Stress-induced reinstatement of drug seeking: 20 years of progress
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT In human addicts, drug relapse and craving are often provoked by stress. Since 1995, this clinical scenario has been studied using a rat model of stress-induced reinstatement of
drug seeking. Here, we first discuss the generality of stress-induced reinstatement to different drugs of abuse, different stressors, and different behavioral procedures. We also discuss
neuropharmacological mechanisms, and brain areas and circuits controlling stress-induced reinstatement of drug seeking. We conclude by discussing results from translational human laboratory
studies and clinical trials that were inspired by results from rat studies on stress-induced reinstatement. Our main conclusions are (1) The phenomenon of stress-induced reinstatement, first
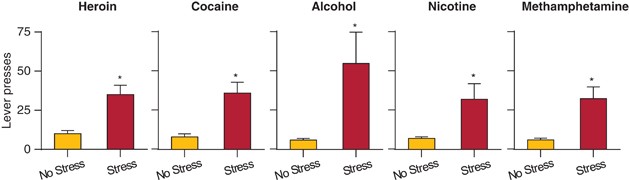
shown with an intermittent footshock stressor in rats trained to self-administer heroin, generalizes to other abused drugs, including cocaine, methamphetamine, nicotine, and alcohol, and is
also observed in the conditioned place preference model in rats and mice. This phenomenon, however, is stressor specific and not all stressors induce reinstatement of drug seeking. (2)
Neuropharmacological studies indicate the involvement of corticotropin-releasing factor (CRF), noradrenaline, dopamine, glutamate, kappa/dynorphin, and several other peptide and
neurotransmitter systems in stress-induced reinstatement. Neuropharmacology and circuitry studies indicate the involvement of CRF and noradrenaline transmission in bed nucleus of stria
terminalis and central amygdala, and dopamine, CRF, kappa/dynorphin, and glutamate transmission in other components of the mesocorticolimbic dopamine system (ventral tegmental area, medial
prefrontal cortex, orbitofrontal cortex, and nucleus accumbens). (3) Translational human laboratory studies and a recent clinical trial study show the efficacy of alpha-2 adrenoceptor
agonists in decreasing stress-induced drug craving and stress-induced initial heroin lapse. SIMILAR CONTENT BEING VIEWED BY OTHERS ROLE OF MESOLIMBIC CANNABINOID RECEPTOR 1 IN STRESS-DRIVEN
INCREASES IN COCAINE SELF-ADMINISTRATION IN MALE RATS Article 15 May 2023 CANNABINOID USE IS ENHANCED BY STRESS AND CHANGES CONDITIONED STRESS RESPONSES Article 10 February 2022 BEHAVIORAL
AND ACCUMBENS SYNAPTIC PLASTICITY INDUCED BY CUES ASSOCIATED WITH RESTRAINT STRESS Article 05 July 2021 INTRODUCTION A central feature of drug addiction is the high rate of relapse during
abstinence (Hunt et al, 1971; O'Brien, 2005). Over the years, results from many correlational human studies have suggested that this relapse is often provoked by stress (Box 1)
(Khantzian, 1985; Shiffman and Wills, 1985; Kosten et al, 1986). These clinical studies have led to the development of a rat model, which is based on the operant conditioning reinstatement
model (Stewart and de Wit, 1987), to study mechanisms of stress-induced relapse to drug seeking (Shaham et al, 2000a). In the reinstatement model, laboratory animals are tested for
reinstatement of drug seeking induced by drug priming, discrete cues, discriminative cues, contextual cues, or stressors, following drug self-administration training (typically lever
pressing or nose poking for drug infusions) and subsequent extinction of the drug-reinforced responding (Shaham et al, 2003; Stewart and de Wit, 1987). The first study using the
reinstatement model was published in 1995 using male and female rats that were trained to self-administer heroin for 12 days for 12 h/day. In this study, acute exposure to 10 min of
intermittent footshock stress immediately before the test sessions reinstated heroin seeking after 1–2 weeks of extinction training and after an additional 4- to 6-week drug-free period
(Shaham and Stewart, 1995) (Figure 1). In this review, we summarize results from studies published during the last 20 years in which investigators have used different variations of the
stress-induced reinstatement model to study behavioral, neuropharmacological, and neuroanatomical mechanisms of stress-induced reinstatement of drug seeking. We also summarize results from
human studies that have determined the degree to which the results from the preclinical studies ‘translate’ to the human condition. The material in our review overlaps to some degree with
previous reviews (Bossert et al, 2005, 2013; Lu et al, 2003; Mantsch et al, 2014; Shaham et al, 2003; Shalev et al, 2010, 2002; Sinha et al, 2011b), but provides the only comprehensive
review uniquely dedicated to the phenomenon of stress-induced reinstatement across drug classes since an early review in 2000 (Shaham et al, 2000a). Since 1995, over 200 papers have been
published on this phenomenon (PubMed, Figure 2). Due to space limitations, we do not cover all published papers on this topic in our review. BOX 1. THE STRESS CONCEPT Stress is a complex
psychological construct that despite many decades of research (Cannon, 1935; Selye, 1936) lacks a singular operational definition (Chrousos and Gold, 1992; Cohen et al, 1982). In animal
models of psychiatric disorders, stress can be broadly defined as forced exposure to events or conditions that are normally avoided (Piazza and Le Moal, 1998). In humans, the stress
definition is often extended to incorporate cognitive and emotional responses as suggested by Cohen et al (1986): ‘stress is a condition in which the environmental demands exceed the coping
abilities of the individual.’ In laboratory animals, the precipitating events or conditions can be divided into two main categories (Lu et al, 2003). The first includes aversive conditions
like restraint, footshock, tail pinch, social defeat, or pharmacological stressors like exogenous CRF that the rat would avoid if given the opportunity. The second includes conditions like
food deprivation, social isolation, or maternal deprivation; each consists of the removal of an environmental condition that is important for maintaining the animal’s normal steady-state
conditions, a state that the subject will attempt to ameliorate by seeking food, conspecific partners, or the dam. GENERALITY OF STRESS-INDUCED REINSTATEMENT GENERALITY TO OTHER DRUG AND
NON-DRUG REWARDS Early studies showed that the effect of intermittent footshock on reinstatement of heroin seeking generalized to male rats with a history of cocaine (Erb et al, 1996),
alcohol (Le et al, 1998), nicotine (Buczek et al, 1999), and methamphetamine (Shepard et al, 2004) self-administration (Figure 1). In contrast, several early studies showed that exposure to
an intermittent footshock stressor that reinstates drug seeking (Shaham et al, 2000a) does not reinstate food reward seeking in male rats trained to self-administer food pellets (Ahmed and
Koob, 1997), sucrose pellets (Le et al, 1998; Mantsch and Goeders, 1999), or sucrose solution (Buczek et al, 1999). However, more recently, Chen et al (2014b) showed that intermittent
footshock modestly reinstates palatable food (high carbohydrate pellets) seeking in female rats with a history of ‘cafeteria diet’ consumption during adolescence. There is also evidence that
intermittent footshock stress reinstates operant responding previously reinforced by brain stimulation reward (Shalev et al, 2000); this finding is in agreement with results from an early
study (Deutch and Howarth, 1962). In the studies described above, and in others reviewed below, the intermittent footshock stressor and other stressors (with the exception of 1 day food
deprivation) were administered acutely just before the reinstatement test sessions. The reasons for the ‘selective’ effect of intermittent footshock stress on reinstatement of drug _vs_ food
reward are unknown. As previously discussed, one possibility is that while certain physiological effects of intermittent footshock promote reward seeking, other effects of footshock induce
physiological states that inhibit food intake and seeking (Shaham et al, 2000a). In this regard, one potential mechanism is hypothalamic corticotropin releasing factor (CRF), which is
released after exposure to footshock and other stressors (Bale and Vale, 2004; Sawchenko et al, 2000), and causes inhibition of food intake (Glowa et al, 1992; Morley and Levine, 1982;
Sekino et al, 2004). As discussed below (section ‘Brain areas and circuits of stress-induced reinstatement’), this classical effect of CRF on the hypothalamic-pituitary adrenal (HPA) axis
(Dallman et al, 1995) contrasts with CRF actions on extrahypothalamic systems, which promote stress-induced reinstatement of drug seeking (Erb et al, 2001b). It is also possible that the
‘putative’ selective effect of intermittent footshock on reinstatement of drug _vs_ food seeking is due to the fact that the above negative studies with food were performed under
experimental conditions in which footshock stress-induced reinstatement is often not observed with drugs like cocaine (eg, limited access daily sessions) (see section ‘Methodological
considerations’). GENERALITY TO OTHER BEHAVIORAL PROCEDURES In the studies described in section ‘Generality to other drug and non-drug rewards’, footshock stress-induced reinstatement was
determined using the operant version of the extinction-reinstatement model (Shaham et al, 2003). The effect of this stressor was also demonstrated in the conditioned place preference (CPP)
variation of the model using cocaine (Wang et al, 2000). In the CPP reinstatement model, laboratory animals are trained to associate a distinct environment with drug injections, undergo
extinction training during which they are exposed to the drug environment in the absence of drug, and then tested for reinstatement of drug CPP after stress exposure (Sanchez and Sorg, 2001;
Wang et al, 2000). There is also evidence that intermittent footshock can re-establish morphine or cocaine CPP under experimental conditions in which the drug CPP is no longer observed
after the passage of time without extinction training (Lu et al, 2000, 2001). Reinstatement of drug seeking after extinction has also been assessed in the runway operant model (Ettenberg,
2009; Ettenberg et al, 1996). To date, however, we are not aware of published studies on the effect of intermittent footshock or other stressors on reinstatement of drug seeking in this
model. GENERALITY TO OTHER STRESSORS Over the years, investigators have determined whether stressors other than intermittent footshock reinstate operant drug seeking or drug CPP. We discuss
these results separately for the self-administration and CPP variations of the reinstatement model. Table 1 provides a summary of the text in section ‘Generality to other stressors’.
STRESS-INDUCED REINSTATEMENT OF DRUG SELF-ADMINISTRATION Acute 1-day food deprivation reinstates heroin and cocaine seeking (Shalev et al, 2000, 2003); these results are in agreement with an
earlier finding that food restriction (about 30–40% of daily food ration) reinstates cocaine seeking in rats with a history of exposure to this stressor during the training phase (Carroll,
1985). Acute 1-day food deprivation also reliably reinstates cocaine seeking in mice (Highfield et al, 2002). Exposure to cues previously paired with social defeat stress, but not acute
exposure to social defeat, modestly reinstates alcohol seeking (Funk et al, 2005). Conrad et al (2010) showed that acute exposure (4–5 min) to cold swim stress 24 h before the reinstatement
tests reinstates cocaine seeking. Most recently, Twining et al (2015) showed that acute intraoral delivery of an aversive bitter quinine solution reinstates cocaine seeking. Pharmacological
induction of a stress-like state by ventricular CRF injections reinstates heroin (Shaham et al, 1997), alcohol (Le et al, 2002), and cocaine (Erb et al, 2006) seeking. Similarly, systemic
injections of the alpha-2 adrenoceptor antagonist yohimbine, which induces stress- and anxiety-like responses in humans and laboratory animals (Bremner et al, 1996a and b), reinstate
methamphetamine (Shepard et al, 2004), alcohol (Le et al, 2005), cocaine (Feltenstein and See, 2006), and nicotine (Feltenstein et al, 2012) seeking in rats, and cocaine seeking in monkeys
(Lee et al, 2004). However, the relevance of the potent effect of yohimbine on reinstatement of drug seeking to the general phenomenon of stress-induced reinstatement has been questioned in
a recent study (Chen et al, 2014c) (see Box 2). Another class of putative pharmacological stressors is kappa opioid receptor (KOR) agonists, which induce stress-like states in humans and
laboratory animals (Bruchas et al, 2010; Van't Veer and Carlezon, 2013). In rats, systemic injections of the KOR agonist U50,488 reinstate nicotine and alcohol seeking (Funk et al,
2014; Grella et al, 2014). In monkeys, systemic injections of KOR agonists (spiradoline and enadoline) reinstate cocaine seeking (Valdez et al, 2007). Another potential demonstration of the
effect of pharmacological stressors on reinstatement is the finding that metyrapone potently reinstates heroin seeking (Shaham et al, 1997). Metyrapone is a corticosterone synthesis
inhibitor (Jenkins et al, 1958) that has been used in numerous endocrinology studies (Haynes, 1990). However, the drug also activates extrahypothalamic brain areas, including the central
amygdala (CeA) nucleus (Rotllant et al, 2002), which has an important role in stress-induced reinstatement (section ‘Brain areas and circuits of stress-induced reinstatement’). Finally,
another class of pharmacological stressors, GABAa antagonists or inverse receptor agonists like pentylenetetrazole or FG7142, had no effect on reinstatement of cocaine (Waters and See,
2011), heroin, or alcohol (Y Shaham, AD Le, unpublished observations) seeking. One study showed that a cue previously paired with footshock (a conditioned fear manipulation) reinstates
alcohol seeking (Liu and Weiss, 2003). However, earlier studies reported that conditioned fear exposure has no effect on reinstatement of heroin or cocaine seeking (Shaham et al, 2000a). A
possible reason for the negative results is that the predominant effect of cues paired with shock is freezing (LeDoux, 2000), a behavior that is incompatible with lever-pressing behavior.
Exposure to restraint stress in a novel context had no effect on reinstatement of heroin seeking (Shalev et al, 2000). As intermittent footshock stress is effective when given in the drug
self-administration context, but has no effect on reinstatement of heroin seeking when given in a novel context, a potential account for the negative results with restraint stress is that
the phenomenon of stress-induced reinstatement is context-specific (Shalev et al, 2000). Finally, the effect of footshock on reinstatement of heroin seeking is mimicked by reversible
inactivation of the medial septum (Highfield et al, 2000). Previous studies have shown that septal lesions cause stress-like physiological and psychological responses (Gray, 1987; Holdstock,
1967). STRESS-INDUCED REINSTATEMENT OF CPP Exposure to several stressors induces reinstatement of drug CPP after extinction in rats and mice. Swim stress reinstates cocaine CPP in mice
(Carey et al, 2007; Kreibich and Blendy, 2004; Redila and Chavkin, 2008) and morphine CPP in rats (Li et al, 2013; Ma et al, 2007). Restraint stress reinstates cocaine CPP in rats (Sanchez
et al, 2003) and morphine CPP in mice (Ribeiro Do Couto et al, 2006). Tail pinch and social defeat reinstate morphine CPP in mice (Ribeiro Do Couto et al, 2006). A conditioned fear
manipulation reinstates cocaine CPP in rats (Sanchez and Sorg, 2001). Additionally, as in the operant reinstatement model, systemic injections of the putative pharmacological stressors
U50,488 (a KOR agonist) (Redila and Chavkin, 2008) and yohimbine (Mantsch et al, 2010) reinstate cocaine CPP in mice. However, 1-day food deprivation, which reinstates operant heroin seeking
in rats (Shalev et al, 2000), has no effect on reinstatement of morphine CPP (Ma et al, 2007). METHODOLOGICAL CONSIDERATIONS In the sections above, we summarize results from studies in
which stressors reinstate drug seeking and CPP. However, particularly in the case of the intermittent footshock stressor, whether or not the stressor reinstates operant drug seeking is
dependent on several critical methodological factors. One factor is the context of footshock exposure: under identical experimental conditions, intermittent footshock reinstates heroin
seeking when it is administered in the drug self-administration context but not in a novel context (Shalev et al, 2000). These findings are in agreement with results from conditioned fear
studies where a footshock previously paired with a conditioned stimulus (CS) reinstates the response to the CS after extinction only when rats are exposed to the stressor in the training
context, but not in a novel context (Bouton and King, 1983; Bouton and Swartzentruber, 1991). A second factor is the duration of the withdrawal period. Shalev et al (2001) used a variation
of the reinstatement model in which extinction of operant responding and subsequent footshock-induced reinstatement is determined in the same daily session (Shalev et al, 2002) to
demonstrate the time-dependent changes in this reinstatement after withdrawal from heroin. The stressor reinstated heroin seeking after 6, 12, 25, and 66 withdrawal days but not after 1 day.
In this study, the magnitude of stress-induced reinstatement was maximal after 6 or 12 days. A third factor is the amount of drug intake during drug self-administration training. For both
heroin (Ahmed et al, 2000) and cocaine (Mantsch et al, 2008), intermittent footshock-induced reinstatement of drug seeking is higher in magnitude and more robust/reliable in rats with a
history of extended daily drug access (6–11 h/day) than in rats with a history of limited daily drug access (1–2 h/day). A fourth factor is the availability of discrete infusion cues during
all phases of the experiment (training, extinction, and reinstatement). Shelton and Beardsley (2005) showed that under identical experimental conditions, intermittent footshock reinstates
cocaine seeking in the presence but not in the absence of a discrete infusion cue previously paired with cocaine infusions during training. This finding is in agreement with the notion that
a potential mechanism for footshock-induced reinstatement is that the stressor reinstates the incentive motivational effects of drug cues (Shaham and Stewart, 1995). Additional support for
this notion is that intermittent footshock potentiates cue-induced reinstatement of cocaine and alcohol seeking (Buffalari and See, 2009; Liu and Weiss, 2002a). In the cue-induced
reinstatement procedure, an operant response reinforced by a discrete drug cue is determined after drug self-administration training in the presence of the cue and extinction training in its
absence (Meil and See, 1996). A fifth factor is baseline stress levels before footshock exposure during the reinstatement tests. In general, while this variable has not been experimentally
manipulated like the other factors described above, anecdotal evidence suggests that footshock-induced reinstatement is not readily observed when the baseline stress level is high, because
under these conditions the predominant effect of the stressor during the reinstatement test is prolonged freezing. This idea has led to the development of a modified stress-induced
reinstatement procedure in which rats are first given several days of extinction training, and during the subsequent reinstatement test they are given several hours of extinction training
before footshock exposure (Erb et al, 1996; Kupferschmidt et al, 2011). Presumably, intermittent footshock reliably reinstates drug seeking in this procedure because the rat’s basal stress
level is relatively low several hours after being exposed to non-specific stressors (eg, handling and transferred from the animal facility to the test chambers). SUMMARY The phenomenon of
intermittent footshock stress-induced reinstatement of drug seeking, originally reported with heroin-trained rats, generalizes to other drugs of abuse and is also observed in the CPP
variation of the reinstatement model. This phenomenon also generalizes to certain environmental and pharmacological stressors but not others. Additionally, it appears that the number of
stressors that are effective in the CPP variation of the reinstatement model is more diverse than in the operant variation of the model (Table 1). Furthermore, several methodological factors
are critical for a reliable effect of intermittent footshock stress on reinstatement of drug seeking. These factors include the context of footshock exposure, high drug intake during
training, the withdrawal period duration, the presence of the extinguished discrete drug cues during testing, and basal (baseline) stress levels before footshock exposure in the
reinstatement tests. Finally, in all the published studies on stress-induced reinstatement, the reinstating stressors have been both uncontrollable and unpredictable. Thus, a question for
future research is whether these features of stressors are critical for their effect on reinstatement of drug seeking. Based on previous studies investigating the inhibitory effect of both
stress controllability and predictability on physiological and psychological stress responses in laboratory animals (Maier, 1984; Seligman and Maier, 1967) and humans (Cohen et al, 1986;
Glass and Singer, 1972), we predict that controllable or predictable stressors will have a weak effect on reinstatement of drug seeking. Additionally, in the studies reviewed above, the
stressors were administered acutely before the reinstatement test session. Thus, another question for future research is whether prior exposure to stress, which often leads to subsequent
sensitization of stress responses (Antelman et al, 2000; Kvetnansky et al, 2009) and cross sensitization of the behavioral and neurochemical responses to drugs (Kalivas and Stewart, 1991),
would potentiate the stressors’ effect on reinstatement of drug seeking. BRAIN AREAS AND CIRCUITS OF STRESS-INDUCED REINSTATEMENT Since 1995 many studies have determined the effects of
systemic and intracranial injections of pharmacological agents targeting different receptor classes on stress-induced reinstatement of drug seeking or CPP. Due to space limitations, we
summarize most of the systemic pharmacology data in Tables 2 and 3 but do not discuss them in detail in our review. The brain circuitry data that we discuss below are primarily based on the
use of intermittent footshock in the self-administration- and CPP-based reinstatement models with different drug classes in rats, and the use of swim stress in the CPP-based procedure with
cocaine in mice. During the last several years, many investigators have also determined the neuropharmacological mechanisms underlying yohimbine-induced reinstatement of drug seeking and CPP
(Bossert et al, 2013; Mantsch et al, 2014; See and Waters, 2010). However, as discussed in Box 2, yohimbine’s effects on operant responding during reinstatement testing are likely
independent of the history of contingent drug self-administration and may not be related to the commonly assumed stress-like effects of yohimbine. Therefore, we only mention results from
yohimbine-related studies when a given neuropharmacological manipulation affected both intermittent footshock- and yohimbine-induced reinstatement. BED NUCLEUS OF STRIA TERMINALIS AND CEA
The bed nucleus of stria terminalis (BNST) and the CeA are known to have important roles in stress responses (Davis et al, 2010; LeDoux, 2000) that involve activation of noradrenaline and
CRF systems (Davis et al, 1997; Flavin and Winder, 2013; McGaugh, 2004; Silberman and Winder, 2013). Studies using systemic or ventricular drug injections have shown that CRF receptor
antagonists and alpha-2 adrenoceptor agonists like clonidine (which inhibit noradrenaline cell firing and release) decrease stress-induced reinstatement of drug seeking and drug CPP (Mantsch
et al, 2014; Shaham et al, 2000a; Shalev et al, 2010) (Tables 2 and 3). Conversely, ventricular injections of CRF or noradrenaline mimic the effect of intermittent footshock stress on
reinstatement (Brown et al, 2009; Erb et al, 2006; Le et al, 2000; Shaham et al, 1997). Studies using endocrinology methods (adrenalectomy, corticosterone replacement) have also shown that
CRF’s role in footshock-induced reinstatement does not involve stress-induced activation of the HPA axis, indicating a role of extrahypothalamic CRF systems (Erb et al, 2001a; Lu et al,
2003). Based on these results, investigators have studied the involvement of CRF and noradrenaline in BNST and CeA in stress-induced reinstatement and CPP. We describe these studies below.
(Note: in Box 3 we discuss in more detail the role of corticosterone and the HPA axis in stress-induced reinstatement.) THE CRF PROJECTIONS FROM CEA TO BNST Erb and Stewart (1999) showed
that injections of the CRF receptor antagonist D-Phe CRF12–41 into the BNST (ventral region) but not CeA decrease footshock-induced reinstatement of cocaine seeking. They also showed that
CRF injections into the BNST but not CeA reinstate cocaine seeking; this finding was confirmed by Vranjkovic et al (2014). In agreement with these findings, Wang et al (2006) showed that
BNST but not CeA injections of the selective CRF1 receptor antagonist CP-154,526 (Schulz et al, 1996) decrease footshock-induced reinstatement of morphine CPP. However, while blockade or
activation of CeA CRF receptors had no effect on footshock-induced reinstatement, Erb et al (2001a) reported data suggesting that a CRF projection from CeA to BNST (Sakanaka et al, 1986) has
a role in this reinstatement. They used an asymmetric anatomical disconnection method (Gold, 1966) and showed that injections of tetrototoxin (TTX, a sodium channel blocker that inhibits
neuronal activity) into the CeA in one hemisphere and D-Phe CRF12–41 into the BNST in the contralateral hemisphere decrease footshock-induced reinstatement of cocaine seeking. Taken
together, these results suggest that stress-induced activation of CRF projections from CeA to BNST and subsequent activation of CRF receptors (presumably the CRF1 subtype) in the BNST are
critical for stress-induced reinstatement of drug seeking and CPP. However, the circuitry (projection) data are based on the use of TTX, which also inhibits fiber of passage and non-CRF
neurons that project to BNST (Cassell et al, 1999). Therefore, it is likely that within the BNST, CRF-related mechanisms that are independent of the CeA to BNST CRF projection also have a
role in stress-induced reinstatement. NORADRENALINE IN CEA AND BNST AND INTERACTION BETWEEN NORADRENALINE AND CRF IN BNST Leri et al (2002) showed that injections of a mixture of beta-1 and
beta-2 adrenoceptor antagonists (betaxolol+ICI-118,551) into both the BNST (the injections were in both dorsal and ventral BNST) and the CeA decrease footshock-induced reinstatement of
cocaine seeking. Subsequently, Vranjkovic et al (2014) showed that within the ventral BNST, this effect is mediated by the beta-2 adrenoceptor: local ICI-118,551 but not betaxolol injections
decrease stress-induced reinstatement. Additionally, they showed that ventral BNST injections of the beta-2 adrenoceptor agonist clenbuterol, but not the beta-1 adrenoceptor agonist
dobutamine, mimic the effect of footshock on reinstatement. Additional evidence for a role of BNST and CeA noradrenaline in stress-induced reinstatement is that BNST and CeA injections of
clonidine decrease footshock-induced reinstatement of morphine CPP (Wang et al, 2001) and footshock-induced reinstatement of nicotine seeking (Yamada and Bruijnzeel, 2011). These data,
however, are difficult to interpret in the absence of additional anatomical and pharmacological controls, because clonidine is highly lipophilic and it can readily diffuse to the nearby
areas, or in the case of the BNST, to the ventricles. Finally, based on the earlier results of Leri et al (2002), an unexpected finding of Yamada and Bruijnzeel (2011) is that CeA injections
of the non-selective beta adrenoceptor antagonist propranolol have no effect on footshock-induced reinstatement of nicotine seeking. Ascending noradrenaline systems originate from two
clusters of neurons, the locus coeruleus (LC, A6) and the lateral tegmental nuclei (A1, A2 (located in nucleus tractus solitarii, NTS), A4); these neurons project to many brain areas _via_
the dorsal and ventral noradrenergic bundles, respectively (Kvetnansky et al, 2009; Moore and Bloom, 1979). The lateral tegmental nuclei, which provide the main source of noradrenaline to
BNST and CeA (Aston-Jones et al, 1999; Fritschy and Grzanna, 1991), but not the LC, are critical for stress-induced reinstatement of drug seeking and CPP. 6-OHDA lesions of the ventral
noradrenergic bundle decrease footshock-induced reinstatement of heroin seeking (Shaham et al, 2000b) and morphine CPP (Wang et al, 2001). In contrast, injections of clonidine or its charged
analog ST-91 into the LC have no effect on this reinstatement (Shaham et al, 2000b; Wang et al, 2001). Finally, there is evidence that an interaction between CRF and noradrenaline in the
BNST is critical for footshock-induced reinstatement of cocaine seeking. In an initial study, Brown et al (2009) showed that reinstatement of cocaine seeking induced by ventricular
injections of noradrenaline is decreased by pretreatment with the CRF receptor antagonist D-Phe CRF12–41. In contrast, reinstatement induced by ventricular injections of CRF is not blocked
by the alpha-2 adrenoceptor agonist clonidine. These data suggest that the role of CRF in stress-induced reinstatement is downstream from stress-induced activation of noradrenaline systems.
The likely brain site for this noradrenaline-CRF interaction is the BNST. Vranjkovic et al (2014) showed that reinstatement of cocaine seeking induced by ventral BNST injections of the
beta-2 adrenoceptor agonist clenbuterol is blocked by local injections of the CRF1 receptor antagonist antalarmin (Bornstein et al, 1998). Taken together, stress-induced activation of
lateral tegmental noradrenergic nuclei and subsequent actions of noradrenaline in both the BNST and CeA are critical for stress-induced reinstatement of drug seeking and CPP. Additionally,
within the ventral BNST, stress-induced activation of CRF and subsequent stress-induced reinstatement are downstream from stress-induced activation of lateral tegmental noradrenergic nuclei
that project to this brain area. OTHER EVIDENCE FOR A ROLE OF BNST AND CEA IN STRESS-INDUCED REINSTATEMENT Several other lines of evidence also indicate critical roles for the BNST and CeA
in stress-induced reinstatement of drug seeking and CPP. Reversible inactivation of ventral BNST or CeA with GABAa and GABAb receptor agonists (muscimol+baclofen) decreases footshock-induced
reinstatement of cocaine seeking (McFarland et al, 2004). Additionally, TTX injections into either the BNST or CeA decrease footshock-induced reinstatement of heroin seeking (Shaham et al,
2000a) and this reinstatement is associated with increased CRF mRNA in both brain areas (Shalev et al, 2001). However, both sets of data should be interpreted with caution. As mentioned
above, a methodological issue with TTX inactivation data is that the drug inactivates both cell bodies and fibers of passage. Additionally, the relevance of the CRF mRNA expression data to
CRF’s role in stress-induced reinstatement is unclear, because within the BNST footshock selectively increases expression in dorsal but not in ventral BNST, while the pharmacological data
described above are primarily based on ventral BNST injections. Other relevant evidence is that systemic injections of the beta-2 adrenoceptor antagonist ICI-118,551 decrease swim
stress-induced reinstatement of cocaine CPP and prevent increases in CRF mRNA in BNST (but not CeA) in mice (McReynolds et al, 2014). However, under somewhat different experimental
conditions, exposure to swim stress increases CRF mRNA in CeA in cocaine-experienced mice (Cleck et al, 2008). In agreement with the latter findings, Nawata et al (2012) showed that a CRF
receptor antagonist (alpha-helical-CRF9-41) decreases footshock-induced reinstatement of methamphetamine seeking in rats; additionally, using enzyme-linked immunosorbent assay (ELISA) they
showed that CRF protein levels in CeA are increased after 10 but not 5 days of withdrawal from methamphetamine. The relevance of the ELISA data to the pharmacological data with the CRF
receptor antagonist is unknown. Finally, studies using markers of neuronal activity such as Fos and pCREB (a downstream target of Fos activation) showed that swim stress-induced
reinstatement of cocaine CPP in mice is associated with increased CREB activity (phosphorylation) in amygdala but not in BNST (Kreibich and Blendy, 2004). In a subsequent study, however,
Briand et al (2010) showed that swim stress increases Fos expression in both ventral and dorsal BNST and that reversible inactivation of the BNST with lidocaine decreases swim stress-induced
reinstatement of cocaine CPP. Additionally, Buffalari and See (2011) showed that reversible inactivation with muscimol+baclofen of the BNST decreases yohimbine-induced reinstatement of
cocaine seeking and yohimbine-induced potentiation of cue-induced cocaine seeking. SUMMARY The studies reviewed above indicate that the BNST (primarily the ventral BNST) and CeA have
important roles in stress-induced reinstatement of drug seeking across drug classes and are also involved in stress-induced reinstatement of morphine and cocaine CPP. These roles include
stress-induced activation of the lateral tegmental nuclei that provide the main noradrenergic innervation to BNST and CeA, and potentially the CRF projection from CeA to BNST. Within the
BNST, stress-induced CRF activation and subsequent stress-induced reinstatement are likely downstream from stress-induced activation of lateral tegmental noradrenaline neurons. VENTRAL
TEGMENTAL AREA, MEDIA PREFRONTAL CORTEX, ORBITAL FRONTAL CORTEX, AND NUCLEUS ACCUMBENS Neuronal activity in the mesocorticolimbic dopamine system, which comprises cell bodies in ventral
tegmental area (VTA) that project to medial prefrontal cortex (mPFC), orbital frontal cortex (OFC), nucleus accumbens (NAc), amygdala, BNST, and other brain areas, has an important role in
reinstatement of drug seeking induced by discrete cues (See, 2005), discriminative cues (Weiss, 2005), contextual cues (Marchant et al, 2014), and drug priming (Kalivas and McFarland, 2003;
Pierce and Kumaresan, 2006; Self, 2004). Early studies have shown that different stressors strongly and preferentially activate the dopaminergic projection from VTA to mPFC (the mesocortical
dopamine system) (Bannon and Roth, 1983; Deutch and Roth, 1990; Thierry et al, 1976). Subsequent studies, however, showed that stressors can also activate the dopaminergic projection from
VTA to NAc (a component of the mesolimbic dopamine system) (Cabib and Imperato, 1996; Kalivas and Stewart, 1991). Based on these findings, the first two studies on intermittent
footshock-induced reinstatement of drug seeking explored the role of mesolimbic dopamine. In the first study, Shaham and Stewart (1995) showed that footshock-induced reinstatement of heroin
seeking is associated with increased dopamine release in NAc. In the second study, they showed that systemic injections of the mixed dopamine receptor antagonist flupenthixol decrease this
reinstatement (Shaham and Stewart, 1996). In a subsequent anatomical mapping study, McFarland et al (2004) showed that reversible inactivation with muscimol+baclofen of VTA, dorsal mPFC, NAc
core and shell, and ventral pallidum (a downstream output of NAc _via_ the ‘indirect’ pathway) decreases footshock-induced reinstatement of cocaine seeking. Below, we describe subsequent
mechanistic studies on the role of different brain areas of the mesocorticolimbic system in stress-induced reinstatement of drug seeking and CPP. GLUTAMATE, CRF, AND KAPPA/DYNORPHIN IN VTA
Glutamate input to VTA arrives from several brain regions (Geisler et al, 2007; Omelchenko and Sesack, 2007) and controls the activity of VTA dopamine neurons under basal conditions
(Westerink et al, 1996; White et al, 1995) and after stress exposure (Enrico et al, 1998; Takahata and Moghaddam, 1998). Based on these studies, Wise and colleagues studied the role of VTA
glutamate in stress-induced reinstatement of cocaine and heroin seeking. They showed that footshock-induced reinstatement of both heroin and cocaine seeking is associated with increased VTA
glutamate release and that this reinstatement is decreased by local blockade of ionotropic glutamate receptors with kynurenic acid (Wang et al, 2005, 2012). VTA dopamine and glutamate
transmission is also controlled by local CRF signaling (Kalivas et al, 1987; Ungless et al, 2003) from unspecified sources and, as discussed above, CRF has a critical role in stress-induced
reinstatement of drug seeking and CPP. Based on these findings, Wise and colleagues studied the role of VTA CRF in stress-induced reinstatement of cocaine seeking. In the first study, they
showed that VTA perfusion (_via_ a microdialysis probe) of alpha-helical CRF9–41 decreases footshock-induced reinstatement and that local CRF perfusion reinstates cocaine seeking (Wang et
al, 2005); see also Wang et al (2009) for a replication of the VTA CRF effect on reinstatement. Next, they showed that footshock-induced reinstatement of cocaine seeking is decreased by VTA
perfusion of a selective CRF2 receptor antagonist or an inhibitor of the CRF binding protein but not by a selective CRF1 receptor antagonist. Moreover, they showed that VTA perfusion of CRF
or CRF2 receptor agonists with strong affinity for CRF-BP (binding protein) causes reinstatement of cocaine seeking, whereas CRF receptor agonists that do not bind CRF-BP are ineffective
(Wang et al, 2007). These are surprising findings from two perspectives. The first is that previous studies discussed above (see also Tables 2 and 3) implicate CRF1 but not CRF2 receptors in
stress-induced reinstatement. The second is that anatomical studies indicate that CRF1 receptors are the predominant postsynaptic CRF receptors in VTA and that CRF2 receptor expression is
not detected by traditional _in situ_ hybridization methods (Van Pett et al, 2000). Another issue to consider in studies using putative selective CRF2 antagonists is that their CRF2/CRF1
ratio selectivity is moderate, and some commonly used CRF2 antagonists like antisauvagine-30 have behavioral effects in CRF2 knockout mice, indicating actions on other receptors (Zorrilla et
al, 2013b). More recent studies demonstrated a role of CRF1 receptors in stress-induced reinstatement of cocaine seeking. Blacktop et al (2011) showed that VTA injections of the CRF1
receptor antagonists antalarmin or CP-376395 but not the CRF2 receptor antagonists astressin-2B or antisauvagine-30 decrease footshock-induced reinstatement of cocaine seeking in rats.
Additionally, VTA injections of the CRF1 receptor agonist cortagine but not the CRF2 receptor agonist rat urocortin II mimic the effect of footshock stress on reinstatement. Chen et al
(2014a) showed that local viral-mediated knockdown of VTA CRF1 receptors decreases food deprivation stress-induced reinstatement of cocaine seeking in mice. Vranjkovic et al (2014) also
showed that injections of the beta-2 adrenoceptor antagonist ICI-188,551 into the BNST in one hemisphere and the CRF1 receptor antagonist antalarmin into the VTA of the contralateral
hemisphere decrease footshock-induced reinstatement of cocaine seeking. These data suggest a potential role of the CRF projection from BNST to VTA (Rodaros et al, 2007), which is activated
by noradrenergic projections to BNST (Aston-Jones et al, 1999), in stress-induced reinstatement of cocaine seeking. In this regard, the BNST projection to VTA is also activated during swim
stress-induced reinstatement of cocaine CPP in mice, as assessed by double labeling of the activity marker Fos with the retrograde tracer fluorogold gold (injected in VTA) in ventral BNST
(Briand et al, 2010). Two recent studies by Kauer, Pierce and colleagues demonstrate a role of kappa/dynorphin signaling in VTA in cold swim stress-induced reinstatement of cocaine seeking
in rats (Graziane et al, 2013; Polter et al, 2014). In this stress reinstatement procedure, short (4–5 min) exposure to cold swim reinstates cocaine seeking 24 h later (Conrad et al, 2010).
These studies were inspired by earlier studies on the regulation of VTA dopamine transmission by local kappa/dynorphin signaling (Ford et al, 2007; Margolis et al, 2003) and the findings
that systemic injections of KOR antagonists decrease stress-induced drug seeking in rats and stress-induced reinstatement of drug CPP in mice (Tables 2 and 3) (Beardsley et al, 2005; Redila
and Chavkin, 2008). In the first study, Graziane et al (2013) showed that systemic injections of the KOR antagonist Nor-BNI rescue swim stress-induced abolishment of long-term potentiation
(LTP) at GABAergic synapses on VTA dopaminergic neurons. Next, they showed that VTA injections of Nor-BNI decrease stress-induced reinstatement of cocaine seeking. In the second study,
Polter et al (2014) showed that acute exposure to swim stress blocks normal LTP at GABAergic synapses on VTA dopaminergic neurons for up to 5 days, an effect dependent on KOR, and that
surprisingly, systemic injections of Nor-BNI several hours _after_ swim stress exposure also decrease the stressor’s effect on reinstatement 24 h later. SUMMARY The studies reviewed above
indicate that footshock stress-induced activation of CRF and glutamate transmission in VTA is critical for the stressor’s effect on reinstatement of drug seeking. A recent study by
Vranjkovic et al (2014) further suggests that glutamatergic (Georges and Aston-Jones, 2002) and/or CRF (Rodaros et al, 2007) projections from BNST to VTA are the source of this activation.
There is also evidence that KOR-mediated stress-induced alterations in GABAergic transmission in VTA have a critical role in the effect of cold swim stress on reinstatement of cocaine
seeking. DOPAMINE AND GLUTAMATE IN NAC, MPFC, AND OFC Studies using intracranial injections of different dopamine receptor antagonists have demonstrated an important role of dopamine
transmission in dorsal mPFC (prelimbic area) and OFC in stress-induced reinstatement of cocaine seeking. Capriles et al (2003) showed that dorsal (but not ventral or infralimbic area) mPFC
and OFC injections of the D1-family receptor antagonist SCH23390 but not the D2-family receptor antagonist raclopride decrease footshock-induced reinstatement of cocaine seeking. McFarland
et al (2004) showed that dorsal mPFC injections of the mixed dopamine receptor antagonist flupenthixol decrease this reinstatement. Sanchez et al (2003) showed that SCH23390 injections into
dorsal mPFC decrease restraint stress-induced reinstatement of cocaine CPP. Dorsal mPFC dopamine and neuronal activity in this brain area also have a role in yohimbine-induced reinstatement
of palatable food seeking in male and female rats (Calu et al, 2013; Cifani et al, 2012; Nair et al, 2011) and acute food deprivation-induced reinstatement of heroin seeking (Tobin et al,
2013). As VTA dopamine neurons project to mPFC and OFC (Moore and Bloom, 1979), the results from the studies reviewed above indicate that stress-induced activation of the dopaminergic
projections from VTA to mPFC (Thierry et al, 1976) and OFC is critical for stress-induced reinstatement. This activation involves D1-family receptors which, in the mPFC, are expressed on
pyramidal cells where they promote excitability primarily by increasing NMDA receptor-mediated effects (Lewis and O'Donnell, 2000; Seamans et al, 2001). McFarland et al (2004) provided
additional evidence that the circuit controlling footshock-induced reinstatement of cocaine seeking includes the dopaminergic projection from VTA to dorsal mPFC (prelimbic area), and also
provided evidence that the glutamatergic projection from dorsal mPFC to NAc core (Sesack et al, 1989) and potentially the GABAergic projection from NAc shell to VTA (Zahm et al, 2001) (the
‘direct’ striatal pathway) have a role in this reinstatement. They showed that (1) reversible inactivation of dorsal mPFC decreases both footshock-induced reinstatement and glutamate release
in NAc core, (2) reversible inactivation of NAc shell and core, and as mentioned above the VTA, decreases this reinstatement, and (3) that reversible inactivation of NAc shell also
decreases stress-induced dopamine release in dorsal mPFC. These authors also showed that reversible inactivation of the ventral pallidum, a main output region of the striatal indirect
pathway (Gerfen, 1992), decreases footshock-induced reinstatement. This circuit scheme, however, is speculative in the absence of more direct evidence from studies using classical anatomical
asymmetrical disconnection procedures (Setlow et al, 2002) or more recent projection-specific inhibition using optogenetic (Stefanik et al, 2013) or chemogenetic (Mahler et al, 2014)
methods. Evidence for a role of NAc dopamine in stress-induced reinstatement is somewhat mixed. Shaham and Stewart (1995) showed that footshock-induced reinstatement of heroin seeking is
associated with increased dopamine levels in NAc. In contrast, McFarland et al (2004) showed that footshock-induced reinstatement of cocaine seeking is not associated with increased dopamine
release in NAc and that flupenthixol injections into NAc core have no effect on this reinstatement. However, Xi et al (2004) showed that injections of the preferential D3 receptor
antagonist SB-277011A into NAc (injections targeted primarily NAc shell) decrease footshock-induced reinstatement of cocaine seeking. Additionally, Tobin et al (2013) showed that SCH23390
injections into NAc shell but not core decrease food deprivation-induced reinstatement of heroin seeking. Thus, one possible account of these potentially discrepant results is that NAc shell
but not core dopamine is critical for stress-induced reinstatement of drug seeking. Finally, a very recent study by Wheeler and colleagues suggests that at least for one stressor, a bitter
quinine solution, reinstatement of cocaine seeking is associated with decreased tonic NAc dopamine levels, as assessed by _in vivo_ voltammetry (Twining et al, 2015). They showed that in
cocaine-experienced rats, exposure to quinine reinstates cocaine seeking, and that in drug-naïve rats, the same manipulation decreases tonic dopamine levels in NAc. Both of these effects
were reversed by blockade of VTA CRF1 receptors. Together, with the previous results reviewed above, the results from this study suggest that both increases and decreases in NAc dopamine can
have a role in stress-induced drug seeking. SUMMARY D1 receptor-mediated dopamine transmission in dorsal mPFC and the glutamatergic projection from this brain area to NAc core are critical
for stress-induced reinstatement of cocaine seeking. This reinstatement also likely involves activation of the NAc GABAergic projections to VTA (the direct pathway) and ventral pallidum (the
indirect pathway), as well as increased dopamine transmission in NAc shell. A recent study using intraoral delivery of quinine solution as the stressor suggests that stress-induced
reinstatement can also be triggered by decreases in NAc dopamine tone. MEDIAN AND DORSAL RAPHE NUCLEI Many studies over the last several decades have implicated central serotonin in stress
responses (Chaouloff, 1993; Maier, 1984; Maier and Watkins, 2005; Stone, 1983). This literature has led to studies on the role of serotonin in the two main cell body regions of brain
serotonergic neurons—median raphe nucleus (MRN) and dorsal raphe nucleus (DRN) (Hale and Lowry, 2011; Vertes, 1991; Vertes et al, 1999)—in stress-induced reinstatement of alcohol seeking in
rats and stress-induced reinstatement of cocaine and morphine CPP. ROLE OF MRN IN STRESS-INDUCED REINSTATEMENT OF ALCOHOL SEEKING Le et al (1999) showed that systemic injections of the
serotonin reuptake blocker fluoxetine, which increases brain serotonin levels, decrease footshock-induced reinstatement of alcohol seeking, indicating that serotonin negatively regulates
this reinstatement. This negative regulation involves the MRN. Local injections of the selective 5-HT1a autoreceptor agonist 8-OH-DPAT, which decrease serotonin cell firing and release
(Mongeau et al, 1997), mimic the effect of intermittent footshock on reinstatement (Le et al, 2002). Additionally, these authors showed that MRN injections of the non-selective CRF receptor
antagonist D-Phe CRF12–41 decrease footshock-induced reinstatement of alcohol seeking while MRN injections of very low doses of CRF (10 ng) partially mimic the stressor’s effect on
reinstatement. A physiological mechanism that connects the similar effects of 8-OH-DPAT and low-dose CRF injections in MRN on reinstatement is that low CRF doses, like 8-OH-DPAT, decrease
serotonin cell firing and release (Kirby et al, 2000; Price et al, 1998). However, to date, this effect has only been reported for DRN but not for MRN neurons. Finally, Le et al (2008)
showed that reversible inactivation of the MRN with the GABAa agonist muscimol reinstates alcohol seeking. However, the relevance of this observation to serotonin’s role in stress-induced
reinstatement is questionable because this effect is not reversed by lesions of serotonin-containing neurons in the brain. ROLE OF DRN IN STRESS-INDUCED REINSTATEMENT OF MORPHINE AND COCAINE
CPP Results from CPP reinstatement studies from the Kirby and Chavkin laboratories implicate the DRN serotonergic cell body region in stress-induced reinstatement. Staub et al (2012) showed
that swim stress-induced reinstatement of morphine CPP is associated with increased GABAergic postsynaptic neuronal activity that leads to inhibition of DRN serotonin neurons, and that this
inhibitory effect involves local CRF signaling. In a follow-up study, Li et al (2013) showed that increasing GABAergic inhibitory transmission by DRN muscimol injections reinstates morphine
CPP while inhibition of GABAergic neurons by bicuculline (a GABAa receptor antagonist) decreases swim stress-induced reinstatement. There is also evidence for a role of kappa/dynorphin
signaling in DRN in stress-induced reinstatement of cocaine CPP in mice. Land et al (2009) showed that DRN injections of the KOR antagonist Nor-BNI decrease social stress-induced
reinstatement of cocaine CPP in mice. Their results from experiments examining the role of kappa/dynorphin in DRN in aversive behavior also suggest that the serotonergic projection from DRN
to NAc likely has a role in the local inhibitory effect of Nor-BNI in DRN on stress-induced reinstatement of cocaine CPP; however, this possibility was not empirically tested. In a
subsequent study, the Chavkin laboratory showed that KOR-mediated social stress-induced activation of p38 mitogen-activated protein kinase (MAPK) in DRN serotonergic neurons has a critical
role in stress-induced reinstatement of cocaine CPP (Bruchas et al, 2011). In this study, the authors first showed that exposure to social defeat stress, which induces reinstatement of
cocaine seeking, activates p38MAPK in DRN, and that this effect is reversed by Nor-BNI and is not observed in KOR knockout mice. They then showed that viral-mediated selective deletion of
p38MAPK in DRN serotonin neurons decreases social stress-induced reinstatement of cocaine CPP. SUMMARY The results of the studies reviewed above indicate an important role for an interaction
between CRF and serotonin in MRN in footshock stress-induced reinstatement of alcohol seeking. There is also evidence for a role of DRN serotonergic neurons, which involves both CRF and
kappa/dynorphin. A question for future research is which serotonergic projection areas contribute to the distinct roles of MRN and DRN in stress-induced reinstatement in the operant model
and the CPP model (Figure 3). TRANSLATIONAL HUMAN RESEARCH In this section, we describe results from several human laboratory studies and two double-blind clinical trials in which
pharmacological agents that decrease stress-induced reinstatement of drug seeking were tested for their effects on drug craving and relapse in humans. These include the alpha-2 adrenoceptor
agonists, clonidine, guanfacine, and lofexidine, the alpha-1 adrenoceptor antagonist, prazosin, and the CRF1 receptor antagonist, pexacerfont. Figures 4 and 5 show comparative data on the
effect of alpha-2 adrenoceptor agonists and CRF1 receptor antagonists on stress-induced reinstatement of drug seeking in rats and stress-induced craving and time to first lapse in humans.
HUMAN LABORATORY STUDIES In 1999, Sinha and colleagues introduced a laboratory model to determine, in a cause-effect manner, the effect of stress on drug craving under controlled laboratory
conditions (Sinha, 2001; Sinha et al, 1999). They and other investigators showed that certain stressors, including guided imagery stress, the Trier Social Stress Task, and systemic CRF
injections induce physiological (eg, increased cortisol release) and psychological (eg, increased anxiety) stress responses and increase self-reports of drug craving (Back et al, 2010;
Sinha, 2009; Sinha et al, 1999). From the perspective of drug relapse, an important finding from these studies is that physiological and psychological (including drug craving) stress
responses in the laboratory predict, to some degree, subsequent drug relapse (Back et al, 2010; Higley et al, 2011; Sinha et al, 2006, 2011a). ALPHA-2 ADRENOCEPTOR AGONISTS In several
studies, investigators determined the effects of alpha-2 adrenoceptor agonists on stress- and cue-induced craving in opiate, cocaine, heroin, and nicotine (smokers) users. In an initial
preliminary study, Sinha et al (2007) showed that in opiate users maintained on naltrexone, lofexidine treatment (2.4 mg/day) decreases both stress- and cue-induced opiate craving. Similar
findings were observed in a larger sample study with cocaine users in which Jobes et al (2011) showed that clonidine (0.1 or 0.2 mg) administration decreases stress- and cue-induced cocaine
craving (Figure 3). In another study, Fox et al (2012) determined the effects of guanfacine (up to 3 mg/day) on stress-, cue-, and stress+cue-induced drug craving in cocaine-dependent
individuals who also use alcohol and nicotine (smokers). They showed that guanfacine decreases stress- and cue-induced nicotine craving. Guanfacine also modestly decreased cue-induced
cocaine craving, anxiety, and arousal, but not stress-induced craving. However, the latter negative finding may reflect a floor effect, because the stress manipulation caused a very weak
craving response in the placebo group. More recently, McKee et al (2014) showed that in human smokers guanfacine treatment (3 mg/day) decreases both stress-induced drug craving and
stress-induced smoking in the laboratory. In another study, Fox et al (2014) examined the effect of guanfacine (2 or 3 mg/day) on stress-, cue, and stress+cue-induced craving in male and
female cocaine users who also use alcohol and nicotine (smokers). A surprising finding in this study was that guanfacine selectively decreased cocaine craving, alcohol craving, anxiety, and
negative emotion following exposure to all three experimental conditions in females but not in males. In contrast, guanfacine decreased stress and cue-induced nicotine craving in both males
and females. Finally, in a very recent study Moran-Santa Maria et al (2015a) showed that in cocaine users acute administration of guanfacine (2 mg) has no effect on cocaine craving in
response to stress, cue, or stress+cue. In interpreting these data, two pharmacological factors should be considered. The first is that, unlike the previous guanfacine studies of Sinha and
colleagues in which guanfacine was given chronically for several weeks, in the Moran-Santa Maria et al (2015a) study, the drug was given acutely. The second is that the data of Sinha and
colleagues suggest that the minimal effective dose of guanfacine in the laboratory studies is ~3 mg, which is higher than the dose used in Moran-Santa Maria _et al_ study. Findings from
three other human laboratory studies in which yohimbine was used to induce drug craving and seeking provide additional support to the translational value of stress-induced reinstatement
studies in laboratory animals. In these studies, doses of yohimbine that induced physiological and psychological stress responses increased alcohol craving (Umhau et al, 2011), potentiated
cue-induced cocaine craving (an effect that was more pronounced in females), and increased opiate seeking in a choice-based (hydromorphone _vs_ money) progressive ratio task (Greenwald et
al, 2013). Thus, despite the interpretation issues related to yohimbine’s effects in rat reinstatement studies (Box 2), in humans, the drug induces an aversive stress-like state of which one
manifestation is increased drug craving and seeking. Finally, as discussed elsewhere (Bossert et al, 2013), an unexpected finding in the human studies was that alpha-2 adrenoceptor agonists
decreased both stress- and cue-induced drug craving. This was unexpected because in rat studies the alpha-2 adrenoceptor agonists selectively decreased footshock stress-induced
reinstatement but not drug priming- or cue-induced reinstatement (Erb et al, 2000; Highfield et al, 2001), but see Smith and Aston-Jones (2011) for different results. A possible account for
these different findings is that in rats cue exposure primarily induces an appetitive motivational state (See, 2005) while in humans, cue exposure also induces stress-like physiological and
psychological states that are similar to those induced by stress exposure (Sinha et al, 1999, 2000). ALPHA-1 ADRENOCEPTOR AND CRF1 RECEPTOR ANTAGONISTS Two studies determined the effects of
the alpha-1 adrenoceptor antagonist prazosin or the CRF1 receptor antagonist pexacerfont on stress- and cue-induced alcohol craving. Fox et al (2012) showed that in alcohol users prazosin
(16 mg/day) decreases stress-induced craving and negative emotions. Prazosin also decreased cue-induced negative emotion self-reports, but had no effect on cue-induced craving. The negative
data might be due to a floor effect, because of a weak cue-induced craving in the placebo group. Most recently, Kwako et al (2015) determined the effects of oral pexacerfont (300 mg/day for
7 days and 100 mg/day for 23 days) on stress- and cue-induced alcohol craving in ‘anxious’ alcohol-dependent subjects. This study was inspired by the large and remarkably consistent
preclinical literature on the effect of CRF receptor antagonists on escalation of drug intake, stress-like physiological and psychological withdrawal symptoms, and stress-induced
reinstatement of drug seeking across drug classes (Heilig and Koob, 2007; Koob, 2008; Shalev et al, 2010; Zorrilla et al, 2013a). In sharp contrast to the preclinical data, pexacerfont
treatment had no effect on stress- or cue-induced alcohol craving, emotional responses, or anxiety (Figure 4). Pexacerfont also had no effect on neural responses (fMRI) to alcohol-related or
affective-related cues. CLINICAL TRIALS The data from the laboratory studies described above provide evidence for the human ‘translational’ value of the stress-induced reinstatement model.
However, from a perspective of human drug relapse, a critical test for the predictive validity of the model is whether medications identified in the rat model show efficacy in the drug
user’s environment (Epstein et al, 2006). Kowalczyk et al (2014) recently determined in heroin users whether the clinical efficacy of buprenorphine would be enhanced by clonidine. In this
relapse prevention study, the subjects first received contingency management to facilitate initial abstinence. Subjects who successfully maintained abstinence were randomized to receive
clonidine (0.1 mg for 1 week, 0.2 mg for the next week, and 0.3 mg for the rest of the study, _n_=61) or placebo (_n_=57) for 14 weeks. The main dependent measures were time to lapse
(defined as any opiate positive or missed urines) and time to relapse (defined as any consecutive lapses), as well as baseline craving, and stress- and cue-induced craving in the subjects’
home environment, as assessed by real-time ecological momentary assessment (EMA) (Epstein et al, 2009). The main findings of the study were that clonidine treatment increased the time to
lapse (from 25 to 35 days) (Figure 4) and decreased baseline craving and stress-induced craving. In contrast, clonidine had no effect on relapse rates once a lapse had occurred. In the
context of our review, a study of Krupitsky et al (2013), which likely was not based on the stress-induced reinstatement studies in the animal model, is also relevant. In this 6-month
clinical trial, these authors determined whether guanfacine (1 mg/day) would decrease relapse rates in opiate users maintained on naltrexone. The main finding was that guanfacine had no
effect on relapse rates but decreased self-reports of stress and craving. While the study included a large number of subjects (_n_=75 per group) and was well designed, a potential
methodological issue was the use of a low guanfacine dose (1 mg). The studies described in section ‘Human laboratory studies’ suggest that the effective dose of guanfacine in the laboratory
studies is ~3 mg (Fox et al, 2014; McKee et al, 2014). SUMMARY The results of the human studies with alpha-2 adrenoceptor agonists and prazosin appear to support the translational utility of
the stress-induced reinstatement model. However, unlike the uniform effects of these agonists on stress-induced reinstatement in the rat model, the effects in the human studies are more
variable, and some of the human studies reported negative results (Krupitsky et al, 2013; Moran-Santa Maria et al, 2015a), possibly due to the use of doses that were lower than those used in
the studies that report positive effects. The human studies also suggest potential sex differences in the efficacy of alpha-2 adrenoceptor agonists (Fox et al, 2014), a factor that has not
been considered in the preclinical studies. What might account for the more heterogeneous/variable results in the clinical preclinical studies? We propose that four factors might be
particularly important. The first is the fact that rat or mouse studies are invariably performed using highly homogenous subjects of the same or similar age, sex, drug exposure duration, and
in many cases likely from a limited number of litters (and therefore a homogenous genetic background). In contrast, the human studies are performed with a highly heterogeneous subject
population of different ages, duration of drug use, race, and also includes both sexes. The second factor is the more flexible nature of studies using animal models, which allows
investigators to adjust both experimental parameters, including drug doses, to determine the effects of pharmacological agents on reinstatement under more than one experimental set-up. In
contrast, human studies are typically performed double-blind from start to end, which increases statistical power but may result in negative data due to two main sources that have a
significant role in the studies reviewed above: drug doses that were likely too low and experimental manipulations (stress- or cue-induced reinstatement) that in some studies had minimal
effect on craving, making it difficult to detect a reliable effect of the medication. Third, a significant issue in assessing the efficacy of medications to treat drug addiction is
compliance (Weiss, 2004), an issue that likely also played a role in the studies reviewed above. Fourth, the different types of stressors used in the preclinical studies (exposure to a
physical aversive stimulus such as footshock, restraint, or cold water) _vs_ the stressors used in the clinical studies (exposure to psychological stressors like stress imagery or the Trier
Social Stress Task) is an issue to consider in ‘translational’ studies on stress-induced reinstatement of drug seeking in which different results are observed in human studies vs. animal
models. Finally, the negative results with pexacerfont (Kwako et al, 2015) indicate that it is unrealistic to expect that all potential medications identified in animal models of psychiatric
disorders, including the stress-induced reinstatement model, will readily translate to the human condition. However, these negative data are particularly disappointing because, as mentioned
above, they are based on unparalleled convergence from multiple animal models of drug addiction and results that were independently demonstrated in multiple laboratories using different
drugs of abuse under different experimental conditions (Heilig and Koob, 2007; Koob, 2008; Sarnyai et al, 2001; Shalev et al, 2010). CONCLUSIONS AND FUTURE DIRECTIONS The demonstration that
a brief exposure to uncontrollable and unpredictable intermittent footshock stress reinstates heroin seeking in rats (Shaham and Stewart, 1995) has stimulated research by many preclinical
investigators on behavioral and neuropharmacological mechanisms of stress-induced reinstatement of drug seeking and CPP. The preclinical model of stress-induced reinstatement and the
demonstration that stress can provoke drug craving under controlled experimental conditions (Sinha et al, 1999) have also stimulated translational research to identify potential medications
that specifically target stress-induced craving and relapse in humans. In this review, we summarized this field of research to consolidate the current state of knowledge within a single
review. The results from the studies reviewed suggest at least four future lines of investigation. The first is the investigation of the synaptic and cellular mechanisms of stress-induced
reinstatement. Several studies have explored this question for swim stress-induced reinstatement of cocaine seeking in rats (Chirila et al, 2014; Kreibich and Blendy, 2004; Polter et al,
2014) and social defeat-induced reinstatement of cocaine CPP in mice (Bruchas et al, 2010, 2011; Land et al, 2009). At present, however, the synaptic and cellular mechanisms of
stress-induced reinstatement are largely unknown. The second is the investigation of circuitry mechanisms of stress-induced reinstatement, using neuroscience methods that go beyond classical
neuropharmacological and neuroanatomical methods. In the ‘relapse/reinstatement’ field, investigators recently have begun to incorporate modern chemogenetic (Bossert et al, 2011; Cruz et
al, 2014; Fanous et al, 2012; Mahler et al, 2014) and optogenetic (Lee et al, 2013; Ma et al, 2014; Stefanik et al, 2013) methods to study brain circuits of context-, cue-, and drug
priming-induced reinstatement of drug seeking, but these methods have not yet been used for stress-induced reinstatement. The third is the study of sex differences in stress-induced
reinstatement of drug seeking. In this regard, footshock stress-induced reinstatement of drug seeking is observed in both male and female rats (Shaham and Stewart, 1995). Additionally,
recent studies on sex differences and the role of ovarian hormones in stress-induced reinstatement have used yohimbine as a stressor and the results are mixed. Two studies showed that
yohimbine effect on reinstatement of cocaine and methamphetamine seeking is somewhat stronger in female rats than in male rats (Anker and Carroll, 2010a; Cox et al, 2013). However, other
studies have shown neither sex differences nor a role of ovarian hormones in yohimbine-induced reinstatement of nicotine or palatable food seeking (Cifani et al, 2012; Feltenstein et al,
2012; Pickens et al, 2012). A question for future research is whether sex differences would be observed in the mechanisms that mediate stress-induced reinstatement. Finally, during human
abstinence, stress exposure often occurs in the presence of contexts and cues previously associated with drug use and the drug itself in the case of an initial lapse (Epstein et al, 2009).
Human laboratory studies also indicate that stress can potentiate cue-induced drug craving (Fox et al, 2014; George et al, 2008; Moran-Santa Maria et al, 2015b). Additionally, studies using
the reinstatement model demonstrate that stress exposure potentiates the response to both cues (Buffalari and See, 2009; Liu and Weiss, 2002b) and drug priming injections (Graf et al, 2013).
Thus, from a translational perspective, mechanistic studies on stress-induced potentiation of the response to drug cues or contexts after extinction or without prior extinction may lead to
the identification of new targets for developing medications for relapse prevention. FUNDING AND DISCLOSURE The authors declare no conflict of interest. BOX 3. CORTICOSTERONE, THE HPA AXIS,
AND STRESS-INDUCED REINSTATEMENT As mentioned in the main text, studies using endocrinology methods of adrenalectomy and adrenalectomy plus corticosterone replacement showed that
stress-induced activation of the HPA axis and subsequent release of corticosterone does not have a role in stress-induced reinstatement of drug seeking (Erb et al, 1998; Le et al, 2000;
Shaham et al, 1997; Shalev et al, 2006, 2003; Graf et al, 2011). However, at least in the case of cocaine, basal corticosterone levels are critical for stress-induced reinstatement (Erb et
al, 1998; Shalev et al, 2003), suggesting a permissive role of the stress hormone in this reinstatement. Additionally, exogenous administration of corticosterone reinstates cocaine seeking
(Deroche et al, 1997). More recently, there is evidence for a role of corticosterone in two emerging important phenomena related to stress-induced reinstatement. The first is that as
mentioned in section ‘Generality of stress-induced reinstatement’, footshock stress-induced reinstatement is significantly stronger and more robust in rats with a history of extended (6
h/day) _vs_ limited (1–2 h/day) access to cocaine (Mantsch et al, 2014). Under these conditions, adrenalectomy before self-administration training prevents stress-induced reinstatement while
the same manipulation after training is ineffective (Graf et al, 2011), suggesting that cocaine-induced corticosterone-dependent neuroadaptations are critical for footshock-induced
reinstatement. Second, there is evidence that footshock, under conditions in which it has no effect on reinstatement on its own, strongly potentiates the ability of both cocaine priming and
cocaine cues to reinstate drug seeking (Buffalari and See, 2009; Graf et al, 2013). The mechanism/s underlying the ‘indirect’ potentiation effect of stress is largely unknown but it appears
that corticosterone has a critical role. Graf et al (2013) showed that this effect of mild stress is reversed by adrenalectomy but not by systemic injections of the glucocorticoid receptor
antagonist RU486. Next, these authors showed that the potentiating effect of mild stress on cocaine priming is mediated by a novel mechanism of action in NAc that involves
glucocorticoid-dependent blockade of dopamine clearance by the monoamine transporter, organic cation transporter 3 (OCT3). BOX 2. YOHIMBINE-INDUCED REINSTATEMENT: DOES IT REFLECT
STRESS-INDUCED REINSTATEMENT? Yohimbine is an alpha-2 adrenoceptor antagonist that has been used in numerous psychiatry-related studies as a pharmacological stressor in rodents, monkeys, and
humans (Bremner et al, 1996a and b; Redmond and Huang, 1979). Over the last decade, yohimbine has become the most common stress manipulation in studies on reinstatement of drug and food
seeking (Bossert et al, 2013; Calu et al, 2014; Nair et al, 2009) for three main reasons. The first is that the effect of yohimbine on reinstatement is less variable and more robust than the
effect of intermittent footshock (Le et al, 2005; Shepard et al, 2004). The second is that early studies showed that like intermittent footshock the effect of yohimbine on reinstatement of
food and alcohol is blocked by CRF1 receptor antagonists, which likely act at extrahypothalamic brain areas (Ghitza et al, 2006; Marinelli et al, 2007). The third reason was the intuitive
appeal of using the same stressor in the animal model and in humans (Greenwald et al, 2013; See and Waters, 2010). However, with the increasing use of yohimbine in reinstatement studies,
evidence emerges for behavioral and pharmacological differences between the effects of intermittent footshock and yohimbine on reward seeking. Unlike footshock, yohimbine reinstates food
seeking and also increases alcohol and food self-administration (Cifani et al, 2012; Le et al, 2005). Unlike intermittent footshock, yohimbine does not induce stress-related 22 kHz
ultrasonic distress vocalizations (Mahler et al, 2013). Unlike intermittent footshock, the effect of yohimbine on reinstatement of cocaine seeking or cocaine CPP is not blocked by the
alpha-2 adrenoceptor agonist clonidine (Brown et al, 2009; Mantsch et al, 2010). These differences between yohimbine and intermittent footshock raise the possibility that the effect of
yohimbine on reinstatement of reward seeking might not be due to its ability to induce a stress-like state that motivates drug or food seeking. This issue was addressed in a recent study by
Chen et al (2014c) in which they determined whether yohimbine’s effect on operant responding is dependent on previous reward history or cue contingency, and whether yohimbine (at a dose used
in reinstatement studies) induces an aversive stress-like state, as assessed by the CPP/CPA (conditioned place aversion) method. Chen _et al_ reported that the magnitude of
yohimbine-induced operant responding during the reinstatement tests was critically dependent on the contingency between lever pressing and discrete tone-light cue delivery, but not the
previous history with food reward during training. Specifically, yohimbine potently and dose-dependently increased operant lever-pressing that was _not_ previously reinforced with either
food or drug. They also showed that repeated pairing of yohimbine (2 mg/kg) with a distinct context causes modest CPP to this context. The highly unexpected conclusion from this study was
that yohimbine’s effects on operant responding in reinstatement studies are likely independent of the history of contingent self-administration of food or drug rewards and may not be related
to the commonly assumed stress-like effects of yohimbine. Chen et al (2014c) proposed that the primary action of yohimbine in reinstatement studies is to invigorate responding for cues,
which before pairing with drug or non-drug rewards or after extinction, have weak motivational effects in rodents. REFERENCES * Ahmed SH, Koob GF (1997). Cocaine- but not food-seeking
behavior is reinstated by stress after extinction. _Psychopharmacology_ 132: 289–295. Article CAS PubMed Google Scholar * Ahmed SH, Walker JR, Koob GF (2000). Persistent increase in the
motivation to take heroin in rats with history of drug escalation. _Neuropsychopharmacology_ 22: 413–421. CAS PubMed Google Scholar * Aldrich JV, Patkar KA, McLaughlin JP (2009).
Zyklophin, a systemically active selective kappa opioid receptor peptide antagonist with short duration of action. _Proc Natl Acad Sci USA_ 106: 18396–18401. CAS PubMed PubMed Central
Google Scholar * Aldrich JV, Senadheera SN, Ross NC, Ganno ML, Eans SO, McLaughlin JP (2013). The macrocyclic peptide natural product CJ-15,208 is orally active and prevents reinstatement
of extinguished cocaine-seeking behavior. _J Nat Prod_ 76: 433–438. CAS PubMed Google Scholar * Anker JJ, Carroll ME (2010a). Reinstatement of cocaine seeking induced by drugs, cues, and
stress in adolescent and adult rats. _Psychopharmacology_ 208: 211–222. CAS PubMed Google Scholar * Anker JJ, Carroll ME (2010b). Sex differences in the effects of allopregnanolone on
yohimbine-induced reinstatement of cocaine seeking in rats. _Drug Alcohol Depend_ 107: 264–267. CAS PubMed Google Scholar * Antelman SM, Levine J, Gershon S (2000). Time-dependent
sensitization: the odyssey of a scientific heresy from the laboratory to the door of the clinic. _Mol Psychiatry_ 5: 350–356. CAS PubMed Google Scholar * Aston-Jones G, Delfs JM, Druhan
J, Zhu Y (1999). The bed nucleus of the stria terminalis: A target site for noradrenergic actions in opiate withdrawal. _Ann NY Acad Sci_ 877: 486–498. CAS PubMed Google Scholar * Back
SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL _et al_ (2010). Reactivity to laboratory stress provocation predicts relapse to cocaine. _Drug Alcohol Depend_ 106: 21–27.
CAS PubMed Google Scholar * Bale TL, Vale WW (2004). CRF and CRF receptors: role in stress responsivity and other behaviors. _Annu Rev Pharmacol Toxicol_ 44: 525–557. CAS PubMed Google
Scholar * Bannon MB, Roth RH (1983). Pharmacology of mesocortical dopamine neurons. _Pharmacol Rev_ 35: 53–68. CAS PubMed Google Scholar * Beardsley PM, Howard JL, Shelton KL, Carroll FI
(2005). Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its
antidepressant-like effects in rats. _Psychopharmacology_ 183: 118–126. CAS PubMed Google Scholar * Beardsley PM, Pollard GT, Howard JL, Carroll FI (2010). Effectiveness of analogs of the
kappa opioid receptor antagonist (3R)-7-hydroxy-N-((1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-pipe ridinyl]methyl}-2-methylpropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxami de
(JDTic) to reduce U50,488-induced diuresis and stress-induced cocaine reinstatement in rats. _Psychopharmacology_ 210: 189–198. CAS PubMed Central PubMed Google Scholar * Blacktop JM,
Seubert C, Baker DA, Ferda N, Lee G, Graf EN _et al_ (2011). Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access
self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. _J Neurosci_ 31: 11396–11403. CAS PubMed Central PubMed Google Scholar * Bornstein SR, Webster EL,
Torpy DJ, Richman SJ, Mitsiades N, Igel M _et al_ (1998). Chronic effects of a nonpeptide corticotropin-releasing hormone type I receptor antagonist on pituitary-adrenal function, body
weight, and metabolic regulation. _Endocrinology_ 139: 1546–1555. CAS PubMed Google Scholar * Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y (2005). Neurobiology of relapse to heroin
and cocaine seeking: An update and clinical implications. _Eur J Pharmacol_ 526: 36–50. CAS PubMed Google Scholar * Bossert JM, Marchant NJ, Calu DJ, Shaham Y (2013). The reinstatement
model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. _Psychopharmacology (Berl)_ 229: 453–476. CAS Google Scholar * Bossert JM,
Stern AL, Theberge FR, Cifani C, Koya E, Hope BT _et al_ (2011). Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. _Nat Neurosci_ 14: 420–422.
CAS PubMed Central PubMed Google Scholar * Bouton ME, King DA (1983). Contextual control of the extinction of conditioned fear: tests for the associative value of the context. _J Exp
Psychol Anim Behav Process_ 9: 248–265. CAS PubMed Google Scholar * Bouton ME, Swartzentruber D (1991). Sources of relapse after extinction in Pavlovian and instrumental learning. _Clin
Psychol Rev_ 11: 123–140. Google Scholar * Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF _et al_ (2005). Role for hypocretin in mediating stress-induced reinstatement
of cocaine-seeking behavior. _Proc Natl Acad Sci USA_ 102: 19168–19173. CAS PubMed PubMed Central Google Scholar * Bremner JD, Krystal JH, Southwick SM, Charney DS (1996a). Noradrenergic
mechanisms in stress and anxiety: I. preclinical studies. _Synapse_ 23: 28–38. CAS PubMed Google Scholar * Bremner JD, Krystal JH, Southwick SM, Charney DS (1996b). Noradrenergic
mechanisms in stress and anxiety: II. clinical studies. _Synapse_ 23: 39–51. CAS PubMed Google Scholar * Briand LA, Vassoler FM, Pierce RC, Valentino RJ, Blendy JA (2010). Ventral
tegmental afferents in stress-induced reinstatement: the role of cAMP response element-binding protein. _J Neurosci_ 30: 16149–16159. CAS PubMed Central PubMed Google Scholar * Brown ZJ,
Kupferschmidt DA, Erb S (2012). Reinstatement of cocaine seeking in rats by the pharmacological stressors, corticotropin-releasing factor and yohimbine: role for D1/5 dopamine receptors.
_Psychopharmacology (Berl)_ 224: 431–440. CAS Google Scholar * Brown ZJ, Tribe E, D'Souza N A, Erb S (2009). Interaction between noradrenaline and corticotrophin-releasing factor in
the reinstatement of cocaine seeking in the rat. _Psychopharmacology_ 203: 121–130. CAS PubMed Google Scholar * Bruchas MR, Land BB, Chavkin C (2010). The dynorphin/kappa opioid system as
a modulator of stress-induced and pro-addictive behaviors. _Brain Res_ 1314: 44–55. CAS PubMed Google Scholar * Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB _et
al_ (2011). Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. _Neuron_ 71: 498–511. CAS PubMed Central PubMed
Google Scholar * Buczek Y, Le AD, Wang A, Stewart J, Shaham Y (1999). Stress reinstates nicotine seeking but not sucrose solution seeking in rats. _Psychopharmacology_ 144: 183–188. CAS
PubMed Google Scholar * Buffalari DM, Baldwin CK, See RE (2012). Treatment of cocaine withdrawal anxiety with guanfacine: relationships to cocaine intake and reinstatement of cocaine
seeking in rats. _Psychopharmacology (Berl)_ 223: 179–190. CAS Google Scholar * Buffalari DM, See RE (2009). Footshock stress potentiates cue-induced cocaine-seeking in an animal model of
relapse. _Physiol Behav_ 98: 614–617. CAS PubMed Central PubMed Google Scholar * Buffalari DM, See RE (2011). Inactivation of the bed nucleus of the stria terminalis in an animal model
of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. _Psychopharmacology_ 213: 19–27. CAS PubMed Google Scholar * Cabib S, Imperato A (1996).
Stress, depression and the mesolimbic dopamine system. _Psychopharamacology_ 128: 331–342. CAS Google Scholar * Calu DJ, Chen YW, Kawa AB, Nair SG, Shaham Y (2014). The use of the
reinstatement model to study relapse to palatable food seeking during dieting. _Neuropharmacology_ 76: 395–406. CAS PubMed Google Scholar * Calu DJ, Kawa AB, Marchant NJ, Navarre BM,
Henderson MJ, Chen B _et al_ (2013). Optogenetic inhibition of dorsal medial prefrontal cortex attenuates stress-induced reinstatement of palatable food seeking in female rats. _J Neurosci_
33: 214–226. CAS PubMed Central PubMed Google Scholar * Cannon WB (1935). Stresses and strains of homeostasis. _Am J Med Sci_ 189: 1–14. Google Scholar * Capriles N, Rodaros D, Sorge
RE, Stewart J (2003). A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. _Psychopharmacology_ 168: 66–74. CAS PubMed Google Scholar
* Carey AN, Borozny K, Aldrich JV, McLaughlin JP (2007). Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. _Eur J Pharmacol_ 569:
84–89. CAS PubMed Central PubMed Google Scholar * Carroll ME (1985). The role of food deprivation in the maintenance and reinstatement of cocaine-seeking behavior in rats. _Drug Alcohol
Depend_ 16: 95–109. CAS PubMed Google Scholar * Cassell MD, Freedman LJ, Shi C (1999). The intrinsic organization of the central extended amygdala. _Ann NY Acad Sci_ 877: 217–241. CAS
PubMed Google Scholar * Chaouloff F (1993). Physiopharamcological interactions between stress hormones and central serotonergic systems. _Brain Res Rev_ 18: 1–32. CAS PubMed Google
Scholar * Chen NA, Jupp B, Sztainberg Y, Lebow M, Brown RM, Kim JH _et al_ (2014a). Knockdown of CRF1 receptors in the ventral tegmental area attenuates cue- and acute food deprivation
stress-induced cocaine seeking in mice. _J Neurosci_ 34: 11560–11570. CAS PubMed PubMed Central Google Scholar * Chen YW, Fiscella KA, Bacharach SZ, Calu DJ (2014b). Effect of cafeteria
diet history on cue-, pellet-priming-, and stress-induced reinstatement of food seeking in female rats. _PLoS One_ 9: e102213. PubMed Central PubMed Google Scholar * Chen YW, Fiscella KA,
Bacharach SZ, Tanda G, Shaham Y, Calu DJ (2014c). Effect of yohimbine on reinstatement of operant responding in rats is dependent on cue contingency but not food reward history. _Addict
Biol_ (in press). * Chirila AM, Brown TE, Bishop RA, Bellono NW, Pucci FG, Kauer JA (2014). Long-term potentiation of glycinergic synapses triggered by interleukin 1beta. _Proc Natl Acad Sci
USA_ 111: 8263–8268. CAS PubMed PubMed Central Google Scholar * Chrousos GP, Gold PW (1992). The concepts of stress and stress system disorders. Overview of physical and behavioral
homeostasis. _JAMA_ 267: 1244–1452. CAS PubMed Google Scholar * Ciccocioppo R, Stopponi S, Economidou D, Kuriyama M, Kinoshita H, Heilig M _et al_ (2014). Chronic treatment with novel
brain-penetrating selective NOP receptor agonist MT-7716 reduces alcohol drinking and seeking in the rat. _Neuropsychopharmacology_ 39: 2601–2610. CAS PubMed Central PubMed Google Scholar
* Cifani C, Koya E, Navarre BM, Calu DJ, Baumann MH, Marchant NJ _et al_ (2012). Medial prefrontal cortex neuronal activation and synaptic alterations after stress-induced reinstatement of
palatable food seeking: a study using c-fos-GFP transgenic female rats. _J Neurosci_ 32: 8480–8490. CAS PubMed Central PubMed Google Scholar * Cippitelli A, Brunori G, Gaiolini KA,
Zaveri NT, Toll L (2015). Pharmacological stress is required for the anti-alcohol effect of the alpha3beta4* nAChR partial agonist AT-1001. _Neuropharmacology_ 93: 229–236. CAS PubMed
Central PubMed Google Scholar * Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A _et al_ (2008). Increase of brain endocannabinoid anandamide levels by FAAH inhibition
and alcohol abuse behaviours in the rat. _Psychopharmacology (Berl)_ 198: 449–460. CAS Google Scholar * Cippitelli A, Rezvani AH, Robinson JE, Eisenberg L, Levin ED, Bonaventure P _et al_
(2011). The novel, selective, brain-penetrant neuropeptide Y Y2 receptor antagonist, JNJ-31020028, tested in animal models of alcohol consumption, relapse, and anxiety. _Alcohol_ 45:
567–576. CAS PubMed Google Scholar * Cleck JN, Ecke LE, Blendy JA (2008). Endocrine and gene expression changes following forced swim stress exposure during cocaine abstinence in mice.
_Psychopharmacology (Berl)_ 201: 15–28. CAS Google Scholar * Cohen F, Horowitz MJ, Lazarus RS, Moos RH, Robbins LN, Rose RM _et al_ (1982). Panel report on psychosocial and modifiers of
stress. In: Eliott GR, Eisdorfer C (eds). _Stress and Human Health_. Springer Publishing Co.: New York. pp 147–188. Google Scholar * Cohen S, Evans GW, Stokols D, Krantz DS (1986)
_Behavior, health, and environmental stress_. Plenum Press: New York. Google Scholar * Conrad KL, McCutcheon JE, Cotterly LM, Ford KA, Beales M, Marinelli M (2010). Persistent increases in
cocaine-seeking behavior after acute exposure to cold swim stress. _Biol Psychiatry_ 68: 303–305. CAS PubMed Central PubMed Google Scholar * Cox BM, Young AB, See RE, Reichel CM (2013).
Sex differences in methamphetamine seeking in rats: impact of oxytocin. _Psychoneuroendocrinology_ 38: 2343–2353. CAS PubMed Central PubMed Google Scholar * Cruz FC, Babin KR, Leao RM,
Goldart EM, Bossert JM, Shaham Y _et al_ (2014). Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. _J Neurosci_ 34: 7437–7446. CAS
PubMed Central PubMed Google Scholar * Dallman MF, Akana SF, Levin N, Walker CD, Bradbury MJ, Suemaru S _et al_ (1995). Corticosteroids and the control of function in the
hypothalamo-pituitary-adrenal (HPA) axis. _Ann NY Acad Sci_ 746: 22–31. Google Scholar * Davis M, Walker DL, Lee Y (1997). Roles of the amygdala and bed nucleus of the stria terminalis in
fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. _Ann NY Acad Sci_ 821: 305–331. CAS PubMed Google Scholar * Davis M, Walker DL, Miles L, Grillon C
(2010). Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. _Neuropsychopharmacology_ 35: 105–135. PubMed Google Scholar * de Guglielmo G,
Cippitelli A, Somaini L, Gerra G, Li H, Stopponi S _et al_ (2013). Pregabalin reduces cocaine self-administration and relapse to cocaine seeking in the rat. _Addict Biol_ 18: 644–653. CAS
PubMed Google Scholar * de Guglielmo G, Martin-Fardon R, Teshima K, Ciccocioppo R, Weiss F (2014). MT-7716, a potent NOP receptor agonist, preferentially reduces ethanol seeking and
reinforcement in post-dependent rats. _Addict Biol_. * Deroche V, Marinelli M, Le Moal M, Piazza PV (1997). Glucocorticoids and behavioral effects of psychostimulants. II: cocaine
intravenous self-administration and reinstatement depend on glucocorticoid levels. _J Pharmacol Exp Ther_ 281: 1401–1407. CAS PubMed Google Scholar * Deutch AY, Roth RH (1990). The
determinants of stress-induced activation of the prefrontal cortical dopamine system. _Prog Brain Res_ 85: 367–403. CAS PubMed Google Scholar * Deutch JA, Howarth CI (1962). Evocation by
fear of a habit learned for electrical stimulation of the brain. _Science_ 130: 1057–1058. Google Scholar * Eans SO, Ganno ML, Reilley KJ, Patkar KA, Senadheera SN, Aldrich JV _et al_
(2013). The macrocyclic tetrapeptide [D-Trp]CJ-15,208 produces short-acting kappa opioid receptor antagonism in the CNS after oral administration. _Br J Pharmacol_ 169: 426–436. CAS PubMed
Central PubMed Google Scholar * Economidou D, Mattioli L, Ubaldi M, Lourdusamy A, Soverchia L, Hardiman G _et al_ (2007). Role of cannabinoidergic mechanisms in ethanol
self-administration and ethanol seeking in rat adult offspring following perinatal exposure to Delta(9)-tetrahydrocannabinol. _Toxicol Appl Pharmacol_ 223: 73–85. CAS PubMed Google Scholar
* Enrico P, Bouma M, de Vries JB, Westerink BH (1998). The role of afferents to the ventral tegmental area in the handling stress-induced increase in the release of dopamine in the medial
prefrontal cortex: a dual-probe microdialysis study in the rat brain. _Brain Res_ 779: 205–213. CAS PubMed Google Scholar * Epstein D, Preston K, Stewart J, Shaham Y (2006). Toward a
model of drug relapse: an assessment of the validity of the reinstatement procedure. _Psychopharmacology_ 189: 1–16. CAS PubMed Central PubMed Google Scholar * Epstein DH, Willner-Reid
J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL (2009). Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. _Arch Gen
Psychiatry_ 66: 88–94. PubMed Central PubMed Google Scholar * Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J (2000). Alpha-2 adrenergic agonists block stress-induced
reinstatement of cocaine seeking. _Neuropsychopharmacology_ 23: 138–150. CAS PubMed Google Scholar * Erb S, Petrovic A, Yi D, Kayyali H (2006). Central injections of CRF reinstate cocaine
seeking in rats after postinjection delays of up to 3h: an influence of time and environmental context. _Psychopharmacology_ 187: 112–120. CAS PubMed Google Scholar * Erb S, Salmaso N,
Rodaros D, Stewart J (2001a). A role for the CRF-containing pathway projecting from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement
of cocaine seeking in rats. _Psychopharmacology_ 158: 360–365. CAS PubMed Google Scholar * Erb S, Shaham Y, Stewart J (1996). Stress reinstates cocaine-seeking behavior after prolonged
extinction and drug-free periods. _Psychopharmacology_ 128: 408–412. CAS PubMed Google Scholar * Erb S, Shaham Y, Stewart J (1998). The role of corticotropin-releasing factor and
corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. _J Neurosci_ 18: 5529–5536. CAS PubMed PubMed Central Google Scholar * Erb S, Shaham Y, Stewart J
(2001b). Stress-induced relapse to drug seeking in the rat: Role of the bed nucleus of the stria terminalis and amygdala. _Stress_ 4: 289–303. CAS PubMed Google Scholar * Erb S, Stewart J
(1999). A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. _J
Neurosci_ 19: RC35. CAS PubMed PubMed Central Google Scholar * Ettenberg A (2009). The runway model of drug self-administration. _Pharmacol Biochem Behav_ 91: 271–277. CAS PubMed
Google Scholar * Ettenberg A, MacConell LA, Geist TD (1996). Effects of haloperidol in a response-reinstatement model of heroin relapse. _Psychopharmacology (Berl)_ 124: 205–210. CAS
Google Scholar * Fanous S, Goldart EM, Theberge FR, Bossert JM, Shaham Y, Hope BT (2012). Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving.
_J Neurosci_ 32: 11600–11609. CAS PubMed Central PubMed Google Scholar * Feltenstein MW, Ghee SM, See RE (2012). Nicotine self-administration and reinstatement of nicotine-seeking in
male and female rats. _Drug Alcohol Depend_ 121: 240–246. CAS PubMed Google Scholar * Feltenstein MW, See RE (2006). Potentiation of cue-induced reinstatement of cocaine-seeking in rats
by the anxiogenic drug yohimbine. _Behav Brain Res_ 174: 1–8. CAS PubMed Google Scholar * Figueroa-Guzman Y, Mueller C, Vranjkovic O, Wisniewski S, Yang Z, Li SJ _et al_ (2010). Oral
administration of levo-tetrahydropalmatine attenuates reinstatement of extinguished cocaine seeking by cocaine, stress or drug-associated cues in rats. _Drug Alcohol Depend_ 116: 72–79.
PubMed Central PubMed Google Scholar * Flavin SA, Winder DG (2013). Noradrenergic control of the bed nucleus of the stria terminalis in stress and reward. _Neuropharmacology_ 70: 324–330.
CAS PubMed Central PubMed Google Scholar * Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA (2008). The 5-HT(2C) receptor agonist Ro60-0175 reduces cocaine self-administration
and reinstatement induced by the stressor yohimbine, and contextual cues. _Neuropsychopharmacology_ 33: 1402–1412. CAS PubMed Google Scholar * Ford CP, Beckstead MJ, Williams JT (2007).
Kappa opioid inhibition of somatodendritic dopamine inhibitory postsynaptic currents. _J Neurophysiol_ 97: 883–891. CAS PubMed Google Scholar * Fox HC, Morgan PT, Sinha R (2014). Sex
differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. _Neuropsychopharmacology_ 39: 1527–1537. CAS PubMed Central PubMed Google Scholar *
Fox HC, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan PT _et al_ (2012). Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary
findings. _J Psychopharmacol_ 26: 958–972. PubMed Central PubMed Google Scholar * Fritschy JM, Grzanna R (1991). Selective effects of DSP-4 on locus coeruleus axons: are there
pharmacologically different types of noradrenergic axons in the central nervous system? _Prog Brain Res_ 88: 257–268. CAS PubMed Google Scholar * Funk D, Coen K, Le AD (2014). The role of
kappa opioid receptors in stress-induced reinstatement of alcohol seeking in rats. _Brain Behav_ 4: 356–367. PubMed Central PubMed Google Scholar * Funk D, Harding S, Juzytsch W, Le AD
(2005). Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. _Psychopharmacology_ 183: 341–349. CAS PubMed
Google Scholar * Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C _et al_ (2007). 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-
imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. _J Neurosci_ 27:
2718–2726. CAS PubMed PubMed Central Google Scholar * Geisler S, Derst C, Veh RW, Zahm DS (2007). Glutamatergic afferents of the ventral tegmental area in the rat. _J Neurosci_ 27:
5730–5743. CAS PubMed Central PubMed Google Scholar * George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C _et al_ (2008). Neurokinin 1 receptor antagonism as a possible therapy
for alcoholism. _Science_ 319: 1536–1539. CAS PubMed Google Scholar * Georges F, Aston-Jones G (2002). Activation of ventral tegmental area cells by the bed nucleus of the stria
terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. _J Neurosci_ 22: 5173–5187. CAS PubMed Central PubMed Google Scholar * Gerfen CR (1992). The neostriatal
mosaic: multiple levels of compartmental organization in the basal ganglia. _Annu Rev Neurosci_ 15: 285–320. CAS PubMed Google Scholar * Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y
(2006). The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF(1) receptors. _Neuropsychopharmacology_ 31: 2188–2196. CAS PubMed Google
Scholar * Glass DC, Singer JE (1972) _Urban Stress: Experiments on Noise and Social Stressors_. Academic Press: New York. Google Scholar * Glowa JR, Barrett JE, Gold PW (1992). Effects of
corticotropin releasing hormone on appetitive behaviors. _Peptides_ 13: 609–621. CAS PubMed Google Scholar * Gold RM (1966). Aphagia and adipsia produced by unilateral hypothalamic
lesions in rats. _Am J Physiol_ 211: 1274–1276. CAS PubMed Google Scholar * Graf EN, Hoks MA, Baumgardner J, Sierra J, Vranjkovic O, Bohr C _et al_ (2011). Adrenal activity during
repeated long-access cocaine self-administration is required for later CRF-Induced and CRF-dependent stressor-induced reinstatement in rats. _Neuropsychopharmacology_ 36: 1444–1454. CAS
PubMed Central PubMed Google Scholar * Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR _et al_ (2013). Corticosterone acts in the nucleus accumbens to enhance dopamine
signaling and potentiate reinstatement of cocaine seeking. _J Neurosci_ 33: 11800–11810. CAS PubMed Central PubMed Google Scholar * Gray JA (1987) _The Psychology of Fear and Stress_2nd
edn.Cambridge University Press: New York. Google Scholar * Graziane NM, Polter AM, Briand LA, Pierce RC, Kauer JA (2013). Kappa opioid receptors regulate stress-induced cocaine seeking and
synaptic plasticity. _Neuron_ 77: 942–954. CAS PubMed Central PubMed Google Scholar * Greenwald MK, Lundahl LH, Steinmiller CL (2013). Yohimbine increases opioid-seeking behavior in
heroin-dependent, buprenorphine-maintained individuals. _Psychopharmacology_ 225: 811–824. CAS PubMed Google Scholar * Grella SL, Funk D, Coen K, Li Z, Le AD (2014). Role of the
kappa-opioid receptor system in stress-induced reinstatement of nicotine seeking in rats. _Behav Brain Res_ 265: 188–197. CAS PubMed Central PubMed Google Scholar * Grimwood S, Lu Y,
Schmidt AW, Vanase-Frawley MA, Sawant-Basak A, Miller E _et al_ (2011). Pharmacological characterization of 2-methyl-N-((2'-(pyrrolidin-1-ylsulfonyl)biphenyl-4-yl)methyl)propan-1-ami ne
(PF-04455242), a high-affinity antagonist selective for kappa-opioid receptors. _J Pharmacol Exp Ther_ 339: 555–566. CAS PubMed Google Scholar * Hale MW, Lowry CA (2011). Functional
topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. _Psychopharmacology (Berl)_ 213: 243–264. CAS Google Scholar *
Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L _et al_ (2006). Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental
stress. _Proc Natl Acad Sci USA_ 103: 15236–15241. CAS PubMed Central PubMed Google Scholar * Haynes R (1990). Adrenocorticotropic hormones: adrenocortical steroids and their synthetic
analogs; inhibitors of the synthesis and actions of adrenocortical hormones In: Gilman A, Rall T, Nies A, Taylor P (eds) _Goodman & Gilman's the Pharmacological Basis of
Therapeutics_8th edn.Pergamon Press: New York. pp 1431–1462. Google Scholar * Heilig M, Koob GF (2007). A key role for corticotropin-releasing factor in alcohol dependence. _Trends
Neurosci_ 30: 399–406. CAS PubMed Central PubMed Google Scholar * Highfield D, Clements A, Shalev U, McDonald R, Featherstone R, Stewart J _et al_ (2000). Involvement of the medial
septum in stress-induced relapse to heroin seeking in rats. _Eur J Neurosci_ 12: 1705–1713. CAS PubMed Google Scholar * Highfield D, Mead A, Grimm JW, Rocha BA, Shaham Y (2002).
Reinstatement of cocaine seeking in mice: effects of cocaine priming, cocaine cues and food deprivation. _Psychopharmacology_ 161: 417–424. CAS PubMed Google Scholar * Highfield D, Yap J,
Grimm JW, Shalev U, Shaham Y (2001). Repeated lofexidine treatment attenuates stress-induced, but not drug cues-induced reinstatement of a heroin-cocaine mixture (speedball) seeking in
rats. _Neuropsychopharmacology_ 25: 320–331. CAS PubMed Google Scholar * Higley AE, Crane NA, Spadoni AD, Quello SB, Goodell V, Mason BJ (2011). Craving in response to stress induction in
a human laboratory paradigm predicts treatment outcome in alcohol-dependent individuals. _Psychopharmacology_ 218: 121–129. CAS PubMed Central PubMed Google Scholar * Holdstock T
(1967). Effects of septal stimulation in rats on heart rate and galvanic skin response. _Psychonom Sci_ 9: 38. Google Scholar * Hunt WA, Barnett LW, Branch LG (1971). Relapse rates in
addiction programs. _J Clin Psychol_ 27: 455–456. CAS PubMed Google Scholar * Jackson KJ, McLaughlin JP, Carroll FI, Damaj MI (2013). Effects of the kappa opioid receptor antagonist,
norbinaltorphimine, on stress and drug-induced reinstatement of nicotine-conditioned place preference in mice. _Psychopharmacology_ 226: 763–768. CAS PubMed Google Scholar * Jenkins J,
Meakin J, Nelson D, Thorn G (1958). Inhibition of adrenal steroid 11oxygenation in the dog. _Science_ 128: 478–480. CAS PubMed Google Scholar * Jobes ML, Ghitza UE, Epstein DH, Phillips
KA, Heishman SJ, Preston KL (2011). Clonidine blocks stress-induced craving in cocaine users. _Psychopharmacology_ 218: 83–88. CAS PubMed Central PubMed Google Scholar * Kalivas PW,
Duffy P, Latimer LG (1987). Neurochemical and behavioral effects of corticotropin-releasing factor in the ventral tegmental area of the rat. _J Pharmacol Exp Ther_ 242: 757–763. CAS PubMed
Google Scholar * Kalivas PW, McFarland K (2003). Brain circuitry and the reinstatement of cocaine-seeking behavior. _Psychopharmacology_ 168: 44–56. CAS PubMed Google Scholar * Kalivas
PW, Stewart J (1991). Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. _Brain Res Rev_ 16: 223–244. CAS PubMed Google
Scholar * Khantzian EJ (1985). The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. _Am J Psychiatry_ 142: 1259–1264. CAS PubMed Google Scholar
* Kirby LG, Rice KC, Valentino RJ (2000). Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. _Neuropsychopharmacology_ 22: 148–162. CAS
PubMed Google Scholar * Koob GF (2008). A role for brain stress systems in addiction. _Neuron_ 59: 11–34. CAS PubMed Central PubMed Google Scholar * Kosten TR, Rounsaville BJ, Kleber
HD (1986). A 2.5-year follow-up of depression, life crises, and treatment effects on abstinence among opioid addicts. _Arch Gen Psychiatry_ 43: 733–738. CAS PubMed Google Scholar *
Kowalczyk WJ, Phillips KA, Jobes ML, Kennedy AP, Ghitza UE, Agage DA _et al_ (2014). Clonidine maintenance prolongs opioid abstinence and decouples stress from craving in daily life: a
randomized controlled trial with ecological momentary assessment. _Am J Psychiatry_ (in press). * Kreibich AS, Blendy JA (2004). cAMP response element-binding protein is required for stress
but not cocaine-induced reinstatement. _J Neurosci_ 24: 6686–6692. CAS PubMed Central PubMed Google Scholar * Krupitsky E, Zvartau E, Blokhina E, Verbitskaya E, Tsoy M, Wahlgren V _et
al_ (2013). Naltrexone with or without guanfacine for preventing relapse to opiate addiction in St.-Petersburg, Russia. _Drug Alcohol Depend_ 132: 674–680. CAS PubMed Central PubMed
Google Scholar * Kupferschmidt DA, Brown ZJ, Erb S (2011). A procedure for studying the footshock-induced reinstatement of cocaine seeking in laboratory rats. _J Vis Exp_ pii 2265. *
Kvetnansky R, Sabban EL, Palkovits M (2009). Catecholaminergic systems in stress: structural and molecular genetic approaches. _Physiol Rev_ 89: 535–606. CAS PubMed Google Scholar * Kwako
LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R _et al_ (2015). The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a
randomized controlled experimental medicine study. _Neuropsychopharmacology_ 40: 1053–1063. CAS PubMed Google Scholar * Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D
_et al_ (2009). Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. _Proc Natl Acad Sci USA_ 106:
19168–19173. CAS PubMed Central PubMed Google Scholar * Le AD, Funk D, Harding S, Juzytsch W, Fletcher PJ (2009). The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced
increases in alcohol-seeking in rats. _Psychopharmacology_ 204: 477–488. PubMed Central Google Scholar * Le AD, Funk D, Harding S, Juzytsch W, Fletcher PJ, Shaham Y (2006). Effects of
dexfenfluramine and 5-HT3 receptor antagonists on stress-induced reinstatement of alcohol seeking in rats. _Psychopharmacology (Berl)_ 186: 82–92. CAS Google Scholar * Le AD, Funk D,
Harding S, Juzytsch W, Li Z, Fletcher PJ (2008). Intra-median raphe nucleus (MRN) infusions of muscimol, a GABA-A receptor agonist, reinstate alcohol seeking in rats: role of impulsivity and
reward. _Psychopharmacology_ 195: 605–615. PubMed Google Scholar * Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C _et al_ (2011). Effect of prazosin and guanfacine on
stress-induced reinstatement of alcohol and food seeking in rats. _Psychopharmacology (Berl)_ 218: 89–99. CAS Google Scholar * Le AD, Harding S, Juzytsch W, Fletcher PJ, Shaham Y (2002).
The role of corticotropin-releasing factor in the median raphe in relapse to alcohol seeking in rats. _J Neurosci_ 22: 7844–7849. CAS PubMed PubMed Central Google Scholar * Le AD,
Harding S, Juzytsch W, Funk D, Shaham Y (2005). Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. _Psychopharmacology_
179: 366–373. CAS PubMed Google Scholar * Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y (2000). The role of corticotropin-releasing factor in stress-induced relapse to
alcohol-seeking behavior in rats. _Psychopharmacology_ 150: 317–324. CAS PubMed Google Scholar * Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y (1999). Effects of naltrexone
and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. _Neuropsychopharmacology_ 21: 435–444.
CAS PubMed Google Scholar * Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y (1998). Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in
rats. _Psychopharmacology_ 135: 169–174. CAS PubMed Google Scholar * LeDoux JE (2000). Emotion circuits in the brain. _Annu Rev Neurosci_ 23: 155–184. CAS PubMed Google Scholar * Lee
B, Tiefenbacher S, Platt DM, Spealman RD (2004). Pharmacological blockade of alpha(2)-arenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys.
_Neuropsychopharmacology_ 29: 686–693. CAS PubMed Google Scholar * Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M _et al_ (2013). Maturation of silent synapses in amygdala-accumbens
projection contributes to incubation of cocaine craving. _Nat Neurosci_ 16: 1644–1651. CAS PubMed Central PubMed Google Scholar * Leri F, Flores J, Rodaros D, Stewart J (2002). Blockade
of stress-induced, but not cocaine-induced reinstatement, by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. _J
Neurosci_ 22: 5713–5718. CAS PubMed PubMed Central Google Scholar * Lewis BL, O'Donnell P (2000). Ventral tegmental area afferents to the prefrontal cortex maintain membrane
potential 'up' states in pyramidal neurons via D(1) dopamine receptors. _Cereb Cortex_ 10: 1168–1175. CAS PubMed Google Scholar * Li C, Staub DR, Kirby LG (2013). Role of GABAA
receptors in dorsal raphe nucleus in stress-induced reinstatement of morphine-conditioned place preference in rats. _Psychopharmacology_ 230: 537–545. CAS PubMed Google Scholar * Liu D,
Weiss F (2002a). Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of
corticotropin-releasing factor and opioid mechanisms. _J Neurosci_ 22: 7856–7861. CAS PubMed PubMed Central Google Scholar * Liu X, Weiss F (2002b). Additive effect of stress and drug
cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. _J Neurosci_ 22:
7856–7861. CAS PubMed PubMed Central Google Scholar * Liu X, Weiss F (2003). Stimulus conditioned to foot-shock stress reinstates alcohol-seeking behavior in an animal model of relapse.
_Psychopharmacology_ 168: 184–191. CAS PubMed Google Scholar * Lu L, Ceng X, Huang M (2000). Corticotropin-releasing factor receptor type I mediates stress-induced relapse to opiate
dependence in rats. _Neuroreport_ 11: 2373–2378. CAS PubMed Google Scholar * Lu L, Liu D, Ceng X (2001). Corticotropin-releasing factor receptor type 1 mediates stress-induced relapse to
cocaine-conditioned place preference in rats. _Eur J Pharmacol_ 415: 203–208. CAS PubMed Google Scholar * Lu L, Shepard JD, Scott Hall F, Shaham Y (2003). Effect of environmental
stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. _Neurosci Biobehav Rev_ 27: 457–491. CAS PubMed Google Scholar * Lu L, Zhang B,
Liu Z, Zhang Z (2002). Reactivation of cocaine conditioned place preference induced by stress is reversed by cholecystokinin-B receptors antagonist in rats. _Brain Res_ 954: 132–140. CAS
PubMed Google Scholar * Ma YY, Chu NN, Guo CY, Han JS, Cui CL (2007). NR2B-containing NMDA receptor is required for morphine-but not stress-induced reinstatement. _Exp Neurol_ 203:
309–319. CAS PubMed Google Scholar * Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R _et al_ (2014). Bidirectional modulation of incubation of cocaine craving by silent synapse-based
remodeling of prefrontal cortex to accumbens projections. _Neuron_ 83: 1453–1467. CAS PubMed Central PubMed Google Scholar * Mahler SV, Moorman DE, Feltenstein MW, Cox BM, Ogburn KB,
Bachar M _et al_ (2013). A rodent "self-report" measure of methamphetamine craving? Rat ultrasonic vocalizations during methamphetamine self-administration, extinction, and
reinstatement. _Behav Brain Res_ 236: 78–89. CAS PubMed Google Scholar * Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J _et al_ (2014). Designer receptors show
role for ventral pallidum input to ventral tegmental area in cocaine seeking. _Nat Neurosci_ 17: 577–585. CAS PubMed Central PubMed Google Scholar * Maier SF (1984). Learned helplessness
and animal models of depression. _Prog Neuropsychopharmacol Biol Psychiatry_ 8: 435–446. CAS PubMed Google Scholar * Maier SF, Watkins LR (2005). Stressor controllability and learned
helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. _Neurosci Biobehav Rev_ 29: 829–841. CAS PubMed Google Scholar * Mantsch JR, Baker DA,
Francis DM, Katz ES, Hoks MA, Serge JP (2008). Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following
long-access cocaine self-administration by rats. _Psychopharmacology_ 195: 591–603. CAS PubMed Google Scholar * Mantsch JR, Goeders NE (1999). Ketoconazole blocks the stress-induced
reinstatement of cocaine-seeking behavior in rats: relationship to the discriminative stimulus effects of cocaine. _Psychopharmacology_ 142: 399–407. CAS PubMed Google Scholar * Mantsch
JR, Vranjkovic O, Twining RC, Gasser PJ, McReynolds JR, Blacktop JM (2014). Neurobiological mechanisms that contribute to stress-related cocaine use. _Neuropharmacology_ 76 PT B: 383–394.
PubMed Google Scholar * Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H (2010). Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced
reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for beta-2 adrenergic receptors. _Neuropsychopharmacology_ 35: 2165–2178. CAS PubMed Central PubMed
Google Scholar * Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM (2014). Role of corticostriatal circuits in context-induced reinstatement of drug seeking. _Brain Res_ (in press). *
Margolis EB, Hjelmstad GO, Bonci A, Fields HL (2003). Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. _J Neurosci_ 23: 9981–9986. CAS PubMed PubMed Central Google
Scholar * Maric T, Sedki F, Chafetz D, Schoela N, Shalev U (2011). A role for neuropeptide Y Y5 but not the Y1-receptor subtype in food deprivation-induced reinstatement of heroin seeking
in the rat. _Psychopharmacology (Berl)_ 218: 693–701. CAS Google Scholar * Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y _et al_ (2007). The CRF(1) receptor antagonist
antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. _Psychopharmacology_ 195: 345–355. CAS PubMed Google
Scholar * Martin-Fardon R, Weiss F (2012). (−)-2-oxa-4-aminobicylco[3.1.0]hexane-4,6-dicarboxylic acid (LY379268) and 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine (MTEP) similarly
attenuate stress-induced reinstatement of cocaine seeking. _Addict Biol_ 17: 557–564. CAS PubMed Google Scholar * McFarland K, Davidge SB, Lapish CC, Kalivas PW (2004). Limbic and motor
circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. _J Neurosci_ 24: 1551–1560. CAS PubMed PubMed Central Google Scholar * McGaugh JL (2004). The amygdala
modulates the consolidation of memories of emotionally arousing experiences. _Annu Rev Neurosci_ 27: 1–28. CAS PubMed Google Scholar * McKee SA, Potenza MN, Kober H, Sofuoglu M, Arnsten
AF, Picciotto MR _et al_ (2014). A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. _J Psychopharmacol_ (in
press). * McLaughlin JP, Marton-Popovici M, Chavkin C (2003). Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. _J Neurosci_ 23:
5674–5683. CAS PubMed Central PubMed Google Scholar * McReynolds JR, Vranjkovic O, Thao M, Baker DA, Makky K, Lim Y _et al_ (2014). Beta-2 adrenergic receptors mediate stress-evoked
reinstatement of cocaine-induced conditioned place preference and increases in CRF mRNA in the bed nucleus of the stria terminalis in mice. _Psychopharmacology (Berl)_ 231: 3953–3963. CAS
Google Scholar * Meil WM, See RE (1996). Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. _Behav
Pharmacol_ 7: 754–763. CAS PubMed Google Scholar * Mongeau R, Blier P, de Montigny C (1997). The serotonergic and noradrenergic systems of the hippocampus: their interactions and the
effects of antidepressant treatments. _Brain Res Rev_ 23: 145–195. CAS PubMed Google Scholar * Moore RY, Bloom FE (1979). Central catecholaimine neuron systems: anatomy and physiology of
the norepinephrine and epinephrine systems. _Annu Rev Neurosci_ 2: 113–168. CAS PubMed Google Scholar * Moran-Santa Maria MM, Baker NL, Ramakrishnan V, Brady KT, McRae-Clark A (2015a).
Impact of acute guanfacine administration on stress and cue reactivity in cocaine-dependent individuals. _Am J Drug Alcohol Abuse_ 41: 146–152. PubMed Google Scholar * Moran-Santa Maria
MM, McRae-Clark A, Baker NL, Ramakrishnan V, Brady KT (2015b). Yohimbine administration and cue-reactivity in cocaine-dependent individuals. _Psychopharmacology_ 231: 4157–4165. Google
Scholar * Morley JE, Levine AS (1982). Corticotrophin releasing factor, grooming and ingestive behavior. _Life Sci_ 31: 1459–1464. CAS PubMed Google Scholar * Nair SG, Adams-Deutsch T,
Epstein DH, Shaham Y (2009). The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. _Prog Neurobiol_ 89: 18–45. CAS
PubMed Central PubMed Google Scholar * Nair SG, Golden SA, Shaham Y (2008). Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and
reinstatement of food seeking in rats. _Br J Pharmacol_ 154: 406–416. CAS PubMed Central PubMed Google Scholar * Nair SG, Navarre BM, Cifani C, Pickens CL, Bossert JM, Shaham Y (2011).
Role of dorsal medial prefrontal cortex dopamine D1-family receptors in relapse to high-fat food seeking induced by the anxiogenic drug yohimbine. _Neuropsychopharmacology_ 36: 497–510. CAS
PubMed Google Scholar * Nawata Y, Kitaichi K, Yamamoto T (2012). Increases of CRF in the amygdala are responsible for reinstatement of methamphetamine-seeking behavior induced by
footshock. _Pharmacol Biochem Behav_ 101: 297–302. CAS PubMed Google Scholar * Nielsen CK, Simms JA, Bito-Onon JJ, Li R, Ananthan S, Bartlett SE (2012). The delta opioid receptor
antagonist, SoRI-9409, decreases yohimbine stress-induced reinstatement of ethanol-seeking. _Addict Biol_ 17: 224–234. CAS PubMed Google Scholar * O'Brien CP (2005). Anticraving
medications for relapse prevention: a possible new class of psychoactive medications. _Am J Psychiatry_ 162: 1423–1431. PubMed Google Scholar * Omelchenko N, Sesack SR (2007). Glutamate
synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. _Neuroscience_ 146: 1259–1274. CAS PubMed Google Scholar * Piazza PV, Le Moal M
(1998). The role of stress in drug self-administration. _Trends Pharmacol Sci_ 19: 67–74. CAS PubMed Google Scholar * Pickens CL, Cifani C, Navarre BM, Eichenbaum H, Theberge FR, Baumann
MH _et al_ (2012). Effect of fenfluramine on reinstatement of food seeking in female and male rats: implications for the predictive validity of the reinstatement model. _Psychopharmacology
(Berl)_ 221: 341–353. CAS Google Scholar * Pierce RC, Kumaresan V (2006). The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? _Neurosci
Biobehav Rev_ 30: 215–238. CAS PubMed Google Scholar * Plaza-Zabala A, Martin-Garcia E, de Lecea L, Maldonado R, Berrendero F (2010). Hypocretins regulate the anxiogenic-like effects of
nicotine and induce reinstatement of nicotine-seeking behavior. _J Neurosci_ 30: 2300–2310. PubMed Central PubMed Google Scholar * Polter AM, Bishop RA, Briand LA, Graziane NM, Pierce RC,
Kauer JA (2014). Poststress block of kappa opioid receptors rescues long-term potentiation of inhibitory synapses and prevents reinstatement of cocaine seeking. _Biol Psychiatry_ 76:
785–793. CAS PubMed Central PubMed Google Scholar * Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I (1998). Effects of corticotropin-releasing factor on brain serotonergic activity.
_Neuropsychopharmacology_ 18: 492–502. CAS PubMed Google Scholar * Redila VA, Chavkin C (2008). Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system.
_Psychopharmacology_ 200: 59–70. CAS PubMed Central PubMed Google Scholar * Redmond DEJ, Huang YH (1979). Locus coeruleus and anxiety. _Life Sci_ 25: 2149–2162. CAS PubMed Google
Scholar * Regier PS, Claxton AB, Zlebnik NE, Carroll ME (2014). Cocaine-, caffeine-, and stress-evoked cocaine reinstatement in high vs. low impulsive rats: treatment with allopregnanolone.
_Drug Alcohol Depend_ 143: 58–64. CAS PubMed Central PubMed Google Scholar * Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodriguez-Arias M, Armario A, Minarro J (2006). Social stress
is as effective as physical stress in reinstating morphine-induced place preference in mice. _Psychopharmacology_ 185: 459–470. CAS PubMed Google Scholar * Richards JK, Simms JA,
Steensland P, Taha SA, Borgland SL, Bonci A _et al_ (2008). Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in
Long-Evans rats. _Psychopharmacology (Berl)_ 199: 109–117. CAS Google Scholar * Rodaros D, Caruana DA, Amir S, Stewart J (2007). Corticotropin-releasing factor projections from limbic
forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. _Neuroscience_ 150: 8–13. CAS PubMed Google Scholar * Rotllant D, Ons S, Carrasco J,
Armario A (2002). Evidence that metyrapone can act as a stressor: effect on pituitary-adrenal hormones, plasma glucose and brain c-fos induction. _Eur J Neurosci_ 16: 693–700. PubMed
Google Scholar * Sakanaka M, Shibasaki T, Lederis K (1986). Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex.
_Brain Res_ 382: 213–238. CAS PubMed Google Scholar * Sanchez CJ, Bailie TM, Wu WR, Li N, Sorg BA (2003). Manipulation of dopamine d1-like receptor activation in the rat medial prefrontal
cortex alters stress- and cocaine-induced reinstatement of conditioned place preference behavior. _Neuroscience_ 119: 497–505. CAS PubMed Google Scholar * Sanchez CJ, Sorg BA (2001).
Conditioned fear stimuli reinstate cocaine-induced conditioned place preference. _Brain Res_ 908: 86–92. CAS PubMed Google Scholar * Sarnyai Z, Shaham Y, Heinrichs SC (2001). The role of
corticotropin-releasing factor in drug addiction. _Pharmacol Rev_ 53: 209–244. CAS PubMed Google Scholar * Sawchenko PE, Li HY, Ericsson A (2000). Circuits and mechanisms governing
hypothalamic responses to stress: a tale of two paradigms. _Prog Brain Res_ 122: 61–78. CAS PubMed Google Scholar * Schank JR, Goldstein AL, Rowe KE, King CE, Marusich JA, Wiley JL _et
al_ (2012). The kappa opioid receptor antagonist JDTic attenuates alcohol seeking and withdrawal anxiety. _Addict Biol_ 17: 634–647. CAS PubMed Central PubMed Google Scholar * Schank JR,
King CE, Sun H, Cheng K, Rice KC, Heilig M _et al_ (2014). The role of the neurokinin-1 receptor in stress-induced reinstatement of alcohol and cocaine seeking. _Neuropsychopharmacology_
39: 1093–1101. CAS PubMed Google Scholar * Schank JR, Pickens CL, Rowe KE, Cheng K, Thorsell A, Rice KC _et al_ (2011). Stress-induced reinstatement of alcohol-seeking in rats is
selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. _Psychopharmacology (Berl)_ 218: 111–119. CAS Google Scholar * Schmoutz CD, Zhang Y, Runyon SP, Goeders NE (2012).
Antagonism of the neuropeptide S receptor with RTI-118 decreases cocaine self-administration and cocaine-seeking behavior in rats. _Pharmacol Biochem Behav_ 103: 332–337. CAS PubMed Central
PubMed Google Scholar * Schroeder JP, Epps SA, Grice TW, Weinshenker D (2013). The selective dopamine beta-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking
behavior. _Neuropsychopharmacology_ 38: 1032–1038. CAS PubMed Central PubMed Google Scholar * Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M _et al_ (1996).
CP154,526: a potent and selective non-peptide antagonist of corticotropin releasing factor receptors. _Proc Natl Acad Sci USA_ 93: 10477–10482. CAS PubMed PubMed Central Google Scholar *
Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ (2001). Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. _Proc Natl
Acad Sci USA_ 98: 301–306. CAS PubMed Google Scholar * Sedki F, Eigenmann K, Gelinas J, Schouela N, Courchesne S, Shalev U (2015). A role for kappa-, but not mu-opioid, receptor
activation in acute food deprivation-induced reinstatement of heroin seeking in rats. _Addict Biol_ 20: 423–432. CAS PubMed Google Scholar * See RE (2005). Neural substrates of
cocaine-cue associations that trigger relapse. _Eur J Pharmacol_ 526: 140–146. CAS PubMed Google Scholar * See RE, Waters RP (2010). Pharmacologically-induced stress: a cross-species
probe for translational research in drug addiction and relapse. _Am J Transl Res_ 3: 81–89. PubMed Central PubMed Google Scholar * Sekino A, Ohata H, Mano-Otagiri A, Arai K, Shibasaki T
(2004). Both corticotropin-releasing factor receptor type 1 and type 2 are involved in stress-induced inhibition of food intake in rats. _Psychopharmacology_ 176: 30–38. CAS PubMed Google
Scholar * Self DW (2004). Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. _Neuropharmacology_ 47: 242–255. CAS PubMed Google
Scholar * Seligman MEP, Maier SF (1967). Failure to escape traumatic shock. _J Exp Psychol_ 74: 1–9. CAS PubMed Google Scholar * Selye H (1936). A syndrome produced by diverse nocuous
agents. _Nature_ 138: 32–36. Google Scholar * Sesack SR, Deutch AY, Roth RH, Bunney BS (1989). Topographical organization of the efferent projections of the medial prefrontal cortex in the
rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. _J Comp Neurol_ 290: 213–242. CAS PubMed Google Scholar * Setlow B, Holland PC, Gallagher M (2002).
Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive pavlovian second-order conditioned responses. _Behav Neurosci_ 116: 267–275. PubMed Google Scholar
* Shaham Y, Erb S, Leung S, Buczek Y, Stewart J (1998). CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to
drug seeking in cocaine- and heroin-trained rats. _Psychopharmacology_ 137: 184–190. CAS PubMed Google Scholar * Shaham Y, Erb S, Stewart J (2000a). Stress-induced relapse to heroin and
cocaine seeking in rats: a review. _Brain Res Brain Res Rev_ 33: 13–33. CAS PubMed Google Scholar * Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J (1997). Corticotropin-releasing
factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. _J Neurosci_ 17: 2605–2614. CAS PubMed PubMed Central Google Scholar * Shaham Y,
Highfield D, Delfs J, Leung S, Stewart J (2000b). Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons.
_Eur J Neurosci_ 12: 292–302. CAS PubMed Google Scholar * Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003). The reinstatement model of drug relapse: history, methodology and major
findings. _Psychopharmacology_ 168: 3–20. CAS PubMed Google Scholar * Shaham Y, Stewart J (1995). Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not
withdrawal. _Psychopharmacology_ 119: 334–341. CAS PubMed Google Scholar * Shaham Y, Stewart J (1996). Effects of opioid and dopamine receptor antagonists on relapse induced by stress and
re-exposure to heroin in rats. _Psychopharmacology_ 125: 385–391. CAS PubMed Google Scholar * Shalev U, Erb S, Shaham Y (2010). Role of CRF and other neuropeptides in stress-induced
reinstatement of drug seeking. _Brain Res_ 1314: 15–28. CAS PubMed Google Scholar * Shalev U, Finnie PS, Quinn T, Tobin S, Wahi P (2006). A role for corticotropin-releasing factor, but
not corticosterone, in acute food-deprivation-induced reinstatement of heroin seeking in rats. _Psychopharmacology (Berl)_ 187: 376–384. CAS Google Scholar * Shalev U, Grimm JW, Shaham Y
(2002). Neurobiology of relapse to heroin and cocaine seeking: a review. _Pharmacol Rev_ 54: 1–42. CAS PubMed Google Scholar * Shalev U, Highfield D, Yap J, Shaham Y (2000). Stress and
relapse to drug seeking in rats: studies on the generality of the effect. _Psychopharmacology_ 150: 337–346. CAS PubMed Google Scholar * Shalev U, Marinelli M, Baumann MH, Piazza PV,
Shaham Y (2003). The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. _Psychopharmacology_ 168: 170–176. CAS PubMed Google Scholar * Shalev
U, Morales M, Hope B, Yap J, Shaham Y (2001). Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats.
_Psychopharmacology_ 156: 98–107. CAS PubMed Google Scholar * Shelton KL, Beardsley PM (2005). Interaction of extinguished cocaine-conditioned stimuli and footshock on reinstatement in
rats. _Int J Comp Psychol_ 18: 154–166. Google Scholar * Shepard JD, Bossert JM, Liu SY, Shaham Y (2004). The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of
drug relapse. _Biol Psychiatry_ 55: 1082–1089. CAS PubMed Google Scholar * Shiffman S, Wills TA (1985) _Coping and Substance Abuse_. Academic Press: Orlando. Google Scholar * Sidhpura N,
Weiss F, Martin-Fardon R (2010). Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history
of ethanol dependence. _Biol Psychiatry_ 67: 804–811. CAS PubMed Central PubMed Google Scholar * Silberman Y, Winder DG (2013). Emerging role for corticotropin releasing factor
signaling in the bed nucleus of the stria terminalis at the intersection of stress and reward. _Front Psychiatry_ 4: 42. PubMed Central PubMed Google Scholar * Simms JA, Haass-Koffler CL,
Bito-Onon J, Li R, Bartlett SE (2012). Mifepristone in the central nucleus of the amygdala reduces yohimbine stress-induced reinstatement of ethanol-seeking. _Neuropsychopharmacology_ 37:
906–918. CAS PubMed Google Scholar * Sinha R (2001). How does stress increase risk of drug abuse and relapse. _Psychopharmacology_ 158: 343–359. CAS PubMed Google Scholar * Sinha R
(2009). Modeling stress and drug craving in the laboratory: implications for addiction treatment development. _Addict Biol_ 14: 84–98. PubMed Google Scholar * Sinha R, Catapano D,
O'Malley S (1999). Stress-induced craving and stress response in cocaine dependent individuals. _Psychopharmacology_ 142: 343–351. CAS PubMed Google Scholar * Sinha R, Fox HC, Hong
KI, Hansen J, Tuit K, Kreek MJ (2011a). Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. _Arch Gen
Psychiatry_ 68: 942–952. CAS PubMed Central PubMed Google Scholar * Sinha R, Fuse T, Aubin LR, O'Malley SS (2000). Psychological stress, drug -related cues and cocaine craving.
_Psychopharnacology_ 152: 140–148. CAS Google Scholar * Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ (2006). Stress-induced cocaine craving and hypothalamic-pituitary-adrenal
responses are predictive of cocaine relapse outcomes. _Arch Gen Psychiatry_ 63: 324–331. PubMed Google Scholar * Sinha R, Kimmerling A, Doebrick C, Kosten TR (2007). Effects of lofexidine
on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. _Psychopharmacology_ 190: 569–574. CAS PubMed Google Scholar * Sinha R, Shaham Y,
Heilig M (2011b). Translational and reverse translational research on the role of stress in drug craving and relapse. _Psychopharmacology (Berl)_ 218: 69–82. CAS Google Scholar * Smith RJ,
Aston-Jones G (2011). alpha(2) Adrenergic and imidazoline receptor agonists prevent cue-induced cocaine seeking. _Biol Psychiatry_ 70: 712–719. CAS PubMed Central PubMed Google Scholar
* Staub DR, Lunden JW, Cathel AM, Dolben EL, Kirby LG (2012). Morphine history sensitizes postsynaptic GABA receptors on dorsal raphe serotonin neurons in a stress-induced relapse model in
rats. _Psychoneuroendocrinology_ 37: 859–870. CAS PubMed Google Scholar * Stefanik MT, Kupchik YM, Brown RM, Kalivas PW (2013). Optogenetic evidence that pallidal projections, not nigral
projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking. _J Neurosci_ 33: 13654–13662. CAS PubMed Central PubMed Google Scholar * Stewart J, de Wit H
(1987). Reinstatement of drug-taking behavior as a method of assessing incentive motivational properties of drugs In: Bozarth MA (ed) _Methods of Assessing the Reinforcing Properties of
Abused Drugs_. Springer-Verlag: New York. pp 211–227. Google Scholar * Stone EA (1983). Adaptation to stress and brain noradrenergic receptors. _Neurosci Biobehav Rev_ 7: 503–509. CAS
PubMed Google Scholar * Stopponi S, de Guglielmo G, Somaini L, Cippitelli A, Cannella N, Kallupi M _et al_ (2013). Activation of PPARgamma by pioglitazone potentiates the effects of
naltrexone on alcohol drinking and relapse in msP rats. _Alcohol Clin Exp Res_ 37: 1351–1360. CAS PubMed Google Scholar * Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S,
Kallupi M _et al_ (2011). Activation of nuclear PPARgamma receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. _Biol Psychiatry_ 69:
642–649. CAS PubMed Google Scholar * Stopponi S, Somaini L, Cippitelli A, de Guglielmo G, Kallupi M, Cannella N _et al_ (2012). Pregabalin reduces alcohol drinking and relapse to alcohol
seeking in the rat. _Psychopharmacology (Berl)_ 220: 87–96. CAS Google Scholar * Takahata R, Moghaddam B (1998). Glutamatergic regulation of basal and stimulus-activated dopamine release
in the prefrontal cortex. _J Neurochem_ 71: 1443–1449. CAS PubMed Google Scholar * Thierry AM, Tassin JP, Blanc G, Glowinski J (1976). Selective activation of mesocortical DA system by
stress. _Nature_ 263: 242–244. CAS PubMed Google Scholar * Tobin S, Newman AH, Quinn T, Shalev U (2009). A role for dopamine D1-like receptors in acute food deprivation-induced
reinstatement of heroin seeking in rats. _Int J Neuropsychopharmacol_ 12: 217–226. CAS PubMed Google Scholar * Tobin S, Sedki F, Abbas Z, Shalev U (2013). Antagonism of the dopamine
D1-like receptor in mesocorticolimbic nuclei attenuates acute food deprivation-induced reinstatement of heroin seeking in rats. _Eur J Neurosci_ 37: 972–981. PubMed Google Scholar *
Twining RC, Wheeler DS, Ebben AL, Jacobsen AJ, Robble MA, Mantsch JR _et al_ (2015). Aversive stimuli Drive drug seeking in a state of low dopamine tone. _Biol Psychiatry_ 77: 895–902. CAS
PubMed Google Scholar * Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George TD _et al_ (2011). Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates
with alcoholism severity, but is insensitive to acamprosate. _Neuropsychopharmacology_ 60: 303–311. Google Scholar * Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A (2003).
Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. _Neuron_ 39: 401–407. CAS PubMed Google Scholar * Valdez
GR, Platt DM, Rowlett JK, Ruedi-Bettschen D, Spealman RD (2007). Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms.
_J Pharmacol Exp Ther_ 323: 525–533. CAS PubMed Google Scholar * Van't Veer A, Carlezon WA Jr. (2013). Role of kappa-opioid receptors in stress and anxiety-related behavior.
_Psychopharmacology (Berl)_ 229: 435–452. CAS Google Scholar * Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C _et al_ (2000). Distribution of mRNAs encoding CRF receptors in
brain and pituitary of rat and mouse. _J Comp Neurol_ 428: 191–212. CAS PubMed Google Scholar * Vaughn LK, Mantsch JR, Vranjkovic O, Stroh G, Lacourt M, Kreutter M _et al_ (2012).
Cannabinoid receptor involvement in stress-induced cocaine reinstatement: potential interaction with noradrenergic pathways. _Neuroscience_ 204: 117–124. CAS PubMed Google Scholar *
Vertes RP (1991). A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. _J Comp Neurol_ 313: 643–668. CAS PubMed Google Scholar * Vertes RP, Fortin WJ, Crane
AM (1999). Projections of the median raphe nucleus in the rat. _J Comp Neurol_ 407: 555–582. CAS PubMed Google Scholar * Vranjkovic O, Gasser PJ, Gerndt CH, Baker DA, Mantsch JR (2014).
Stress-induced cocaine seeking requires a beta-2 adrenergic receptor-regulated pathway from the ventral bed nucleus of the stria terminalis that regulates CRF actions in the ventral
tegmental area. _J Neurosci_ 34: 12504–12514. PubMed Central PubMed Google Scholar * Vranjkovic O, Hang S, Baker DA, Mantsch JR (2012). beta-adrenergic receptor mediation of
stress-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: roles for beta1 and beta2 adrenergic receptors. _J Pharmacol Exp Ther_ 342: 541–551. CAS
PubMed Central PubMed Google Scholar * Wang B, Luo F, Zhang WT, Han JS (2000). Stress or drug priming induces reinstatement of extinguished conditioned place preference. _Neuroreport_ 11:
2781–2784. CAS PubMed Google Scholar * Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB (2005). Cocaine experience establishes control of midbrain glutamate and dopamine by
corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. _J Neurosci_ 25: 5389–5396. CAS PubMed PubMed Central Google Scholar * Wang B, You ZB, Rice KC, Wise RA
(2007). Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. _Psychopharmacology_ 193: 283–294. CAS
PubMed Google Scholar * Wang B, You ZB, Wise RA (2009). Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local
corticotropin-releasing factor network. _Biol Psychiatry_ 65: 857–862. CAS PubMed Central PubMed Google Scholar * Wang B, You ZB, Wise RA (2012). Heroin self-administration experience
establishes control of ventral tegmental glutamate release by stress and environmental stimuli. _Neuropsychopharmacology_ 37: 2863–2869. CAS PubMed Central PubMed Google Scholar * Wang
J, Fang Q, Liu Z, Lu L (2006). Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine
conditioned place preference in rats. _Psychopharmacology_ 185: 19–28. CAS PubMed Google Scholar * Wang X, Cen X, Lu L (2001). Noradrenaline in the bed nucleus of the stria terminalis is
critical for stress-induced reactivation of morphine-conditioned place preference in rats. _Eur J Pharmacol_ 432: 153–161. CAS PubMed Google Scholar * Waters RP, See RE (2011). Chronic
cocaine self-administration attenuates the anxiogenic-like and stress potentiating effects of the benzodiazepine inverse agonist, FG 7142. _Pharmacol Biochem Behav_ 99: 408–413. CAS PubMed
Central PubMed Google Scholar * Weiss F (2005). Neurobiology of craving, conditioned reward and relapse. _Curr Opin Pharmacol_ 5: 9–19. CAS PubMed Google Scholar * Weiss RD (2004).
Adherence to pharmacotherapy in patients with alcohol and opioid dependence. _Addiction_ 99: 1382–1392. PubMed Google Scholar * Westerink BH, Kwint HF, deVries JB (1996). The pharmacology
of mesolimbic dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. _J Neurosci_ 16: 2605–2611. CAS PubMed Central PubMed
Google Scholar * White FJ, Hu XT, Zhang XF, Wolf ME (1995). Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system.
_J Pharmacol Exp Ther_ 273: 445–454. CAS PubMed Google Scholar * Xi ZX, Gilbert J, Campos AC, Kline N, Ashby CR Jr., Hagan JJ _et al_ (2004). Blockade of mesolimbic dopamine D3 receptors
inhibits stress-induced reinstatement of cocaine-seeking in rats. _Psychopharmacology (Berl)_ 176: 57–65. CAS Google Scholar * Yamada H, Bruijnzeel AW (2011). Stimulation of
alpha2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats. _Neuropharmacology_ 60: 303–311. CAS PubMed Google
Scholar * Zahm DS, Williams EA, Latimer MP, Winn P (2001). Ventral mesopontine projections of the caudomedial shell of the nucleus accumbens and extended amygdala in the rat: double
dissociation by organization and development. _J Comp Neurol_ 436: 111–125. CAS PubMed Google Scholar * Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F (2006). Activation
of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. _J Neurosci_ 26:
9967–9974. CAS PubMed PubMed Central Google Scholar * Zhou L, Ghee SM, Chan C, Lin L, Cameron MD, Kenny PJ _et al_ (2012). Orexin-1 receptor mediation of cocaine seeking in male and
female rats. _J Pharmacol Exp Ther_ 340: 801–809. CAS PubMed Central PubMed Google Scholar * Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW (2007). Effects of the CRF receptor
antagonist D-Phe CRF(12-41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. _Neuropharmacology_ 53: 958–966. CAS
PubMed Central PubMed Google Scholar * Zorrilla EP, Heilig M, de Wit H, Shaham Y (2013a). Behavioral, biological, and chemical perspectives on targeting CRF(1) receptor antagonists to
treat alcoholism. _Drug Alcohol Depend_ 128: 175–186. CAS PubMed Central PubMed Google Scholar * Zorrilla EP, Roberts AJ, Rivier JE, Koob GF (2013b). Anxiolytic-like effects of
antisauvagine-30 in mice are not mediated by CRF2 receptors. _PLoS One_ 8: e63942. CAS PubMed Central PubMed Google Scholar Download references ACKNOWLEDGEMENTS The writing of this
review was supported by extramural (JRM, DAB, and AL) and intramural (YS) funds from the National Institute on Drug Abuse, NIH. Dr David Baker and Dr John Mantsch receive compensation from
and have financial holdings in Promentis Pharmaceuticals. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biomedical Sciences, Marquette University, Milwaukee, Wisconsin, USA
John R Mantsch & David A Baker * Center for Addiction and Mental Health, Campbell Family Mental Health Research Institute, University of Toronto, Toronto, ON, Canada Douglas Funk &
Anh D Lê * Intramural Research Program, NIDA-NIH, Baltimore, MD, USA Yavin Shaham Authors * John R Mantsch View author publications You can also search for this author inPubMed Google
Scholar * David A Baker View author publications You can also search for this author inPubMed Google Scholar * Douglas Funk View author publications You can also search for this author
inPubMed Google Scholar * Anh D Lê View author publications You can also search for this author inPubMed Google Scholar * Yavin Shaham View author publications You can also search for this
author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to John R Mantsch or Yavin Shaham. POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT
SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 POWERPOINT SLIDE FOR FIG. 5 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Mantsch, J., Baker, D., Funk, D.
_et al._ Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. _Neuropsychopharmacol_ 41, 335–356 (2016). https://doi.org/10.1038/npp.2015.142 Download citation * Received: 24
March 2015 * Revised: 01 May 2015 * Accepted: 08 May 2015 * Published: 15 May 2015 * Issue Date: January 2016 * DOI: https://doi.org/10.1038/npp.2015.142 SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative
