information, Data S2A). Similar to the pathway analysis, unbiased gene ontology biological
process and UniProt keywords annotation of the Khib proteome showed that Khib proteins were associated with metabolic process and RNA processing (Supplementary information, Figure S6). We
also performed a protein complex enrichment analysis of the Khib proteome with a manually curated CORUM database41. In accord with the pathway analysis, the top enriched protein complexes
are associated with ribosome, spliceosome and proteasome (Supplementary information, Data S2B). In addition to these complexes, we identified significant enrichment of Khib in the CCT
micro-complex (adjusted _P_ = 6.09 × 10−10), the H2AX complex (adjusted _P_ = 2.29 × 10−8), the TNF-α/NF-κB signal transduction pathway (adjusted _P_ = 5.95 × 10−8) and the
DNA-PK-Ku-eIF2-NF90-NF45 complex (adjusted _P_ = 6.44 × 10−8) (Supplementary information, Data S2B). The TNF-α/NF-κB signal transduction pathway plays a pivotal role in various biological
processes, and dysregulation of this pathway is associated with many diseases42. Our data showed that 9 out of 14 subunits in this complex were 2-hydroxyisobutyrylated (Supplementary
information, Data S2B). The DNA-PK-Ku-eIF2-NF90-NF45 complex is involved in DNA double-strand break repair43. We found that 87.5% of proteins (7 out of 8) in this complex were
2-hydroxyisobutyrylated (Supplementary information, Data S2B). These results suggest that Khib can be involved in diverse cellular functions and networks that include protein synthesis and
degradation, cellular signaling and DNA repair. KHIB IN ENZYMES Of the 346 2-hydroxyisobutyrylated enzymes, 219 enzymes have multiple Khib sites. Of these, 21 enzymes were heavily modified
and had more than 10 Khib sites (Figure 5C; Supplementary information, Data S3). The DNA-dependent protein kinase catalytic subunit (PRKDC), the core component of the
DNA-PK-Ku-eIF2-NF90-NF45 complex, has 37 Khib sites (Figure 5C). Glycolysis is a catabolic process that converts glucose into pyruvate via 10 enzymatic steps44. Strikingly, 5 out of the 10
key enzymes required for glycolysis were heavily modified, including phosphoglycerate kinase 1 (PGK1), alpha-enolase (ENO1), pyruvate kinase isoform M2 (PKM2), glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) and fructose-bisphosphate aldolase A (ALDOA) (Figure 5C). ENO1 is a highly-conserved enzyme that coverts 2-phosphoglycerate to the high-energy intermediate
phosphoenolpyruvate. Remarkably, its activity center residue, K343, was 2-hydroxyisobutyrylated (Figure 5D). Previous experiments showed that mutations on this site (Lys to Ala or Met)
abolished enzyme activity45. In addition, the crystal structure of ENO1 shows that two magnesium ions are located in the active pocked within 4Å to the epsilon nitrogen atom of K343 (Figure
5D), and both magnesium ions are thought to participate in the catalytic reaction45. Therefore, the K343hib could not only inactivate the epsilon nitrogen atom, but is also likely to occupy
the space of magnesium ions, thus disrupting the interaction network of the active-site residues and hampering enzymatic activity. In addition to these enzyme active sites, we also found
that many substrates or cofactor binding sites were 2-hydroxyisobutyrylated (Figure 5E). For example, K186 of adenosylhomocysteinase; K147 of ALDOA; K171 of glucose-6-phosphate
1-dehydrogenase; and K147 of glutamate dehydrogenase 1 are known to be important for substrate binding (www.uniprot.com). The K745 residue of epidermal growth factor receptor (EGFR), K112 of
heat shock protein HSP 90-alpha A2, K168 of endoplasmin and K220 of PGK1 are critical residues for ATP binding (www.uniprot.com). Khib on these positions is most likely to affect the
protein functions by disrupting the binding interaction. DISCUSSION Emerging evidence indicates that diverse newly-discovered lysine acylations are associated with normal cellular physiology
and disease. Histone butyrylation, crotonylation and β-hydroxybutyrylation are associated with gene expression in diverse cellular systems10,46,47,48. These modifications can also have
specific “readers” to mediate their functions49,50,51,52. Lysine propionylation, butyrylation, malonylation, succinylation, glutarylation all contribute to multiple heritable genetic
diseases36,40,53,54,55. These lines of evidence suggest that lysine acylations are a family of PTMs that are physiologically relevant. Lysine acetylation not only occurs on core histones,
but also on diverse other proteins in nuclei, cytosol and mitochondria, many of which are metabolic enzymes. Previous studies have suggested that the lysine acetylation pathway has diverse
functions. Given the significant structural changes associated with Khib and also its widespread abundance, its close association with gene expression, it seems that the Khib pathway is also
highly likely to function similar to lysine acetylation and to have diverse regulatory roles. However, study of the Khib pathway is hindered by a lack of knowledge of its regulatory enzymes
and key substrates, the major focus of this work. Our findings on the Khib activities of Esa1p/TIP60, HDAC2 and HDAC3 broaden the enzymatic activities of the previously annotated HATs and
HDACs. TIP60 is a cellular lysine acetyltransferase that is involved in the regulation of gene expression and DNA damage response. It exerts cellular functions principally through
acetylation of histones and critical cellular proteins like p53, p21 and ATM kinase56. To our knowledge, TIP60 has not yet been found to have acyltransferase activity other than acetylation.
Thus, many of the annotated acetylation-regulatory enzymes have an expanded repertoire of acyl-transferase activities, and our result provides additional complexity of enzymatic activities
of these enzymes. Our study of the comprehensive global lysine 2-hydroxyisobutyrylome has provided a data set of 6 548 reliable Khib sites on 1 725 proteins in HeLa cells. This data set
represents the first Khib proteome in mammalian cell to date, and illustrates the broad landscape of the Khib pathway. Notably, 14 Khib sites were located at substrate or cofactor-binding
positions, suggesting Khib on these positions may negatively regulate protein functions. Second, this study provides a valuable resource for understanding the molecular mechanism whereby
Khib is associated with enzyme functions and human diseases. We detected 1 252 Khib sites on 346 enzymes in the HeLa Khib proteome (Supplementary information, Data S3) and many of these
enzymes are heavily 2-hydroxyisobutyrylated, suggesting important roles of Khib in diverse cellular processes and development of disease. Third, our further analysis of the Khib proteome has
shed additional light on the crosstalk between Khib and phosphorylation. We identified 115 Khib sites in the HeLa Khib proteome located within five residues of reported phosphorylation
sites (Supplementary information, Data S4) by mapping the Khib proteome against the phosphorylome in UniProt database. Given that phosphorylation can serve as a functional on/off switch,
these data suggest potential roles for Khib in tuning protein functions through interplay with nearby phosphorylation sites. Our study also sheds new light on the ways that microbial
metabolites might affect human health and disease. The human gut harbors trillions of microorganisms that produce multiple metabolites, which have been demonstrated to play important roles
in the development of diverse diseases8. For example, high levels of 2-hydroxyisobutyrate in the urine of obese people have been found associated with reduced bacterial diversity in “obese”
gut microbiota12. However, the underlying mechanisms of how the microbial metabolites affect human health and disease are poorly understood. Emerging evidence has demonstrated that
fluctuations in the availability of short-chain fatty acids could directly impinge on levels of their corresponding PTMs10,53,57. In this context, given the potentially important roles of
Khib in various cellular processes revealed in this study, it is highly likely that microbial metabolites, including 2-hydroxyisotutarate, play a regulatory role at the level of PTMs such as
Khib in a variety of host tissues and can ultimately affect host protein functions and the overall phenotype. Identification of key regulatory elements for the Khib pathway, including
enzymes and substrate proteins, will mark a key step forward toward description of this pathway. It is expected that histone Khib pathway will have unique binding modules in a similar
fashion to histone lysine acetylation and crotonylation49,50. It seems highly likely that the relative abundance of histone Kac and Khib in a given substrate protein such as a histone
protein is regulated by metabolic status and concentrations of acyl-CoAs in a similar fashion as histone lysine crotonylation10. We now require functional studies of these enzymes and
substrates, together with the discovery and characterization of their binding proteins (or “readers”), and investigations of the governing principles that acyl-CoA metabolism might have on
these proteins. Such future studies will greatly enhance our understanding of the Khib pathway and molecular regulation of cellular functions mediated by its cognate short-chain fatty acid.
MATERIALS AND METHODS PLASMIDS, SIRNA, ANTIBODIES AND CELL LINES Tip60 was cloned into pcDNA3.0 with a Flag tag. The construction was verified by DNA sequencing. siRNA was purchased from
Dharmacon (L-006301-00-0005). The antibodies used here were anti-Pan Kac (PTM Biolabs, PTM-101), anti-Pan Khib (PTM Biolabs, PTM-801), anti-H4K8ac (PTM Biolabs, PTM-120), anti-H4K8hib (PTM
Biolabs, PTM-805), anti-H4 (Abcam, ab31830), anti-Flag (Sigma-Aldrich, F7425), anti-Tubulin (Abcam, ab6160) and anti-Tip60 (Abcam, 23886). Cell lines were purchased from ATCC (www.atcc.org)
and used without further authentication, including HEK293 (ATCC CRL-1573), HEK293T (ATCC CRL-3216), HeLa (ATCC CCL-2) and U2OS (ATCC HTB-96). No mycoplasma contamination was detected using a
MycoAlert Mycoplasma Detection Kit (Lonza, LT07-118). STABLE ISOTOPE LABELING OF CELLS AND TRANSFECTIONS HEK293T cells were grown in lysine-free DMEM supplemented with 10% dialyzed FBS, and
either light (12C614N2-L-Lysine) or heavy (13C614N2-L-Lysine) lysine (100 mg/L). Cells were grown for more than seven generations to achieve more than 98% labeling efficiency. For the
transfections, HeLa and HEK293 cells were cultured in DMEM medium supplemented with 10% FBS, 50 U/mL penicillin and 50 mg/mL streptomycin in a 5% CO2 atmosphere at 37 °C. Transfection to
achieve transient overexpression was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. siRNA transfection was performed with Lipofectamine
RNAiMAX (Invitrogen) according to manufacturer's instructions. For HDAC1, HDAC2 and HDAC3 overexpression, five pools of HEK293T cells were transfected with an empty vector PCDNA3.1, a
vector encoding PCDNA3.1-HA-HDAC1, a vector encoding PCDNA3.1-HA-HDAC2, a vector encoding PCDNA3.1-HA-HDAC3 and mixed vectors encoding PCDNA3.1-HA-HDAC2 and PCDNA3.1-HA-HDAC3 for 48 h.
DEPLETION OF HDAC1, HDAC2 AND HDAC3 Two pools of HEK293T cells were transfected twice with HDAC1 siRNA (Genepharma, siHDAC1: (#1) 5′-GCUCCUCUGACAAACGAAUTT-3′; (#2)
5′-CCGGUCAUGUCCAAAGUAATT-3′; (#3) 5′-GCUGUACAUUGACAUUGAUTT-3′) and a negative control siRNA (Genepharma, 5′-UUCUCCGAACGUGUCACGUTT-3′), respectively. About 48 h after the second transfection,
the cell lysates were probed with anti-Kac and anti-Khib antibodies. HEK293T cells were transfected with an empty vector PX458 and an HDAC2 knockout vector PX458-sgRNA (HDAC2),
respectively, to get two stable cell lines. Depletion of HDAC2 and HDAC3 was further achieved by using the HDAC2 knockout HEK293T cell line transfected twice with a siRNA mix against HDAC3
(Genepharma, siHDAC3: (#1) 5′-CCGCCAGACAAUCUUUGAATT-3′; (#2) 5′-GAGCUUCCCUAUAGUGAAUTT-3′; (#3) 5′-GGGAAUGCGUUGAAUAUGUTT-3′). The HDAC2 knockout HEK293T cells transfected twice with siRNA
(Genepharma, 5′-UUCUCCGAACGUGUCACGUTT-3′) were used as negative control. About 48 h after the last transfection, the Khib and Kac levels were assessed by western blot using extractions of
whole-cell lysates. PREPARATION OF CELL LYSATE Cells were sonicated for 3 min on ice using a high-intensity ultrasonic processor (Scientz) in lysis buffer (8 M urea, 2 mM EDTA, 3 μM TSA, 50
mM NAM, 5 mM DTT and 1% Protease Inhibitor Cocktail III). The remaining debris was removed by centrifugation at 18 000× _g_ at 4 oC for 3 min. The protein concentration was determined using
a 2-D Quant kit according to the manufacturer's instructions. TRYPSIN DIGESTION OF CELL LYSATE Proteins in the cell lysate were reduced with 10 mM DTT for 1 h at 37 oC, alkylated with
20 mM iodoacetamide for 45 min at room temperature in darkness, and the excess iodoacetamide was blocked by 20 mM cysteine. Then the protein sample was diluted by adding 100 mM NH4HCO3 to
reduce the urea concentration to < 2 M. Trypsin was added at 1:50 trypsin-to-protein mass ratio for the first digestion overnight and 1:100 trypsin-to-protein mass ratio for a second 4
h-digestion. Finally, 18 mg of proteins from the HeLa cells lysate was digested for subsequent experiments. PEPTIDE FRACTIONATION WITH HIGH-PH REVERSE-PHASE CHROMATOGRAPHY The peptides from
HeLa cells lysate were then divided into three equal parts, and each of them (6 mg) was fractionated into six fractions by high pH reverse-phase HPLC using an Agilent 300 Extend C18 column
(5 μm particles, 4.6 mm ID, 250 mm length). Briefly, peptides were first separated using a gradient of 2%-60% acetonitrile in 10 mM ammonium bicarbonate at pH 10 over 90 min into 90
fractions. The peptides were then combined into six fractions and dried by vacuum centrifugation. IMMUNOAFFINITY ENRICHMENT To enrich Khib peptides, total peptides dissolved in NETN buffer
(100 mM NaCl, 1 mM EDTA, 50 mM Tris-HCl, 0.5% NP-40, pH 8.0) were incubated with pre-washed pan anti-Khib beads (PTM Biolabs Inc., Chicago, IL) at 4 oC overnight with gentle shaking. The
beads were washed four times with NETN buffer and twice with ddH2O. The bound peptides were eluted from the beads with 0.1% trifluoroacetic acid, and the eluted fractions were combined and
vacuum-dried. MASS SPECTROMETRY Samples were dissolved in 0.1% formic acid and loaded onto a reversed-phase pre-column (Acclaim PepMap 100, Thermo Scientific). Peptide separation was
performed using a reversed-phase analytical column (Acclaim PepMap RSLC, Thermo Scientific) with a gradient of 5-80% HPLC buffer B (0.1% formic acid in 90% acetonitrile, v/v) in buffer A
(0.1% formic acid in water, v/v) at a flow rate of 300 nl/min over 60 min on an EASY-nLC 1000 UPLC system. The samples were analyzed by Q Exactive Plus Mass Spectrometers (ThermoFisher
Scientific). A data-dependent procedure that alternated between one full mass scan followed by the top 20 most intense precursor ions was applied with 30 s dynamic exclusion. Intact peptides
were detected with a resolution of 70 000 at 200 _m/z_, and ion fragments were detected with a resolution of 17 500 at 28% normalized collision energy. DATABASE SEARCH AND DATA FILTER
CRITERIA The resulting MS/MS data were processed using MaxQuant with integrated Andromeda search engine (v.1.3.0.5)58. Tandem mass spectra were searched against the UniProt Human protein
database (88 277 entries, http://www.uniprot.org) concatenated with reverse decoy database. Trypsin/P was specified as cleavage enzyme allowing up to two missing cleavages and five
modifications per peptide. Carbamidomethylation on cysteine was specified as fixed modification. Oxidation on methionine, 2-hydroxyisobutyrylation on lysine, acetylation on lysine and
acetylation on protein N-terminus were specified as variable modifications. False discovery rate thresholds for protein, peptide and modification site were specified at 1%. Minimum peptide
length was set at 7. All the other parameters in MaxQuant were set to default values. Khib identified on peptides from reverse or contaminant protein sequences, peptides with an Andromeda
score below 40, site localization probability below 0.75 and redundant Khib sites were removed. In addition, Khib sites on the peptide C-terminus were eliminated, unless the peptide
C-terminus was also the corresponding protein C-terminus. For the SILAC sample data, Khib site ratios were normalized by the quantified protein expression levels. _IN VITRO_ ACETYLATION AND
2-HYDROXYISOBUTYRYLATION ASSAY Recombinant human Tip60 and Histone H4 proteins were purchased from Cayman (10783) and NEB (M2504S), respectively. For each reaction, 250 ng of Tip60 protein,
2.5 μg of H4 protein and 10 μM of CoA were added in reaction buffer (50 mM Tris-CI, pH 8.0, 10% glycerol, 10 mM butyric acid, 0.1 mM EDTA, 1 mM DTT and 1 mM PMSF). The reaction mixtures were
incubated at 30 oC for 1 h, followed by addition of SDS sample buffer. The levels of acetylation and 2-hydroxyisobutyrylation were assessed by western blot. PICNUA4 _IN VITRO_ ASSAY The NCP
with 147 bp 601 “wisdom” sequence was prepared using luger's protocol. PicNuA4 complex was prepared using an unpublished method. The reaction system was 1 μM NCP, 0.01 μM picNuA4, 50
μM 2-hib-CoA, 50 mM NaCl, 10 mM HEPES (pH 7.0), 0.1 mg/mL BSA. The assay was performed at room temperature, utilizing picNuA4 to start the reactions with a time-based gradient. SDS-loading
buffer was used to stop the reactions with incubation at 100 °C for 1 min. The signal was detected by western blot. PROTEIN FUNCTION ANNOTATION ANALYSIS Enrichment analysis for KEGG pathway
and PFAM domain was performed using a hypergeometric test in GOstats package in R59. Protein complexes were enriched basing on that manually curated CORUM protein complex database41 for all
mammals using a hypergeometric test. Unbiased gene ontology terms and UniProt keywords enrichment analyses were performed using a web tool (https://agotool.sund.ku.dk)60. PROTEIN-PROTEIN
INTERACTION NETWORK ANALYSIS The STRING database (v10, http://www.string-db.org/) was used for analyzing the protein-protein interaction network of the Tip60-regulated Khib proteome.
Interactions with the highest confidence score (above 0.9) were selected and the network was visualized in Cytoscape (v.3.2.1). CORRELATION BETWEEN KHIB AND MUTATION/PHOSPHORYLATION In-house
developed scripts were applied to map Khib to known mutations and phosphorylation sites. Disease-related mutations were extracted from UniProt (http://www.uniprot.org) and COSMIC (Catalogue
of Somatic Mutations in Cancer, http://cancer.sanger.ac.uk/cosmic) databases. Protein dysfunction-related mutations, substrate/cofactor-binding sites and reported phosphorylation sites were
extracted from UniProt database. CHEMICAL SYNTHESIS OF 2-HYDROXYISOBUTYRYL-COA The method used to synthesize 2-hydroxyisobutyryl-CoA was modified from a previous study61. About 0.88 g (10
mmol) 2-hydroxyisobutyric acid and 1 mL thiophenol were dissolved in 50 mL pre-cooled dimethylformamide (DMF), to which 2.52 g (12.2 mmol) dicyclohexylcarbodiimide in 50 mL DMF was added
drop by drop and the mixture was stirred for 3 h on ice bath. About 40 mL of cold water was then added and the solution was filtered. The filtrate was extracted with 100 mL of ether and the
organic layer was washed three times with saturated NaCl. The ether extract was dried using anhydrous sodium sulfate and ether evaporated. The residue was then purified by silica gel column
chromatography with an elution solvent consisting of ethylacetate:hexane (20:1). The roughly purified product was further purified by silica thin-layer chromatography with an elution solvent
consisting of ethylacetate:hexane (1:4). The target band was collected and the purified S-phenyl 2-hydroxy-2-methylpropanethioate was used as the reactant for the next step. 16 mg of
S-phenyl 2-hydroxy-2-methylpropanethioate was dissolved in 500 μL 0.1 M NaHCO3 (pH 8.0) and 200 μL dioxane mixture was added to a solution of 10 mg sodium salt of CoA dissolved in 1 mL
NaHCO3 (pH 8.0) at 0 °C. The mixture was reacted overnight, and then was neutralized by adding 1 N HCl to pH 7.0 to stop the reaction. About 8 mL ether was used to extract the unreacted
reactant and this step was repeated five times. Then 8 mL of ethylacetate was used to extract the water phase and this step was repeated eight times. The final product remained in the water
phase. Water was allowed to evaporate at 30 °C in order to achieve the solid end product. DATA AVAILABILITY The mass spectrometry proteomics data have been deposited in the ProteomeXchange
Consortium via the PRIDE62 partner repository with the data set identifier PXD005414. AUTHOR CONTRIBUTIONS YZ and JD designed and coordinated the whole project. HH performed the proteomics
experiments, data analysis and bioinformatics analysis, and helped coordinate the project. SQ performed the immunoprecipitation and Tip60-related experiments. JH and ZL were involved in
yeast acyltransferases assay and verification experiments. XW, LG, WZ, LD and WG were involved in the HDAC assay and verification experiments. PX and ZC performed the modeling experiments.
FL and JW synthesized 2-hydroxyisobutyryl-CoA. HH, YZ and JD wrote the manuscript. All authors discussed the results and commented on the manuscript. COMPETING FINANCIAL INTERESTS YZ is on
the science advisory board of PTM Biolabs. ACCESSION CODES ACCESSIONS PROTEIN DATA BANK * 2PSN * 4R3U * 5J9W REFERENCES * Kanehisa M, Goto S . KEGG: kyoto encyclopedia of genes and genomes.
_Nucleic Acids Res_ 2000; 28:27–30. Article CAS Google Scholar * Fierz B, Muir TW . Chromatin as an expansive canvas for chemical biology. _Nat Chem Biol_ 2012; 8:417–427. Article CAS
Google Scholar * Walsh CT, Garneau-Tsodikova S . Gatto GJ Jr . Protein posttranslational modifications: the chemistry of proteome diversifications. _Angew Chem Int Ed Engl_ 2005;
44:7342–7372. Article CAS Google Scholar * DeBerardinis RJ, Thompson CB . Cellular metabolism and disease: what do metabolic outliers teach us? _Cell_ 2012; 148:1132–1144. Article CAS
Google Scholar * Moellering RE, Cravatt BF . Functional lysine modification by an intrinsically reactive primary glycolytic metabolite. _Science_ 2013; 341:549–553. Article CAS Google
Scholar * Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F . From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. _Cell_ 2016; 165:1332–1345.
Article CAS Google Scholar * Russell WR, Hoyles L, Flint HJ, Dumas ME . Colonic bacterial metabolites and human health. _Curr Opin Microbiol_ 2013; 16:246–254. Article CAS Google
Scholar * Lee WJ, Hase K . Gut microbiota-generated metabolites in animal health and disease. _Nat Chem Biol_ 2014; 10:416–424. Article CAS Google Scholar * den Besten G, van Eunen K,
Groen AK, Venema K, Reijngoud DJ, Bakker BM . The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. _J Lipid Res_ 2013; 54:2325–2340.
Article CAS Google Scholar * Sabari BR, Tang Z, Huang H, _et al_. Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. _Mol Cell_ 2015;
58:203–215. Article CAS Google Scholar * Sabari BR, Zhang D, Allis CD, Zhao YM . Metabolic regulation of gene expression through histone acylations. _Nat Rev Mol Cell Biol_ 2017;
18:90–101. Article CAS Google Scholar * Calvani R, Miccheli A, Capuani G, _et al_. Gut microbiome-derived metabolites characterize a peculiar obese urinary metabotype. _Int J Obes_ 2010;
34:1095–1098. Article CAS Google Scholar * Li M, Wang B, Zhang M, _et al_. Symbiotic gut microbes modulate human metabolic phenotypes. _Proc Natl Acad Sci USA_ 2008; 105:2117–2122.
Article CAS Google Scholar * Koppel N, Balskus EP . Exploring and understanding the biochemical diversity of the human microbiota. _Cell Chem Biol_ 2016; 23:18–30. Article CAS Google
Scholar * Dai L, Peng C, Montellier E, _et al_. Lysine 2-hydroxyisobutyrylation is a widely distributed active histone mark. _Nat Chem Biol_ 2014; 10:365–370. Article CAS Google Scholar
* Allfrey VG, Faulkner R, Mirsky AE . Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. _Proc Natl Acad Sci USA_ 1964; 51:786–794. Article
CAS Google Scholar * Allis CD, Glover CV, Bowen JK, Gorovsky MA . Histone variants specific to the transcriptionally active, amitotically dividing macronucleus of the unicellular
eucaryote, Tetrahymena thermophila. _Cell_ 1980; 20:609–617. Article CAS Google Scholar * Brownell JE, Zhou J, Ranalli T, _et al_. Tetrahymena histone acetyltransferase A: a homolog to
yeast Gcn5p linking histone acetylation to gene activation. _Cell_ 1996; 84:843–851. Article CAS Google Scholar * Brownell JE, Allis CD . An activity gel assay detects a single,
catalytically active histone acetyltransferase subunit in Tetrahymena macronuclei. _Proc Natl Acad Sci USA_ 1995; 92:6364–6368. Article CAS Google Scholar * Gu W, Shi XL, Roeder RG .
Synergistic activation of transcription by CBP and p53. _Nature_ 1997; 387:819–823. Article CAS Google Scholar * Kim SC, Sprung R, Chen Y, _et al_. Substrate and functional diversity of
lysine acetylation revealed by a proteomics survey. _Mol Cell_ 2006; 23:607–618. Article CAS Google Scholar * Choudhary C, Kumar C, Gnad F, _et al_. Lysine acetylation targets protein
complexes and co-regulates major cellular functions. _Science_ 2009; 325:834–840. Article CAS Google Scholar * Zhao S, Xu W, Jiang W, _et al_. Regulation of cellular metabolism by protein
lysine acetylation. _Science_ 2010; 327:1000–1004. Article CAS Google Scholar * Chen Y, Zhao W, Yang JS, _et al_. Quantitative acetylome analysis reveals the roles of SIRT1 in regulating
diverse substrates and cellular pathways. _Mol Cell Proteomics_ 2012; 11:1048–1062. Article CAS Google Scholar * Sapountzi V, Logan IR, Robson CN . Cellular functions of TIP60. _Int J
Biochem Cell Biol_ 2006; 38:1496–1509. Article CAS Google Scholar * Chen Y, Sprung R, Tang Y, _et al_. Lysine propionylation and butyrylation are novel post-translational modifications in
histones. _Mol Cell Proteomics_ 2007; 6:812–819. Article CAS Google Scholar * Allard S, Utley RT, Savard J, _et al_. NuA4, an essential transcription adaptor/histone H4 acetyltransferase
complex containing Esa1p and the ATM-related cofactor Tra1p. _EMBO J_ 1999; 18:5108–5119. Article CAS Google Scholar * Boudreault AA, Cronier D, Selleck W, _et al_. Yeast enhancer of
polycomb defines global Esa1-dependent acetylation of chromatin. _Genes Dev_ 2003; 17:1415–1428. Article CAS Google Scholar * Xu P, Li CM, Chen ZH, _et al_. The NuA4 core complex
acetylates nucleosomal histone H4 through a double recognition mechanism. _Mol Cell_ 2016; 63:965–975. Article CAS Google Scholar * Mitchell L, Lambert JP, Gerdes M, _et al_. Functional
dissection of the NuA4 histone acetyltransferase reveals its role as a genetic hub and that Eaf1 is essential for complex integrity. _Mol Cell Biol_ 2008; 28:2244–2256. Article CAS Google
Scholar * Smith ER, Eisen A, Gu WG, _et al_. ESA1 is a histone acetyltransferase that is essential for growth in yeast. _Proc Natl Acad Sci USA_ 1998; 95:3561–3565. Article CAS Google
Scholar * Gao C, Bourke E, Scobie M, _et al_. Rational design and validation of a Tip60 histone acetyltransferase inhibitor. _Sci Rep_ 2014; 4:5372. Article CAS Google Scholar * Peng C,
Lu Z, Xie Z, _et al_. The first identification of lysine malonylation substrates and its regulatory enzyme. _Mol Cell Proteomics_ 2011; 10:M111.012658. Article Google Scholar * Du J, Zhou
Y, Su X, _et al_. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. _Science_ 2011; 334:806–809. Article CAS Google Scholar * Jiang H, Khan S, Wang Y, _et al_. SIRT6
regulates TNF-alpha secretion through hydrolysis of long-chain fatty acyl lysine. _Nature_ 2013; 496:110–113. Article CAS Google Scholar * Park J, Chen Y, Tishkoff DX, _et al_.
SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. _Mol Cell_ 2013; 50:919–930. Article CAS Google Scholar * Rardin MJ, He W, Nishida Y, _et al_. SIRT5 regulates
the mitochondrial lysine succinylome and metabolic networks. _Cell Metab_ 2013; 18:920–933. Article CAS Google Scholar * Nishida Y, Rardin MJ, Carrico C, _et al_. SIRT5 regulates both
cytosolic and mitochondrial protein malonylation with glycolysis as a major target. _Mol Cell_ 2015; 59:321–332. Article CAS Google Scholar * Svinkina T, Gu HB, Silva JC, _et al_. Deep,
quantitative coverage of the lysine acetylome using novel anti-acetyl-lysine antibodies and an optimized proteomic workflow. _Mol Cell Proteomics_ 2015; 14:2429–2440. Article CAS Google
Scholar * Colak G, Pougovkina O, Dai L, _et al_. Proteomic and biochemical studies of lysine malonylation suggest its malonic aciduria-associated regulatory role in mitochondrial function
and fatty acid oxidation. _Mol Cell Proteomics_ 2015; 14:3056–3071. Article CAS Google Scholar * Ruepp A, Waegele B, Lechner M, _et al_. CORUM: the comprehensive resource of mammalian
protein complexes--2009. _Nucleic Acids Res_ 2010; 38:D497–501. Article CAS Google Scholar * Bouwmeester T, Bauch A, Ruffner H, _et al_. A physical and functional map of the human
TNF-alpha/NF-kappa B signal transduction pathway. _Nat Cell Biol_ 2004; 6:97–105. Article CAS Google Scholar * Ting NS, Kao PN, Chan DW, Lintott LG, Lees-Miller SP . DNA-dependent protein
kinase interacts with antigen receptor response element binding proteins NF90 and NF45. _J Biol Chem_ 1998; 273:2136–2145. Article CAS Google Scholar * Lunt SY, Vander Heiden MG .
Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. _Annu Rev Cell Dev Biol_ 2011; 27:441–464. Article CAS Google Scholar * Pancholi V . Multifunctional
alpha-enolase: its role in diseases. _Cell Mol Life Sci_ 2001; 58:902–920. Article CAS Google Scholar * Goudarzi A, Zhang D, Huang H, _et al_. Dynamic competing histone H4 K5K8
acetylation and butyrylation are hallmarks of highly active gene promoters. _Mol Cell_ 2016; 62:169–180. Article CAS Google Scholar * Xie Z, Zhang D, Chung D, _et al_. Metabolic
regulation of gene expression by histone lysine beta-hydroxybutyrylation. _Mol Cell_ 2016; 62:194–206. Article CAS Google Scholar * Tan M, Luo H, Lee S, _et al_. Identification of 67
histone marks and histone lysine crotonylation as a new type of histone modification. _Cell_ 2011; 146:1016–1028. Article CAS Google Scholar * Li YY, Sabari BR, Panchenko T, _et al_.
Molecular coupling of histone crotonylation and active transcription by AF9 YEATS domain. _Mol Cell_ 2016; 62:181–193. Article CAS Google Scholar * Zhao D, Guan HP, Zhao S, _et al_.
YEATS2 is a selective histone crotonylation reader. _Cell Res_ 2016; 26:629–632. Article CAS Google Scholar * Andrews FH, Shinsky SA, Shanle EK, _et al_. The Taf14 YEATS domain is a
reader of histone crotonylation. _Nat Chem Biol_ 2016; 12:396–398. Article CAS Google Scholar * Flynn EM, Huang OW, Poy F, _et al_. A subset of human bromodomains recognizes butyryllysine
and crotonyllysine histone peptide modifications. _Structure_ 2015; 23:1801–1814. Article CAS Google Scholar * Hirschey MD, Zhao YM . Metabolic regulation by lysine malonylation,
succinylation, and glutarylation. _Mol Cell Proteomics_ 2015; 14:2308–2315. Article CAS Google Scholar * Tan M, Peng C, Anderson KA, _et al_. Lysine glutarylation is a protein
posttranslational modification regulated by SIRT5. _Cell Metab_ 2014; 19:605–617. Article CAS Google Scholar * Pougovkina O, Te Brinke H, Wanders RJ, Houten SM, de Boer VC . Aberrant
protein acylation is a common observation in inborn errors of acyl-CoA metabolism. _J Inherit Metab Dis_ 2014; 37:709–714. Article CAS Google Scholar * Squatrito M, Gorrini C, Amati B .
Tip60 in DNA damage response and growth control: many tricks in one HAT. _Trends Cell Biol_ 2006; 16:433–442. Article CAS Google Scholar * Sabari BR, Zhang D, Allis CD, Zhao Y . Metabolic
regulation of gene express through histone acylations. _Nat Rev Mol Cell Biol_ 2017; 18:90–101. Article CAS Google Scholar * Cox J, Mann M . MaxQuant enables high peptide identification
rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. _Nat Biotechnol_ 2008; 26:1367–1372. Article CAS Google Scholar * Falcon S, Gentleman R .
Using GOstats to test gene lists for GO term association. _Bioinformatics_ 2007; 23:257–258. Article CAS Google Scholar * Scholz C, Lyon D, Refsgaard JC, Jensen LJ, Choudhary C, Weinert
BT . Avoiding abundance bias in the functional annotation of post-translationally modified proteins. _Nat Methods_ 2015; 12:1003–1004. Article CAS Google Scholar * Padmakumar R, Gantla S,
Banerjee R . A rapid method for the synthesis of methylmalonyl-coenzyme A and other CoA-esters. _Anal Biochem_ 1993; 214:318–320. Article CAS Google Scholar * Vizcaino JA, Csordas A,
del-Toro N, _et al_. 2016 update of the PRIDE database and its related tools. _Nucleic Acids Res_ 2016; 44:D447–D456. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS Our
work was supported by the National Institutes of Health (NIH) award GM105933, DK107868 and GM115961 (YZ). This work was also supported by the National Key Research and Development Program of
China (2017YFA0505103 to JD), Research Fund for the Doctoral Program of Higher Education of China (20120002110022 to JD) and the National Natural Science Foundation of China (81570060 to
LD). AUTHOR INFORMATION Author notes * He Huang, Zhouqing Luo, Shankang Qi and Jing Huang: These four authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Ben May Department
for Cancer Research, The University of Chicago, Chicago, 60637, IL, USA He Huang, Shankang Qi & Yingming Zhao * Center for Synthetic Biology Engineering Research, Shenzhen Institutes of
Advanced Technology, Chinese Academy of Sciences, Shenzhen, 518055, Guangdong, China Zhouqing Luo & Junbiao Dai * MOE Key Laboratory of Bioinformatics and Center for Synthetic and
Systems Biology, School of Life Sciences, Tsinghua University, Beijing, 100084, China Zhouqing Luo, Jing Huang, Peng Xu, Zhucheng Chen & Junbiao Dai * Department of General Practice and
Lab of PTM, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Collaborative Innovation Center of Biotherapy, Chengdu, Sichuan, 610041, China Xiuxuan Wang, Li Gao
& Lunzhi Dai * School of Pharmaceutical Sciences, Tsinghua University, Beijing, 100084, China Fangyi Li & Jian Wang * Department of Biochemistry and Molecular Biology, Health Science
Center and Beijing Key Laboratory of Protein Posttranslational Modifications and Cell Function, Peking University, Beijing, 100191, China Wenhui Zhao * Department of Pathology and Cell
Biology, Institute for Cancer Genetics, Herbert Irving Comprehensive Cancer Center, College of Physicians and Surgeons, Columbia University, 1130 Nicholas Avenue, New York, 10032, NY, USA
Wei Gu Authors * He Huang View author publications You can also search for this author inPubMed Google Scholar * Zhouqing Luo View author publications You can also search for this author
inPubMed Google Scholar * Shankang Qi View author publications You can also search for this author inPubMed Google Scholar * Jing Huang View author publications You can also search for this
author inPubMed Google Scholar * Peng Xu View author publications You can also search for this author inPubMed Google Scholar * Xiuxuan Wang View author publications You can also search for
this author inPubMed Google Scholar * Li Gao View author publications You can also search for this author inPubMed Google Scholar * Fangyi Li View author publications You can also search for
this author inPubMed Google Scholar * Jian Wang View author publications You can also search for this author inPubMed Google Scholar * Wenhui Zhao View author publications You can also
search for this author inPubMed Google Scholar * Wei Gu View author publications You can also search for this author inPubMed Google Scholar * Zhucheng Chen View author publications You can
also search for this author inPubMed Google Scholar * Lunzhi Dai View author publications You can also search for this author inPubMed Google Scholar * Junbiao Dai View author publications
You can also search for this author inPubMed Google Scholar * Yingming Zhao View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS
Correspondence to Lunzhi Dai, Junbiao Dai or Yingming Zhao. ADDITIONAL INFORMATION ( SUPPLEMENTARY INFORMATION is linked to the online version of the paper on the _Cell Research_ website.)
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION, FIGURE S1 Synthesis of 2-hydroxyisobutyryl-CoA. (PDF 502 kb) SUPPLEMENTARY INFORMATION, FIGURE S2 Competition assay of Tip60's
acyltransferase activity towards Acetyl-CoA and 2-Hydroxyisobutyryl-CoA. (PDF 242 kb) SUPPLEMENTARY INFORMATION, FIGURE S3 Spectra of the H4Khib peptides identified in the _in vitro_ assay.
(PDF 599 kb) SUPPLEMENTARY INFORMATION, FIGURE S4 The ratios of quantifiable histone H4 N-terminal Khib sites between Tip60 overexpressed (OE) 293T cells and wildtype (WT) 293T cells. (PDF
225 kb) SUPPLEMENTARY INFORMATION, FIGURE S5 Specificity of the pan anti-Khib antibody. (PDF 273 kb) SUPPLEMENTARY INFORMATION, FIGURE S6 Gene Ontology (GO) terms and UniProt keywords
enrichment analysis on the HeLa Khib proteome. (PDF 193 kb) SUPPLEMENTARY INFORMATION, TABLE S1 The ratios of quantifiable histone Khib sites between HDAC3 overexpressed 293T cells and
control 293T cells. (PDF 170 kb) SUPPLEMENTARY INFORMATION, DATA S1 List of Khib Sites Identified in HeLa cells, Related to Figure 1. (XLSX 2051 kb) SUPPLEMENTARY INFORMATION, DATA S2 KEGG
Pathway and Protein Complex Analysis of the Khib Proteins Identified in HeLa cells. (XLSX 27 kb) SUPPLEMENTARY INFORMATION, DATA S3 Khib Enzymes Identified in HeLa cells, Related to Figure
2. (XLSX 89 kb) SUPPLEMENTARY INFORMATION, DATA S4 Potential Crosstalk between Reported Phosphorylation Sites and Khib Sites. (XLSX 20 kb) RIGHTS AND PERMISSIONS Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Huang, H., Luo, Z., Qi, S. _et al._ Landscape of the regulatory elements for lysine 2-hydroxyisobutyrylation pathway. _Cell Res_ 28, 111–125 (2018).
https://doi.org/10.1038/cr.2017.149 Download citation * Received: 18 May 2017 * Revised: 02 August 2017 * Accepted: 16 August 2017 * Published: 01 December 2017 * Issue Date: January 2018 *
DOI: https://doi.org/10.1038/cr.2017.149 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * post-translational modification * lysine
2-hydroxyisobutyrylation * regulatory element * acyltransferase * deacylase * substrate
