Candidate gene studies of a promising intermediate phenotype: failure to replicate
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Many candidate gene studies use ‘intermediate phenotypes’ instead of disease diagnoses. It has been proposed that intermediate phenotypes have simpler genetic architectures such
that individual alleles account for a larger percentage of trait variance. This implies that smaller samples can be used to identify genetic associations. Pharmacogenomic drug challenge
studies may be an especially promising class of intermediate phenotype. We previously conducted a series of 12 candidate gene analyses of acute subjective and physiological responses to
amphetamine in 99–162 healthy human volunteers (_ADORA2A_, _SLC6A3_, _BDNF_, _SLC6A4_, _CSNK1E_, _SLC6A2_, _DRD2_, _FAAH_, _COMT_, _OPRM1_). Here, we report our attempt to replicate these
findings in over 200 additional participants ascertained using identical methodology. We were unable to replicate any of our previous findings. These results raise critical issues related to
non-replication of candidate gene studies, such as power, sample size, multiple testing within and between studies, publication bias and the expectation that true allelic effect sizes are
similar to those reported in genome-wide association studies. Many of these factors may have contributed to our failure to replicate our previous findings. Our results should instill caution
in those considering similarly designed studies. SIMILAR CONTENT BEING VIEWED BY OTHERS MODEL-BASED ASSESSMENT OF REPLICABILITY FOR GENOME-WIDE ASSOCIATION META-ANALYSIS Article Open access
30 March 2021 ALCOHOL USE DISORDER AND BODY MASS INDEX SHOW GENETIC PLEIOTROPY AND SHARED NEURAL ASSOCIATIONS Article 31 March 2025 MULTIVARIATE GENOME-WIDE ASSOCIATION META-ANALYSIS OF
OVER 1 MILLION SUBJECTS IDENTIFIES LOCI UNDERLYING MULTIPLE SUBSTANCE USE DISORDERS Article 22 March 2023 INTRODUCTION A central goal of psychiatric genetics is to identify the small subset
of polymorphisms that influence behavior out of the millions of polymorphisms that could, in principle, have such an effect. One approach is to focus on ‘candidate genes,’ which are
typically genes for proteins involved in neurotransmission or with similarly well-understood functions. Many candidate gene studies have focused on intermediate phenotypes, for example,
laboratory-based measures of normal behaviors. In contrast to endophenotypes, which must meet specific criteria (Gottesman and Gould, 2003), the term intermediate phenotype is sometimes used
for traits that have not been formally shown to meet the criteria for endophenotypes (see Goldman and Ducci, 2007). It has been argued that intermediate phenotypes have a simpler genetic
architecture than disease phenotypes, which would allow for the use of smaller samples (Goldman and Ducci, 2007). Drug response phenotypes, some of which can be considered intermediate
phenotypes, have sometimes yielded large effect alleles (Daly, 2010), which stimulated our interest in intermediate phenotypes that focus on subjective drug responses. Based on this
reasoning, we investigated variability in acute response to a stimulant drug, _d_-amphetamine, in a large sample of healthy volunteers under highly controlled conditions. _D_-amphetamine
response is known to be heritable in humans (Crabbe et al, 1983; Nurnberger et al, 1982) and behavioral responses to _d_-amphetamine are also heritable in mice (Alexander et al, 1996; Grisel
et al, 1997; Kamens et al, 2005; Zombeck et al, 2010). Our study benefited from excellent experimental control and a reasonably large number of participants (_N_=398). The participants were
normal-weight, psychiatrically and physically healthy young adults, with no history of substance dependence. We screened for drug use before each session, limited testing to the follicular
phase in women, and counterbalanced the order of sessions. The study was double-blind, placebo controlled and included two (or in some participants, three) doses of the drug. Over the 5
years that it took to collect these data, we conducted several interim analyses (_N_=99–162) that focused on carefully selected candidate genes: _ADORA2A_ (Hohoff et al, 2005), _SLC6A3_
(Hamidovic et al, 2010b; Lott et al, 2005), _BDNF_ (Flanagin et al, 2006), _SLC6A4_ (Lott et al, 2006), _CSNK1E_ (Veenstra-VanderWeele et al, 2006), _SLC6A2_ (Dlugos et al, 2007; Dlugos et
al, 2009) _DRD2_ (Hamidovic et al, 2009), _FAAH_ (Dlugos et al, 2010), _COMT_ (Hamidovic et al, 2010a), and _OPRM1_ (Dlugos et al, 2011). These genes were examined using either the first 99
or the first 162 participants. The resulting publications have been cited over 200 times and have helped to inspire multiple similar studies. In the present report, we have attempted to
replicate our previously published associations in over 200 more recently collected participants that were recruited, screened and tested in an identical manner. Unlike many other attempts
to replicate results from candidate gene studies, ours consists of multiple candidate genes, relatively large initial and replication cohorts and identical methodology. Thus, we avoided
multiple sources of heterogeneity that are sometimes used to explain the failure of candidate gene studies to replicate. MATERIALS AND METHODS Here, we present the results of a new sample of
young adults tested with three doses of _d_-amphetamine (0, 5, 10, 20 mg), under double-blind conditions exactly like those in the earlier studies. Because our goal was to replicate our
previously reported associations, we first reanalyzed the data published previously for each gene (which we refer to as the ‘original’ sample), and then conducted an identical analysis with
the new (‘replication’) sample using only the more recently collected participants. Replication was defined as obtaining a significant difference (in the same direction) in the replication
sample when performing the same statistical test that was used in the original publication. STUDY DESIGN Healthy young adults completed separate sessions during which they received placebo,
10 mg, or 20 mg of _d_-amphetamine. Some participants (_N_=299) also participated in a fourth session with 5 mg. The study was performed under double-blind conditions with drug order
counterbalanced. Earlier participants were genotyped at single SNPs and VNTRs, as well as using the Addictions Array (Hodgkinson et al, 2008). More recently, genotyping was performed for all
381 participants on the Affymetrix 6.0 array with imputation from the HapMap 3 (Frazer et al, 2007) and 1000 Genomes panels (Durbin et al, 2010), as previously described (Hart et al, 2012).
VNTRs were directly genotyped in all 381 participants because they could not be reliably imputed using the SNP data. PARTICIPANTS The complete sample (ie, original and replication samples
combined) consisted of 398 healthy volunteers aged 18–35 years old who were recruited locally and screened through a physical examination, electrocardiogram, modified Structured Clinical
Interview for DSM-IV, psychiatric symptom checklist (SCL90) and health questionnaire that included sections on current and lifetime drug use. Exclusion criteria were: past year Axis I
Disorder, history of mania or psychosis, less than a high-school level education, smoking >10 cigarettes per week, drinking more than three cups of coffee per day, lack of English
fluency, a body mass index out of the range of 19–26 kg/m2, any regular prescription medication except oral contraceptive or medical contraindication to amphetamine administration. Women not
taking oral contraceptives were only tested in the follicular phase of their menstrual cycle (White et al, 2002). The final sample consisted of 381 participants (17 participants could not
be included in the final analysis as discussed in Hart et al, 2012). Qualifying participants also provided a blood sample, or in some cases a saliva sample, for DNA analysis. PHENOTYPING
PROCEDURE Participants attended three or four 4-h sessions, conducted from 0900 to 1300 hours. They were tested individually in a comfortably furnished room located in the hospital. Sessions
were separated by at least 48 h, and participants were instructed to abstain from drugs and alcohol for 24 h, nicotine for 12 h, and to fast for 12 h before each session. Before each
session, participants provided urine (ToxCup, Branan Medical Corporation, Irvine, CA, USA) and breath samples (Alcosensor III, Intoximeters, St Louis, MO, USA; piCO+ Smokerlyzer, Bedfont,
Rochester, UK) to confirm drug, alcohol, and nicotine abstinence, and female participants were tested for pregnancy. After compliance checks, participants completed subjective effects
questionnaires (see below) and heart rate and blood pressure were recorded. They then ingested a capsule containing _d_-amphetamine (5, 10, or 20 mg) or placebo, under double blind
conditions. During the next 3.5 h, participants relaxed in the laboratory, with reading materials or TV. They completed additional subjective effects measures 30, 60, 90, 150, and 180 min
after the capsule, and physiological measures were also obtained at these times. At 120 min, they completed behavioral tasks described below. This study was approved by the Institutional
Review Board of The University of Chicago and was carried out in accordance with the Helsinki Declaration of 1975. DEPENDENT MEASURES Subjective measures consisted of three standardized
questionnaires: the Profile of Mood States (POMS; Johanson and Uhlenhuth, 1980), Drug Effects Questionnaire (DEQ; Chait et al, 1985), and Addiction Research Center Inventory (ARCI; Martin et
al, 1971). The POMS consists of 72 adjectives used to describe mood, ranging from ‘not at all’ (0) to ‘extremely’ (4). The subscales included from this questionnaire were ‘Friendliness,’
‘Elation,’ ‘Vigor,’ ‘Anger,’ ‘Anxiety,’ ‘Confusion,’ ‘Depression,’ and ‘Fatigue.’ In some cases, the composite ‘Positive Mood’ (Elation − Depression) and Arousal
[(Anxiety+Vigor)—(Fatigue+Confusion)] scales were analyzed. The DEQ consists of five 100 cm visual-analog scales describing five subjective responses to the drug: ‘Feel Drug,’ ‘Want More,’
‘Feel High,’ ‘Like Drug,’ and ‘Dislike Drug.’ The ARCI is an empirically derived 52-item true/false questionnaire consisting of six subscales that measures effects of six classes of drugs:
Amphetamine, Benzedrine, Marijuana, Lysergic Acid (LSD), Morphine-Benzedrine Group (MBG), and Pentobarbital-Chlorpromazine-Alcohol Group (PCAG). These measures were summarized in some
analyses by either calculating the peak change score (PCS) or area under the curve (AUC). Behavioral tasks included the Stop Task (Logan et al, 1984), a measure of behavioral inhibition, and
the Digit Symbol Substitution Task (DSST; Wechsler, 1958), a measure of motor-speed processing. GENOTYPING AND QUALITY CONTROL DNA was extracted from blood at the General Clinical Research
Center at the University of Chicago. In the few cases where blood was not available, DNA was extracted from saliva samples with the Oragene OG-250 or OG-500 kit (Oragene, DNA Genotek,
Kanata, Ontario, Canada). DNA from 15 participants could not be genotyped on the Affymetrix 6.0 array for technical reasons. We identified two participants who completed the study twice; we
excluded their second sessions from the final data set. Thus, we had genotype and phenotype data from 381 participants in the final sample. We were concerned that non-replication might
reflect some systematic error in the replication sample (eg, misalignment of genotypes and phenotypes). Sample swaps can also often be detected as discordant genotypic and self-reported sex;
however, we observed that genotypic sex was 100% consistent with self-reported sex. Genotyping was performed in several stages throughout the course of the 5-year study. Participants were
genotyped at single SNPs or VNTRs using PCR-based methods or on the Addictions Array (Hodgkinson et al, 2008); these genotypes were analyzed in our earlier publications. More recently,
participants were genotyped on the Affymetrix 6.0 array as described in Hart et al (2012). We verified each individual’s self-reported ancestry using the SmartPCA component of EIGENSOFT
(Patterson et al, 2006), which generated ancestry principal components (PCs) that were included as covariates in reanalysis of original studies that included non-Caucasians. We imputed
non-genotyped SNPs with the IMPUTE2 software package (Howie et al, 2009), using the 1000 Genomes (Durbin et al, 2010) and HapMap3 (Frazer et al, 2007) phased genotypes as reference panels.
Rs47958, rs6265, rs135745, rs36017, and rs4680 were genotyped on the Affymetrix 6.0 array, and rs5751876, rs1861647, rs4648317, rs12364283, rs3766246, rs2295633, and rs460000 were imputed.
We checked the concordance of imputed genotypes by comparing them to the genotypes from the original studies. In all cases, the imputed genotypes had 96% or greater concordance with the
direct genotypes, which demonstrated that these SNPs were well imputed. VNTR GENOTYPING SLC6A3 3′ UTR VNTR Polymerase chain reactions were performed in a total volume of 25 μl containing: 1
× PCR buffer, 1.5 mM MgCl2, 5% DMSO, 0.2 mM dNTPs, 0.4 mM of each primer (F: 5′-GGT GTA GGG AAC GGC CTG AGA-3′; R: 5′-CTT CCT GGA GGT CAC GGC TCA AGG-3′), 1.25 U _Taq_ DNA polymerase
(Fermentas, Glen Burnie, MD), and 100 ng DNA. Cycling conditions were 95 °C for 5 min, followed by 30 cycles of 94 °C for 30 s, 62 °C for 30 s, and 72 °C for 30 s. PCR products were resolved
on a 2% agarose gel. SLC6A4 INTRON 2 VNTR Polymerase chain reactions were performed in a total volume of 25 μl containing: 1 × PCR buffer, 1.0 mM MgCl2, 0.2 mM dNTPs, 0.4 mM of each primer
(F: 5′-TGG ATT TCC TTC TCT CAG TGA TTG G-3′; R: 5′-TCA TGT TCC TAG TCT TAC GCC AGT-3′), 1 U _Taq_ DNA polymerase (Fermentas, Glen Burnie, MD), and 100 ng DNA. Cycling conditions were 95 °C
for 2 min, followed by 35 cycles of 95 °C for 1 min, 62.5 °C for 1 min, and 72 °C for 2 min, with a final extension step of 72 °C for 10 min. PCR products were resolved on a 2% agarose gel.
ORIGINAL DATA SETS In some cases the genotypes from prior studies were still available, which allowed us to exactly recreate the original analyses. In other cases, the original genotype
information was no longer available and so we used genotypes obtained from the Affymetrix 6.0 array, by imputation, or by direct genotyping (VNTRs). In such cases, the sample was slightly
different because DNA from a few of the earliest participants was no longer available. All phenotype data were available for reanalysis. RESULTS Table 1 summarizes the results of the
original and the replication analyses. _The main conclusion is that none of our previous findings could be replicated using the newer data._ The demographic characteristics of the sample
separated by 100’s of sequentially tested participants are summarized in Table 2. This table shows that the sample was relatively uniform over the data collection period, except for race,
which was mixed in the first 100 participants but was deliberately limited to Caucasian-only in the remainder; this issue is addressed in the section titled ‘Population stratification
analyses’ (below). In the next sections, we summarize the findings of the original and the replication analyses for 10 genes that were the subject of 12 of our previous publications. For the
purpose of this paper, we reanalyzed the original data using methods that were identical to the original publications, and then conducted the same analysis with the replication sample. The
methods used in the original publications (including data reduction, selection of outcome measures, selection of covariates and data presentation) varied across studies, so these are
described separately in each section. To facilitate comparison, we present the results in the same format that they appeared in the original publications. Results for the combined analyses
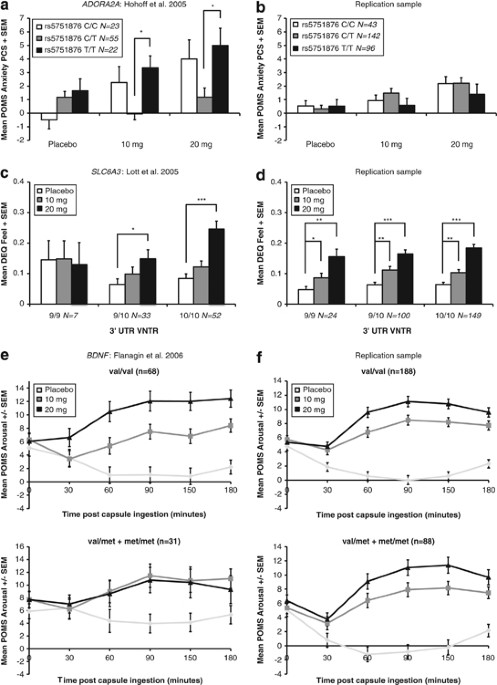
(original and replication samples) are in Supplementary Table 1. Phenotypic means and standard deviations for each study are in Supplementary Table 2. _ADORA2A_ (HOHOFF ET AL, 2005) The
original analysis for adenosine receptor genes (_ADORA1_, _ADORA2A_) consisted of 99 mixed-ancestry participants genotyped at three polymorphisms in _ADORA2A_ (rs5760405, rs5751876,
rs35320474) and one polymorphism in _ADORA1_ (rs10920568). These genes were examined in relation to subjective (POMS subscales) and physiological responses to amphetamine using 3 × 5 × 3
repeated-measures ANCOVAs (Dose × Time × Genotype), with predrug scores used as covariates. _Post hoc_ Dunnet’s _t_-tests were used to assess the effect of specific genotypes. Hohoff et al
(2005) identified a significant Drug × Genotype interaction (_P_=0.041) between ratings on the POMS Anxiety subscale (PCS) and _ADORA2A_ rs5751876 genotype. We obtained the same result
(Figure 1a). _Post hoc_ tests revealed that the rs5751876 T/T group had higher anxiety during the 10- and 20-mg sessions as compared with the C/T group (10 mg, _P=_0.004; 20 mg, _P_=0.028).
We conducted the same analysis used in the original publication in the replication sample (_N_=281). In the replication sample, the three genotype groups did not differ on the POMS Anxiety
scale (Figure 1b), indicating a failure to replicate the original result from the original sample. Because we were concerned about the apparent differences in means between the original and
replication samples, we plotted the distributions for each sample to verify that there was no overall difference in POMS Anxiety scores between the two samples. When the genotypic groups
were combined, the distributions were very similar for the original sample and the replication sample (POMS Anxiety PCS, 20 mg; Supplementary Figure S1). _SLC6A3_ (LOTT ET AL, 2005) The
original analysis for the dopamine transporter gene (_SLC6A3_) consisted of 100 mixed-ancestry participants genotyped at the _SLC6A3_ 3′ UTR VNTR polymorphism. Two common alleles exist
(9-repeat and 10-repeat); four participants with rare alleles were excluded from the analysis. This polymorphism was examined in relation to subjective drug effects (POMS, DEQ, and ARCI
subscales) and physiological responses to amphetamine using 3 × 5 × 3 repeated-measures ANCOVAs (Dose × Time × Genotype), with predrug scores used as covariates. _Post hoc t_-tests were
performed when a significant Drug × Genotype effect was found. Lott et al (2005) identified significant Drug × Genotype interactions between _SLC6A3_ 3′ UTR VNTR genotype and ratings on the
DEQ Feel (_P_=0.006) and ARCI LSD (_P_=0.007) subscales, as well as a significant association with diastolic blood pressure (_P_=0.037). We repeated this analysis and obtained the same
results (Figure 1c; Supplementary Figures S2A and C). _Post hoc_ tests revealed that the 9/10 and 10/10 groups differed significantly for DEQ Feel at 20 mg compared with placebo (9/9: ns,
9/10: _P_=0.003, 10/10: 4 × 10−9); there was no significant difference for the 9/9 group. The same effect was seen for the ARCI LSD scale (9/9: ns, 9/10: _P_=2.9 × 10−4, 10/10: _P_=1.13 ×
10−4). The 9/10 group differed significantly for diastolic blood pressure for the 20-mg session compared with placebo (9/9: ns, 9/10: _P_=0.004, 10/10: ns). The 10/10 group did show
significantly increased diastolic blood pressure at 10 mg compared with placebo (_P_=0.005). We conducted the same analysis used in the original publication in the replication sample
(_N_=284). In the replication sample, the all genotype groups showed significant responses to amphetamine on DEQ Feel (Figure 1d) and diastolic blood pressure (Supplementary Figure S2D),
indicating a failure to replicate the results from the original sample. However, there was a modest but significant Dose × Genotype interaction for ARCI LSD (_P_=0.02; Supplementary Figure
S2B). _BDNF_ (FLANAGIN ET AL, 2006) The original analysis for the brain-derived neurotrophic factor gene (_BDNF_) consisted of 99 mixed-ancestry participants genotyped at the val66met
polymorphism (rs6265) in _BDNF_. Due to low minor allele frequency, the met/met group was pooled with the val/met heterozygote group in the original study. Associations of rs6265 with
subjective mood scales and physiological measures were assessed with 3 × 5 × 2 repeated-measures ANCOVAs (Dose × Time × Genotype), with predrug scores used as covariates. Flanagin et al
(2006) identified significant Drug × Genotype interactions between genotype at rs6265 and POMS Arousal (_P_=0.01; Figure 1e) and ARCI BG (_P_=0.023; Supplementary Figures S3A and C), as well
as Dose × Genotype × Time interaction with heart rate (trend, _P_=0.057; Supplementary Figures S3E and G), and we repeated this analysis and obtained the same results. We conducted the same
analysis used in the original publication in the replication sample (_N_=290). In the replication sample, the two genotype groups did not differ on any measure (POMS Arousal: Figure 1f;
ARCI BG: Supplementary Figures S3B and D; heart rate: Supplementary Figures S3F and H), indicating a failure to replicate the results from the original sample. _SLC6A4_ (LOTT ET AL, 2006)
The original analysis of the serotonin transporter gene consisted of 101 mixed-ancestry participants genotyped at the serotonin transporter (_SLC6A4_) Intron 2 VNTR and 5-HTTLPR
polymorphisms. The two common Intron 2 VNTR alleles were analyzed (10 or 12 repeats) and individuals with rare alleles were excluded. These polymorphisms were analyzed in relation to
subjective response to amphetamine (DEQ Feel, POMS Anxiety, and ARCI MBG subscales) using 3 × 5 × 3 repeated-measures ANCOVAs (Dose × Time × Genotype), with predrug scores used as
covariates. _Post hoc_ analyses were conducted with paired _t_-tests. Lott et al (2006) identified a significant Drug × Genotype interaction between ratings of ARCI MBG in response to 20 mg
amphetamine. Our reanalysis of the original data produced the same result (_P_=0.046; Figure 2a). _Post hoc_ tests with mean change scores from baseline revealed significantly greater mean
ratings on the ARCI MBG subscale in the 10/10 group as compared with the 12/12 and 10/12 groups (_P_=0.002, _P_=0.006, respectively). We conducted the same analysis used in the original
publication in the replication sample (_N_=279). In the replication sample, we did not identify any difference between the three genotype groups (Figure 2b), indicating a failure to
replicate the results from the original sample. _CSNK1E_ (VEENSTRA-VANDERWEELE ET AL, 2006) The original analysis of the casein-kinase I epsilon gene (_CSNK1E_) consisted of 91 participants
genotyped at three polymorphisms in _CSNK1E_ (rs135745, rs1005473, rs199764). This polymorphism was analyzed in relation to subjective responses to amphetamine (DEQ Feel, POMS Anxiety, ARCI
MBG subscales) using 3 × 5 × 3 repeated-measures ANOVAs (Dose × Time × Genotype). Predrug scores were subtracted from the score at each time point to yield change scores. _Post hoc_ analyses
were conducted to assess the effect of dose on the Genotype × Dose interaction; these consisted of 2 × 5 × 3 repeated-measures ANOVAs (Dose × Time × Genotype). Veenstra-VanderWeele et al
(2006) identified significant Drug × Genotype interactions between DEQ Feel and ARCI MBG change scores and genotype at rs135745 (_P_=0.038; _P_=0.008), an effect that was specific to the
10-mg dose (_P_=0.001; _P_=0.004); we repeated this analysis and obtained the same result (Figure 2c; Supplementary Figure S4A). We conducted the same analysis used in the original
publication in the replication sample (_N_=279), but did not identify any differences between the three genotype groups on any measure (Figure 2d; Supplementary Figure S4B), indicating a
failure to replicate the results from the original sample. _SLC6A2_ (DLUGOS ET AL, 2007) The original analysis of the norepinephrine transporter gene (_SLC6A2_) consisted of 99 participants
genotyped at eight SNPs in _SLC6A2_ (rs35915, rs168924, rs168924, rs2242446, rs36017, rs2270935, rs47958, rs171798). These SNPs, along with eight haplotypes comprised of these SNPs, were
examined in relation to subjective responses to amphetamine using the non-parametric Kruskal–Wallis test. Dlugos et al (2007) identified a significant association between ratings of POMS
Positive Mood (PCS; _P_=0.019) and POMS Elation (PCS; _P_=0.01) following amphetamine administration (20 mg) and genotype at rs49758, and we repeated this analysis and obtained the same
results (Figure 2e). _Post hoc_ tests revealed significantly higher ratings of Positive Mood in response to 20 mg amphetamine in the C/C group (_P_=0.003) and the A/C group (_P_=0.007), but
not in the A/A group (_P_=0.6), as well as significantly higher ratings of Elation in the C/C (_P_=1.34 × 10−4) and A/C (_P_=0.001), but not the A/A group (_P_=0.4). Additionally, Dlugos _et
al_ found that the rs36017–rs2270935–rs47958 GCC and CCA haplotypes were significantly associated with ratings of POMS Positive Mood (20 mg PCS), and we repeated the analysis and obtained
the same results (GCC, _P_=0.032; CCA, _P_=0.016). We conducted the same analysis used in the original publication in the replication sample (_N_=289), but in the replication sample, the
genotype groups did not differ on any measure (Figure 2f). Furthermore, neither the GCC haplotype (_P_=0.453) nor the CCA haplotype (_P_=0.573) was associated with ratings of POMS Positive
Mood in the replication sample. Thus, we failed to replicate the results from the original sample. _SLC6A2_ (DLUGOS ET AL, 2009) The original analysis of the norepinephrine transporter gene
(_SLC6A2_) consisted of 162 Caucasian participants genotyped at 11 SNPs in _SLC6A2_ (rs2397771, rs3785143, rs192303, rs36024, rs36021, rs3785152, rs36017, rs10521329, rs3785155, rs1861647,
rs5569). These SNPs, along with two haplotypes comprised of these SNPs, were analyzed in relation to POMS Elation and Vigor subscales (PCS) in response to amphetamine using 3 × 3
repeated-measures ANOVAs or ANCOVAs (Dose × Genotype). Gender was used as a covariate in the analyses of POMS Elation, as it was seen to be associated with this subscale. _Post hoc_ one-way
ANOVAs were performed. Dlugos et al (2009) identified associations between POMS Vigor and Elation following amphetamine administration and _SLC6A2_ SNP genotypes. We obtained the same
results using the same data (Figure 3a; Supplementary Figures S5A, C and E). Specifically, significant Drug × Genotype interactions were identified for rs36017 and Vigor (_P_=0.041) and
rs1861647 and Vigor (_P_=0.006). Although not statistically significant, trends were seen for rs36017 and Elation (_P_=0.154) and rs1861647 (_P_=0.137). _Post hoc_ analyses revealed that
individuals with the C/C genotype at rs36017 had significantly higher ratings of POMS Vigor following 20 mg amphetamine when compared with the C/G and G/G groups (_P_=0.003, _P_=0.019,
respectively; Figure 3a). Similarly, this group had significantly higher ratings of POMS Elation in response to 20 mg amphetamine when compared with the C/G group (_P_=0.013; Supplementary
Figure S5A). The rs181647 A/A group had significantly higher ratings of POMS Vigor (_P_=0.01; Supplementary Figure S5C) and POMS Elation (_P_=0.017; Supplementary Figure S5E) when compared
with the G/G group. Additionally, Dlugos _et al_ found that the rs36017–rs10521329–rs3785155 CCG and rs1861647–rs5569 GC haplotypes were significantly associated with ratings of POMS Vigor
(20 mg PCS), and we repeated this analysis and obtained similar results (rs36017–rs10521329–rs3785155 CCG, _P_=0.097; rs1861647–rs5569 GC, _P_=0.0142). We conducted the same analysis used in
the original publication in the replication sample (_N_=170), but did not identify any differences between the three genotype groups for either SNP on any measure (Figure 3f; Supplementary
Figures S5B, D and F). Neither the CCG haplotype (_P_=0.667) nor the GC haplotype (_P_=0.571) was associated with ratings of POMS Vigor in the replication sample. Thus, we failed to
replicate the results from the original sample. _DRD2_ (HAMIDOVIC ET AL, 2009) The original analysis of the dopamine D2 receptor gene (_DRD2_) consisted of 93 Caucasian participants
genotyped at 12 SNPs in _DRD2_ (rs2242592, rs1079596, rs1125394, rs27471857, rs4648317, rs4350392, rs1799978, rs12364283, rs71003679, rs4648318, rs4274224, rs4581480). In addition to 10 and
20 mg of amphetamine, this study also included the 5-mg dose. These SNPs were analyzed in relation to performance on the Stop Task following amphetamine administration using 4 × 3
repeated-measures ANOVAs (Dose × Genotype). Paired-samples _t_-tests were used to assess the effect of drug on each genotype group when a significant Drug × Genotype interaction was found.
Hamidovic et al (2009) identified a significant Drug × Genotype interaction between genotype at rs12364283 and scores on the Stop Task in response to amphetamine (_P_=0.008), and we repeated
this analysis and obtained the same result (Figure 3c). _Post hoc_ tests revealed that amphetamine decreased stop reaction time (Stop RT) in the A/A group as compared with placebo (5 mg,
_P_=0.02; 10 mg, _P_=0.001; 20 mg, _P_=0.05), but did not decrease Stop RT in the combined A/G+G/G group, and the 10-mg amphetamine dose significantly increased Stop reaction time compared
with placebo in the combined A/G+G/G group (_P_=0.043; Figure 3c). We conducted the same analysis used in the original publication in the replication sample (_N_=122), which was reduced in
size because we excluded participants that possessed low quality Stop RT data. We did not identify any differences between the genotype groups (Figure 3d), indicating a failure to replicate
the results from the original sample. _FAAH_ (DLUGOS ET AL, 2010) The original analysis of the fatty acid amide hydrolase gene (_FAAH_) consisted of 159 Caucasian participants genotyped at
four SNPs in _FAAH_ (rs6703669, rs3766246, rs324420, rs2295633). These SNPs were analyzed in relation to subjective responses to amphetamine (POMS Arousal, Fatigue subscales; AUC) using 3 ×
3 repeated-measures ANOVAs/ANCOVAs (Dose × Genotype). _Post hoc_ analyses were carried out with one-way ANOVAs. Gender was used as a covariate in the analyses of POMS Arousal, as it was
found to be associated with this subscale. Dlugos et al (2010) identified associations between two SNPs in _FAAH_ and scores on the POMS Arousal and Fatigue subscales in response to
amphetamine. Significant Drug × Genotype interactions were found for rs2295633 and POMS Arousal (_P_=0.02) as well as Fatigue (_P_=0.01). We repeated this analysis and obtained the same
results (Figure 3e; Supplementary Figure S6A). _Post hoc_ tests revealed that the C/C group had significantly higher ratings of Arousal in response to 10 mg amphetamine as compared with the
C/T group (_P_=0.003); the C/C group also showed significantly reduced Fatigue (_P_=0.005). Additionally, significant Drug × Genotype interactions were found for rs3766246 and POMS Arousal
(_P_=0.013) and POMS Fatigue (_P_=0.009). We obtained the same results in our repeat analysis. Participants in the C/C group reported higher ratings of Arousal and lower ratings of Fatigue
when compared with participants in the C/T group (_P_=0.009 and _P_=0.01, respectively). Additionally, Dlugos _et al_ found that the rs3766246–rs324420–rs2295633 CCC and TAT haplotypes were
significantly associated with ratings of Fatigue at 10 mg, and we repeated the analysis and obtained the same results (CCC, _P_=0.003; TAT, _P_=0.012). We conducted the same analysis used in
the original publication in the replication sample (_N_=173). In the replication sample, the genotype groups for both SNPs did not differ on any measure (Figure 3f; Supplementary Figure
S6B). Furthermore, neither the CCC nor the TAT haplotype was significantly associated with ratings of fatigue in the replication sample (CCC, _P_=0.667; TAT, _P_=1.0). Thus, we failed to
replicate the results from the original sample. _COMT_ (HAMIDOVIC ET AL, 2010A) The original analysis of the catechol-_O_-methyltransferase gene (_COMT_) consisted of 161 Caucasian
participants genotyped at the val158met polymorphism (rs4680). This SNP was analyzed in relation to subjective and behavioral responses to amphetamine administration (POMS subscales, DSST;
AUC) using 3 × 3 repeated-measures ANOVAs (Dose × Genotype). Paired-samples _t_-tests were used to assess the effect of drug on each genotype group when a significant Drug × Genotype
interaction was found. Hamidovic et al (2010a) identified a significant Drug × Genotype interaction (_P_=0.008) between scores of the DSST in response to amphetamine. We repeated this
analysis and obtained the same result (Figure 4a). _Post hoc_ analyses revealed that met/met carriers did not respond to amphetamine, while val/val carriers showed enhanced performance in
the 10 mg and 20 mg drug sessions as compared with placebo (10 mg, _P_=5.4 × 10−5; 20 mg, _P_=1.3 × 10−4; Figure 4a). Val/met carriers showed an intermediate response to drug in the 20-mg
session (_P_=0.002). We conducted the same analysis used in the original publication in the replication sample (_N_=176), but did not identify any differences between the three genotype
groups (Figure 4b), indicating a failure to replicate the results from the original sample. _SLC6A3_ (HAMIDOVIC ET AL, 2010B) The original analysis of the dopamine transporter gene
(_SLC6A3_) consisted of 152 Caucasian participants genotyped at four SNPs in _SLC6A3_ (rs460000, rs3756450, rs37022, rs6869645). Due to low minor allele frequency, the minor allele
homozygotes were pooled with the heterozygotes for all four SNPs. These SNPs were analyzed in relation to subjective effects and cognitive performance in response to amphetamine using 3 × 2
repeated-measures ANOVAs (Dose × Genotype). Paired-samples _t_-tests were used to assess the effect of drug on each genotype group when a significant Drug × Genotype interaction was found.
Hamidovic et al (2010b) identified a significant Drug × Genotype interaction for the ARCI Amphetamine and ARCI MBG scales (AUC) and genotype at rs460000 (_P_=0.015, _P_=0.025, respectively).
We repeated this analysis and obtained the same result (Supplementary Figure S7A; Figure 4c). _Post hoc_ tests demonstrated that the C/C group had greater response to amphetamine when
compared with the A/A+A/C group (ARCI Amphetamine placebo _vs_ 20 mg _P_=3.1 × 10−13 _vs P_=1.9 × 10−7; ARCI MBG placebo _vs_ 20 mg _P_=1.5 × 10−11 _vs P_=2 × 10−6). We conducted the same
analysis used in the original publication in the replication sample (_N_=169). In the replication sample, there was a significant Drug × Genotype interaction between ratings on the ARCI
Amphetamine subscale and genotype at rs460000; however, a _post hoc_ one-way ANOVA revealed that this effect was driven by the placebo session (_P_=0.028; Supplementary Figure S7B). There
was no evidence of association with ARCI MBG, with all groups responding similarly across all sessions (Figure 4d), indicating a failure to replicate the results from the original sample.
_OPRM1_ (DLUGOS ET AL, 2011) The original analysis of the opioid receptor, mu 1 gene (_OPRM1_) consisted of 162 Caucasian participants genotyped at seven SNPs in _OPRM1_ (rs1799971,
rs510769, rs660756, rs1918760, rs2281617, rs1998220, rs1998220). These SNPs, along with seven haplotypes comprised of these SNPs, were analyzed in relation to the subjective response to
amphetamine (ARCI subscales; PCS) using 3 × 3 repeated-measures ANOVAs/ANCOVAs (Dose × Genotype). _Post hoc_ analyses consisted of one-way ANOVAs/ANCOVAs. Gender was used as a covariate in
the analyses of ARCI BG, as it was seen to be associated with this subscale. Dlugos et al (2011) identified significant Drug × Genotype interactions between rs510769 and ARCI MBG (_P_=0.031)
and ARCI Amphetamine (_P_=0.019), as well as between rs2281617 and ARCI MBG (_P_=0.01) and ARCI BG (_P_=0.008). We repeated this analysis and obtained the same result (Supplementary Figures
S8A and C; Figure 4e; Supplementary Figure S8E). _Post hoc_ tests revealed that these associations were specific to the 10 mg session. The rs510769 G/G group had increased ratings on the
ARCI MBG scale as compared with the A/A group (_P_=0.02; Supplementary Figure S8A), and the A/G and G/G groups had increased ARCI Amphetamine ratings as compared with the A/A group
(_P_=0.005, _P_=0.003, respectively; Supplementary Figure S8C). The rs2281617 C/C group had increased ratings on the ARCI MBG and ARCI BG scales compared with the C/T+T/T group (_P_=3.4 ×
10−4, _P_=1.3 × 10−4, respectively; Figure 4e; Supplementary Figure S8E). Dlugos _et al_, also identified significant associations between the rs1799171–rs510769 AG and AA haplotypes and
ratings on the ARCI Amphetamine scale, the rs1799171–rs510769 AA haplotype and ratings on the ARCI MBG scale, the rs1918760–rs2281617–rs1998220 ATA haplotype and ratings on the ARCI MBG
scale, and the rs1918760–rs2281617–rs1998220 ATA and GCG haplotypes and ratings on the ARCI BG subscale. We repeated the analysis and obtained similar results (rs1799171–rs510769 AG and AA
ARCI Amphetamine _P_=0.019, _P_=0.005; rs1799171–rs510769 AA and ARCI MBG _P_=0.031; rs1918760–rs2281617–rs1998220 ATA and ARCI MBG _P_=0.01; rs1918760–rs2281617–rs1998220 ATA and ARCI BG
_P_=0.048; rs1918760–rs2281617–rs1998220 GCG and ARCI BG _P_=0.069). We conducted the same analysis used in the original publication in the replication sample (_N_=171). In the replication
sample, the genotype groups for both SNPs did not show significant differences for any measures (Supplementary Figures S8B and D; Figure 4f; Supplementary Figure S8F). Furthermore, we failed
to identify any associations with haplotypes in the replication sample (rs1799171–rs510769 AG and AA ARCI Amphetamine _P_=0.222, _P_=0.190; rs1799171–rs510769 AA and ARCI MBG _P_=0.590;
rs1918760–rs2281617–rs1998220 ATA and ARCI MBG _P_=0.233; rs1918760–rs2281617–rs1998220 ATA and ARCI BG _P_=0.975; rs1918760–rs2281617–rs1998220 GCG and ARCI BG _P_=0.159). Taken together,
these results reflect a failure to replicate the results from the original sample. POPULATION STRATIFICATION ANALYSES The original sample of 99 participants, which was used in the analysis
of _ADORA2A_ (Hohoff et al, 2005), _SLC6A3_ (Lott et al, 2005), _BDNF_ (Flanagin et al, 2006), _SLC6A4_ (Lott et al, 2006), _CSNK1E_ (Veenstra-VanderWeele et al, 2006), and _SLC6A2_ (Dlugos
et al, 2007), included 41 participants who self-reported being non-Caucasians (subsequent studies of _SLC6A2_, _DRD2_, _FAAH_, _COMT_, and _OPRM1_ excluded non-Caucasians). We used
genome-wide SNP data (Hart et al, 2012) to evaluate whether population stratification contributed to the associations identified using the non-Caucasian participants. Because nine samples
were not available for genotyping with the Affymetrix 6.0 microarray, this analysis required us to exclude those nine participants. We report the initial _P_-values (‘Original’), the effect
of removing those nine individuals (‘Original—9 participants’) and any additional effect of using the ancestry PCs as covariates (‘Original – 9 participants+PCs’) in Table 3. Adjustment for
ancestry principal components appeared to have little impact on the initial results. When we included the ancestry PCs as covariates, some associations became slightly stronger (eg, _SLC6A3_
and ARCI LSD), while others became slightly weaker (eg, _CSNK1E_ and ARCI MBG; Table 3). Taken together, these results demonstrate that none of the original associations appears to be
primarily due to population stratification. DISCUSSION Our results show an unexpectedly widespread failure to replicate our previously published findings. This study is striking because we
were attempting to replicate apparently robust findings related to well-studied candidate genes. We used a relatively large number of new participants for the replication, and their data
were collected and analyzed using identical procedures. Thus, our study did not suffer from the heterogeneity in phenotyping procedures implicated in previous failures to replicate other
candidate gene studies (Ho et al, 2010; Mathieson et al, 2012). The failure of our associations to replicate suggests that most or all of our original results were false positives. One
possible cause of these false positives could have been that that six of our original studies included 41 non-Caucasian participants (Table 3). To address this concern, we repeated the
original analyses with the addition of ancestry PCs as covariates; there were no major differences. Therefore, while population stratification can sometimes lead to false positive results,
it does not appear that the inclusion of non-Caucasians significantly contributed to the observed failure to replicate our previously published results. It is worth considering whether we
should have viewed our original results with greater skepticism. Genome-wide association studies (GWAS) of a wide variety of phenotypes suggest that the effects of individual alleles are
very small, such that the modestly sized samples typically used in candidate-gene studies such as ours would be severely under-powered (McCarthy et al, 2008). Both the original and the
replication samples were too small to detect alleles with the small effect sizes seen in GWAS. Our original studies suggested that we were detecting alleles that contributed ∼5% of the total
phenotypic variance we reported. Given that there are millions of polymorphisms in the human genome, such large effects might have aroused greater scrutiny, but we were reassured by the
commonly held belief that polymorphisms in our candidate genes represented a privileged subset of polymorphisms and by the notion that intermediate phenotypes might have a simpler genetic
architecture. We were not alone—many other candidate gene studies have and continue to report similarly large effect sizes and to espouse similar beliefs. Three of our previously reported
associations were due to the _lack_ of a drug effect in a particular genotype group; in all cases, the rare homozygote groups did not show a significant drug response, which could reflect a
lack of power rather than a true lack of response. For example, the 3′ UTR VNTR polymorphism in _SLC6A3_ was associated with ratings of DEQ Feel in Lott et al (2005), but in the original
analysis the minor allele 9/9 genotype group (_N_=7) did not show response to amphetamine, while the heterozygote (_N_=33) and major allele 10/10 (_N_=52) groups did. In the replication
sample, the 9/9 group (_N_=24), like the 9/10 and 10/10 groups, showed a significant drug response. Similarly, a lack of drug effect in rare allele homozygote groups contributed to the
associations in Flanagin et al (2006) (_BDNF_) and Hamidovic et al (2010a) (_COMT_); these results reflect poor power to detect the effect of amphetamine due to a small number of rare allele
homozygotes. This phenomenon has been noted in other candidate gene studies of the _SLC6A3_ 3′ UTR where a lack of effect was observed in the 9/9 genotype group (Joober et al, 2007; Stein
et al, 2005), suggesting that this may be a widespread problem. The fundamental issue is that small genotype groups may not show a response to treatment due to a lack of power. One
potentially valuable strategy to avoid this problem is prospective genotyping, which allows for more balanced genotype groups and is thus helpful when evaluating rare alleles. Two related
problems that are common among candidate gene studies like ours are insufficient correction for multiple testing (both within and across studies) and publication bias. Although several of
our previous reports applied corrections for the number of tests performed _within_ that publication, others did not. Furthermore, we never corrected for all comparisons performed _across_
all 12 studies. Similar failures to fully correct for multiple testing are common in the candidate gene literature, where large data sets are often repeatedly analyzed. If we corrected for
all 322 primary tests performed in this study, the Bonferroni-corrected significance threshold would be 0.00015. While this _P_-value is overly stringent because both SNPs and phenotypes are
inter-correlated, it gives some sense for the cumulative burden of multiple testing across all 12 studies. Multiple testing across studies is more problematic than multiple testing within
studies, as it is often not readily apparent that a data set has been analyzed repeatedly from the reading of one study. Better standards for reporting prior analyses of a given data set
might be helpful, but in the end running more tests will inevitably inflate the number of false positives, whether the tests use one data set repeatedly or many separate data sets. Thus
standards that tend to preclude multiple analyses of the same data set are too simplistic to fully address this problem. The problem of publication bias, which is the tendency to
preferentially publish significant results and to repress non-significant ones, is related to the failure to correct for multiple testing because the true number of hypotheses tested in a
given data set is concealed. It has been argued that publication bias against non-significant results contributes to non-replication of candidate gene associations (Bosker et al, 2011;
Munafò et al, 2007). Our original results reflect a minor degree of publication bias: in our early investigations we performed preliminary analyses on a small number of genes that did not
yield significant results and thus we did not publish them. This increase in multiple testing was not taken into account when determining significance thresholds. Similarly, we sometimes
considered several alternative methods for calculating phenotypes (eg, peak change score summarization _vs_ area under the curve, which tend to be highly but incompletely correlated). It
seems very likely that the candidate gene literature frequently reflects this sort of publication bias, which represents a special case of uncorrected multiple testing. Proper correction for
multiple phenotypes is a concern and a source of debate for multidimensional phenotypes, such as brain imaging (Poldrack and Mumford, 2009; Bennett et al, 2011). One feature of our studies
was the use of subjective drug effects as outcome measures. While we initially regarded the use of subjective drug effects as a strength, it may be that other phenotypes provide a more
sensitive indicator of drug response. Although subjective drug effects are dose and time dependent and provide a unique, face-valid indicator of the drug’s effect on behavior, they are also
highly variable within and across participants, and are subject to the biases present in any self-report measure. Instead, measures such as functional magnetic resonance imaging (fMRI) may
provide a more precise and objective index of biological response to a drug. One example of the success of fMRI phenotypes has been the association of the amygdala response to threat stimuli
and the 5-HTTLPR polymorphism, which was initially suggested to account for 10% of the phenotypic variance (Munafò et al, 2008) but was later determined to account for ∼1% (Murphy et al,
2012). Other examples of potentially promising phenotypes include alcohol-induced flushing (Macgregor et al, 2009; Wall et al, 2005), nicotine metabolism (Benowitz et al, 2003; Lerman et al,
2006), and electroencephalography (Hodgkinson et al, 2010). Ultimately, the optimal phenotype for any particular scientific or clinical question depends on the sensitivity and selectivity
of the measure and practical issues such as cost and throughput. Using our results as an example, we demonstrate that a rigorously assessed and biologically based intermediate phenotype has
the potential to yield false positives, in part because of the common practices used in candidate gene studies. How can this problem be addressed in the future? Clearly more stringent
thresholds for significance that better address multiple testing within and between studies are important. Because an initial study is best for hypothesis generation, replication studies,
such as this one, are also essential. More stringent standards will require correspondingly larger samples. Although many journals have added requirements for replication (Anonymous, 2005;
Barsh et al, 2012; Hewitt 2012; http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1552-485X/homepage/ForAuthors.html; http://www.blackwellpublishing.com/pdf/G2B-Association-Studies.pdf),
replication is not infallible (Sullivan, 2007). Candidate gene studies with samples of several hundred or less subjects are only adequately powered to detect alleles with effects that are
significantly larger than those observed in most GWAS. Thus, these studies are only productive if one assumes that an intermediate phenotype is fundamentally different from a disease trait.
Our study does not support this hypothesis, although it just one example. If the genetic architecture of intermediate phenotypes is indeed similar to disease traits, very large samples will
be needed to achieve sufficient power. One key factor for a successful candidate gene study is to have strong prior information that the polymorphism being examined is likely to be a true
positive. Whereas ‘traditional’ candidate gene studies such as ours have focused on heavily studied genes (sometimes with a specific focus on coding SNPs, eg Flanagin et al, 2006; Hamidovic
et al, 2010a; Dlugos et al, 2011), a more recent trend is to focus on the SNPs that have experimentally validated effects on gene expression; such SNPs are termed expression quantitative
trait loci (eQTL). Recent studies have shown that SNPs associated with complex traits are enriched for eQTLs (Schadt et al, 2008; Nicolae et al, 2010; Fehrmann et al, 2011; Gamazon et al,
2012). Similarly, recent GWAS studies have begun to provide unambiguous associations between SNPs and disease traits (Furberg et al, 2010; Ripke et al, 2011); these SNPs are likely to be the
subject of the next wave of candidate gene studies. While focusing on polymorphisms that have known biological effects can only improve candidate gene studies, the fundamental question
remains: is it realistic to assume that the effect of these SNPs will be large enough to allow for detection when examining intermediate phenotypes with only modestly sized samples? In
conclusion, in an effort to examine the validity and replicability of our previous work, we performed a replication study of 12 of our previously published candidate gene association
studies. We were motivated to perform this replication study because we believed that we had an ideal sample to explore replication in a broad range of different candidate genes. _We failed
to replicate any of our previously published results, suggesting that our previously published findings were likely false positives_. More broadly, our results should instill caution in
other investigators who, in some cases inspired by our previous publications, have undertaken similarly designed and powered studies. The final judgment about the usefulness of intermediate
phenotypes will depend on the results from many studies. Our experience provides one example in which a promising intermediate phenotype did not perform as expected. We conclude that future
candidate gene studies focused on intermediate phenotypes similar to ours should strongly consider the possibility that effect sizes may be similar to those observed in GWAS. REFERENCES *
Alexander RC, Wright R, Freed W (1996). Quantitative trait loci contributing to phencyclidine-induced and amphetamine-induced locomotor behavior in inbred mice. _Neuropsychopharmacology_ 15:
484–490. Article CAS PubMed Google Scholar * _American Journal of Medical Genetics Part B: Neuropsychiatric Genetics_. Editorial Policy on Association Studies
http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1552-485X/homepage/ForAuthors.html. * Anonymous (2005). Framework for a fully powered risk engine. _Nat Genet_ 37: 1153. Article Google
Scholar * Barsh GS, Copenhaver GP, Gibson G, Williams SM (2012). Guidelines for genome-wide association studies. _Plos Genet_ 8: e1002812. Article CAS PubMed PubMed Central Google
Scholar * Bennett C, Baird AA, Miller MB, Wolford GL (2011). Neural correlates of interspecies perspective taking in the post-Mortem Atlantic Salmon: an argument for proper multiple
comparisons correction. _J Serendipitous Unexpected Results_ 1: 1–5. Google Scholar * Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P (2003). Nicotine metabolite ratio as a predictor of
cigarette consumption. _Nicotine Tob Res_ 5: 621–624. Article CAS PubMed Google Scholar * Bosker FJ, Hartman CA, Nolte IM, Prins BP, Terpstra P, Posthuma D _et al_ (2011). Poor
replication of candidate genes for major depressive disorder using genome-wide association data. _Mol Psychiatry_ 16: 516–532. Article CAS PubMed Google Scholar * Chait L, Fischman MW,
Schuster CR (1985). 'Hangover' effects the morning after marijuana smoking. _Drug Alcohol Depend_ 15: 229–238. Article CAS PubMed Google Scholar * Crabbe J, Jarvik L, Liston E,
Jenden D (1983). Behavioral responses to amphetamines in identical twins. _Acta Genet Med Gemellol (Roma)_ 32: 139–149. Article CAS Google Scholar * Daly AK (2010). Genome-wide
association studies in pharmacogenomics. _Nat Rev Genet_ 11: 241–246. Article CAS PubMed Google Scholar * Dlugos A, Freitag C, Hohoff C, McDonald J, Cook E, Deckert J _et al_ (2007).
Norepinephrine transporter gene variation modulates acute response to D-amphetamine. _Biol Psychiatry_ 61: 1296–1305. Article CAS PubMed Google Scholar * Dlugos AM, Hamidovic A,
Hodgkinson C, Shen PH, Goldman D, Palmer AA _et al_ (2011). OPRM1 gene variants modulate amphetamine-induced euphoria in humans. _Genes Brain Behav_ 10: 199–209. Article CAS PubMed Google
Scholar * Dlugos AM, Hamidovic A, Hodgkinson CA, Goldman D, Palmer AA, de Wit H (2010). More aroused, less fatigued: fatty acid amide hydrolase gene polymorphisms influence acute response
to amphetamine. _Neuropsychopharmacology_ 35: 613–622. Article CAS PubMed Google Scholar * Dlugos AM, Hamidovic A, Palmer AA, Wit H (2009). Further evidence of association between
amphetamine response and SLC6A2 gene variants. _Psychopharmacology (Berl)_ 206: 501–511. Article CAS Google Scholar * Durbin R, Abecasis G, Altshuler D, Auton A, Brooks L, Gibbs R _et al_
(2010). A map of human genome variation from population-scale sequencing. _Nature_ 467: 1061–1073. Article CAS PubMed Google Scholar * Fehrmann RSN, Jansen RC, Veldink JH, Westra H-J,
Arends D, Bonder MJ _et al_ (2011). Trans-eQTLs reveal that independent genetic variants associated with a complex phenotype converge on intermediate genes, with a major role for the HLA.
_Plos Genet_ 7: e1002197. Article CAS PubMed PubMed Central Google Scholar * Flanagin B, Cook E, de Wit H (2006). An association study of the brain-derived neurotrophic factor Val66Met
polymorphism and amphetamine response. _Am J Med Genet B_ 141: 576–583. Article Google Scholar * Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA _et al_ (2007). A second
generation human haplotype map of over 3.1 million SNPs. _Nature_ 449: 851–861. Article CAS PubMed Google Scholar * Furberg H, Kim Y, Dackor J, Boerwinkle E, Franceschini N, Ardissino D
_et al_ (2010). Genome-wide meta-analyses identify multiple loci associated with smoking behavior. _Nat Genet_ 42: 441–447. Article CAS Google Scholar * Gamazon ER, Badner JA, Cheng L,
Zhang C, Zhang D, Cox NJ _et al_ (2012). Enrichment of cis-regulatory gene expression SNPs and methylation quantitative trait loci among bipolar disorder susceptibility variants. _Mol
Psychiatry_http://www.blackwellpublishing.com/pdf/G2B-Association-Studies.pdf. * Goldman D, Ducci F (2007). Deconstruction of vulnerability to complex diseases: enhanced effect sizes and
power of intermediate phenotypes. _ScientificWorldJournal_ 7: 124–130. Article CAS PubMed PubMed Central Google Scholar * Gottesman II, Gould TD (2003). The endophenotype concept in
psychiatry: etymology and strategic intentions. _Am J Psychiatry_ 160: 636–645. Article PubMed Google Scholar * Grisel JE, Belknap JK, O'Toole LA, Helms ML, Wenger CD, Crabbe JC
(1997). Quantitative trait loci affecting methamphetamine responses in BXD recombinant inbred mouse strains. _J Neurosci_ 17: 745–754. Article CAS PubMed PubMed Central Google Scholar *
Hamidovic A, Dlugos A, Palmer A, de Wit H (2010a). Catechol-O-methyltransferase val158met genotype modulates sustained attention in both the drug-free state and in response to amphetamine.
_Psychiatr Genet_ 20: 85. PubMed PubMed Central Google Scholar * Hamidovic A, Dlugos A, Palmer AA, de Wit H (2010b). Polymorphisms in dopamine transporter (SLC6A3) are associated with
stimulant effects of d-amphetamine: an exploratory pharmacogenetic study using healthy volunteers. _Behav Genet_ 40: 255–261. Article PubMed PubMed Central Google Scholar * Hamidovic A,
Dlugos A, Skol A, Palmer AA, de Wit H (2009). Evaluation of genetic variability in the dopamine receptor D2 in relation to behavioral inhibition and impulsivity/sensation seeking: an
exploratory study with d-amphetamine in healthy participants. _Exp Clin Psychopharmacol_ 17: 374–383. Article CAS PubMed PubMed Central Google Scholar * Hart AB, Engelhardt BE, Wardle
MC, Sokoloff G, Stephens M, de Wit H _et al_ (2012). Genome-wide association study of d-amphetamine response in healthy volunteers identifies putative associations, including cadherin 13
(_CDH13_). _PLoS ONE_ 7: e42646. Article CAS PubMed PubMed Central Google Scholar * Hewitt JK (2012). Editorial policy on candidate gene association and candidate gene-by-environment
interaction studies of complex traits. _Behav Genet_ 42: 1–2. Article PubMed Google Scholar * Ho MK, Goldman D, Heinz A, Kaprio J, Kreek MJ, Li MD _et al_ (2010). Breaking barriers in the
genomics and pharmacogenetics of drug addiction. _Clin Pharmacol Ther_ 88: 779–791. Article CAS PubMed Google Scholar * Hodgkinson CA, Enoch M-A, Srivastava V, Cummins-Oman JS, Ferrier
C, Iarikova P _et al_ (2010). Genome-wide association identifies candidate genes that influence the human electroencephalogram. _Proc Natl Acad Sci USA_ 107: 8695–8700. Article CAS PubMed
PubMed Central Google Scholar * Hodgkinson CA, Yuan Q, Xu K, Shen P-H, Heinz E, Lobos EA _et al_ (2008). Addictions biology: haplotype-based analysis for 130 candidate genes on a single
array. _Alcohol Alcohol_ 43: 505–515. Article CAS PubMed PubMed Central Google Scholar * Hohoff C, Mcdonald JM, Baune BT, Cook EH, Deckert J, de Wit H (2005). Interindividual variation
in anxiety response to amphetamine: possible role for adenosine A 2Areceptor gene variants. _Am J Med Genet_ 139B: 42–44. Article CAS PubMed Google Scholar * Howie BN, Donnelly P,
Marchini J (2009). A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. _Plos Genet_ 5: e1000529. Article PubMed PubMed Central
Google Scholar * Johanson C, Uhlenhuth E (1980). Drug preference and mood in humans: diazepam. _Psychopharmacology (Berl)_ 71: 269–273. Article CAS Google Scholar * Joober R, Grizenko N,
Sengupta S, Amor LB, Schmitz N, Schwartz G _et al_ (2007). Dopamine transporter 3'-UTR VNTR genotype and ADHD: a pharmaco-behavioural genetic study with methylphenidate.
_Neuropsychopharmacology_ 32: 1370–1376. Article CAS PubMed Google Scholar * Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ (2005). Sensitivity to psychostimulants
in mice bred for high and low stimulation to methamphetamine. _Genes Brain Behav_ 4: 110–125. Article CAS PubMed Google Scholar * Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields
PG, Pinto A _et al_ (2006). Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. _Clin Pharmacol Ther_ 79: 600–608. Article CAS PubMed Google Scholar
* Logan G, Cowan W, Davis K (1984). On the ability to inhibit simple and choice reaction time responses: a model and a method. _J Exp Psychol_ 10: 276–291. CAS Google Scholar * Lott D,
Kim S, Cook E, de Wit H (2005). Dopamine transporter gene associated with diminished subjective response to amphetamine. _Neuropsychopharmacology_ 30: 602–609. Article CAS PubMed Google
Scholar * Lott D, Kim S, Cook E, de Wit H (2006). Serotonin transporter genotype and acute subjective response to amphetamine. _Amer J Addiction_ 15: 327–335. Article Google Scholar *
Macgregor S, Lind PA, Bucholz KK, Hansell NK, Madden PAF, Richter MM _et al_ (2009). Associations of ADH and ALDH2 gene variation with self report alcohol reactions, consumption and
dependence: an integrated analysis. _Hum Mol Genet_ 18: 580–593. Article CAS PubMed Google Scholar * Martin W, Sloan J, Sapira J, Jasinski D (1971). Physiologic, subjective, and
behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. _Clin Pharmacol Ther_ 12: 245–258. Article CAS PubMed Google Scholar * Mathieson
I, Munafò MR, Flint J (2012). Meta-analysis indicates that common variants at the DISC1 locus are not associated with schizophrenia. _Mol Psychiatry_ 17: 634–641. Article CAS PubMed
Google Scholar * McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, Ioannidis JPA _et al_ (2008). Genome-wide association studies for complex traits: consensus, uncertainty and
challenges. _Nat Rev Genet_ 9: 356–369. Article CAS PubMed Google Scholar * Munafò MR, Brown SM, Hariri AR (2008). Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a
meta-analysis. _Biol Psychiatry_ 63: 852–857. Article PubMed Google Scholar * Munafò MR, Matheson IJ, Flint J (2007). Association of the DRD2 gene Taq1A polymorphism and alcoholism: a
meta-analysis of case-control studies and evidence of publication bias. _Mol Psychiatry_ 12: 454–461. Article PubMed Google Scholar * Murphy SE, Norbury R, Godlewska BR, Cowen PJ, Mannie
ZM, Harmer CJ _et al_ (2012). The effect of the serotonin transporter polymorphism (5-HTTLPR) on amygdala function: a meta-analysis. _Mol Psychiatry_ (e-pub ahead of print). * Nicolae DL,
Gamazon E, Zhang W, Duan S, Dolan ME, Cox NJ (2010). Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. _Plos Genet_ 6: e1000888. Article PubMed
PubMed Central Google Scholar * Nurnberger J, Gershon ES, Simmons S, Ebert M, Kessler L, Dibble E _et al_ (1982). Behavioral, biochemical and neuroendocrine responses to amphetamine in
normal twins and ‘well-state’ bipolar patients. _Psychoneuroendocrinology_ 7: 163–176. Article CAS PubMed Google Scholar * Patterson N, Price AL, Reich D (2006). Population structure and
eigenanalysis. _Plos Genet_ 2: e190. Article PubMed PubMed Central Google Scholar * Poldrack RA, Mumford JA (2009). Independence in ROI analysis: where is the voodoo? _Soc Cogn Affect
Neurosci_ 4: 208–213. Article PubMed PubMed Central Google Scholar * Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA _et al_ (2011). Genome-wide association study
identifies five new schizophrenia loci. _Nat Genet_ 43: 969–976. Article CAS Google Scholar * Schadt EE, Molony C, Chudin E, Hao K, Yang X, Lum PY _et al_ (2008). Mapping the genetic
architecture of gene expression in human liver. _PLoS Biol_ 6: e107. Article PubMed PubMed Central Google Scholar * Stein MA, Waldman ID, Sarampote CS, Seymour KE, Robb AS, Conlon C _et
al_ (2005). Dopamine transporter genotype and methylphenidate dose response in children with ADHD. _Neuropsychopharmacology_ 1–9. * Sullivan PF (2007). Spurious genetic associations. _Biol
Psychiatry_ 61: 1121–1126. Article CAS PubMed Google Scholar * Veenstra-VanderWeele J, Qaadir A, Palmer AA, Cook EH, de Wit H (2006). Association between the Casein Kinase 1 Epsilon gene
region and subjective response to D-amphetamine. _Neuropsychopharmacology_ 31: 1056–1063. Article CAS PubMed Google Scholar * Wall TL, Shea SH, Luczak SE, Cook TAR, Carr LG (2005).
Genetic associations of alcohol dehydrogenase with alcohol use disorders and endophenotypes in white college students. _J Abnorm Psychol_ 114: 456–465. Article PubMed Google Scholar *
Wechsler D (1958). The measurement and appraisal of adult intelligence. _J Med Educ_ 33: 706. Google Scholar * White TL, Justice AJH, de Wit H (2002). Differential subjective effects of
d-amphetamine by gender, hormone levels and menstrual cycle phase. _Pharmacology, Biochemistry and Behavior_ 73: 729–741. Article CAS PubMed Google Scholar * Zombeck JA, Swearingen SP,
Rhodes JS (2010). Acute locomotor responses to cocaine in adolescents vs adults from four divergent inbred mouse strains. _Genes Brain Behav_ 9: 892–898. Article CAS PubMed PubMed Central
Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by NIH Grants DA007255 (ABH), DA02812 (HdW), and DA021336 and DA024845 (AAP). We thank Barbara E Engelhardt for
providing imputed SNP genotypes and Margaret C Wardle for organization and preprocessing of the phenotype data. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Human Genetics,
University of Chicago, Chicago, IL, USA Amy B Hart & Abraham A Palmer * Department of Psychiatry and Behavioral Neuroscience, University of Chicago, Chicago, IL, USA Harriet de Wit &
Abraham A Palmer Authors * Amy B Hart View author publications You can also search for this author inPubMed Google Scholar * Harriet de Wit View author publications You can also search for
this author inPubMed Google Scholar * Abraham A Palmer View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Abraham A
Palmer. ETHICS DECLARATIONS COMPETING INTERESTS HdW has received a research grant from Unilever for a project unrelated to this study. ABH and AAP declare no potential conflict of interest.
ADDITIONAL INFORMATION Supplementary Information accompanies the paper on the Neuropsychopharmacology website SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE S1 (PDF 400 KB) SUPPLEMENTARY
FIGURE S2 (PDF 363 KB) SUPPLEMENTARY FIGURE S3 (PDF 435 KB) SUPPLEMENTARY FIGURE S4 (PDF 341 KB) SUPPLEMENTARY FIGURE S5 (PDF 427 KB) SUPPLEMENTARY FIGURE S6 (PDF 320 KB) SUPPLEMENTARY
FIGURE S7 (PDF 315 KB) SUPPLEMENTARY FIGURE S8 (PDF 381 KB) SUPPLEMENTARY FIGURE LEGENDS (DOC 31 KB) SUPPLEMENTARY TABLE 1 (XLS 43 KB) SUPPLEMENTARY TABLE 2 (XLS 104 KB) POWERPOINT SLIDES
POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Hart, A., de Wit, H. & Palmer, A. Candidate Gene Studies of a Promising Intermediate Phenotype: Failure to Replicate. _Neuropsychopharmacol_ 38, 802–816 (2013).
https://doi.org/10.1038/npp.2012.245 Download citation * Received: 13 September 2012 * Revised: 07 November 2012 * Accepted: 26 November 2012 * Published: 03 December 2012 * Issue Date:
April 2013 * DOI: https://doi.org/10.1038/npp.2012.245 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * _D_-amphetamine * intermediate phenotype *
candidate gene * genetic association * replication
