Childhood abuse and deprivation are associated with distinct sex-dependent differences in brain morphology
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Childhood adversity (CA) has been associated with long-term structural brain alterations and an increased risk for psychiatric disorders. Evidence is emerging that subtypes of CA, varying in
the dimensions of threat and deprivation, lead to distinct neural and behavioral outcomes. However, these specific associations have yet to be established without potential confounders such
as psychopathology. Moreover, differences in neural development and psychopathology necessitate the exploration of sexual dimorphism. Young healthy adult subjects were selected based on
history of CA from a large database to assess gray matter (GM) differences associated with specific subtypes of adversity. We compared voxel-based morphometry data of subjects reporting
specific childhood exposure to abuse (n=127) or deprivation (n=126) and a similar sized group of controls (n=129) without reported CA. Subjects were matched on age, gender, and educational
level. Differences between CA subtypes were found in the fusiform gyrus and middle occipital gyrus, where subjects with a history of deprivation showed reduced GM compared with subjects with
a history of abuse. An interaction between sex and CA subtype was found. Women showed less GM in the visual posterior precuneal region after both subtypes of CA than controls. Men had less
GM in the postcentral gyrus after childhood deprivation compared with abuse. Our results suggest that even in a healthy population, CA subtypes are related to specific alterations in brain
structure, which are modulated by sex. These findings may help understand neurodevelopmental consequences related to CA.
Childhood adversities (CAs) are among the most consistently documented risk factors for psychiatric disorders (Cuijpers et al, 2011; McLaughlin et al, 2012). They are in particular linked to
affective, addiction, and personality disorders and possibly associated with distinct phenotypes within these disorders (Gilbert et al, 2009; Heim et al, 2010; McLaughlin et al, 2012). To
learn more about the effects of these adversities on brain structure, it is essential to study healthy individuals where the consequences of CA are not confounded by the possible effects of
psychopathology (Dannlowski et al, 2012). Indeed such studies have highlighted numerous long-term structural differences in the brain related to CA (Dannlowski et al, 2012; Lim et al, 2014;
Lupien et al, 2009). Most consistently, changes in gray matter (GM) volume in the prefrontal cortex, sensory association cortices, anterior cingulate gyrus, the amygdala, hippocampus,
insula, striatum, and the cerebellum have been found in healthy individuals when compared with controls with no history of adverse childhood experiences (Dannlowski et al, 2012; Edmiston et
al, 2011).
Recently, it has been suggested that different types of adverse childhood experiences may lead to distinct morphological alterations in the brain, possibly corresponding to different types
of psychopathology in adulthood (Humphreys and Zeanah, 2014; McLaughlin et al, 2014a; Sheridan and McLaughlin, 2014). For example, cortical thinning in the somatosensory cortex representing
the female genital area was found exclusively in women that had experienced sexual abuse compared with other forms of childhood trauma (Heim et al, 2013). In subjects with a specific history
of emotional abuse, thinning in areas involved in emotion processing such as the medial prefrontal cortex and precuneus have been found (Heim et al, 2013; van Harmelen et al, 2010). In
addition, it was proposed that a distinction could also be made between adversity in the form of deprivation, such as absence of the expected social input, and direct threat, such as in
active abuse. These two types of adversity potentially lead to different (neural) outcomes (Edmiston et al, 2011; Humphreys and Zeanah, 2014; McLaughlin et al, 2014a; Sheridan and
McLaughlin, 2014). Deprivation could lead to changes in the association cortex, involved in higher cognitive and social processes, whereas abuse could give rise to alterations in circuits
involved in emotional learning (McLaughlin et al, 2014a; Sheridan and McLaughlin, 2014). Although these approaches seem promising, practical problems such as co-existing psychopathology and
the frequent co-occurrence of different subtypes of adversity have complicated the understanding of their specific impact on neural development.
Our first aim was therefore to assess healthy subjects for specific regional GM volume differences corresponding to different types of CA. To this end, we selected subjects from a database
of more than 2700 subjects by their reported history of specific childhood events. We then created two matched groups that had experienced items from either the ‘abuse’ or ‘deprivation’
categories. Because of the healthy nature of our population, we also included relatively mild indicators of deprivation, such as death of a close relative (eg, caring grandparent).
We hypothesized that subjects with a history of deprivation would specifically show GM reductions compared with a matched control group in somatosensory brain regions and association cortex
(McLaughlin et al, 2014a; Sheridan and McLaughlin, 2014). Subjects with a history of abuse were hypothesized to differ from the controls especially in brain regions involved in emotion
processing and emotional learning, such as the amygdala and hippocampus, ventromedial and dorsolateral prefrontal cortex, and the precuneus (Heim et al, 2013; McLaughlin et al, 2014a;
Sheridan and McLaughlin, 2014; van Harmelen et al, 2010).
An important secondary question was to find out whether the two distinct types of CA were associated with different GM correlates in the two sexes. Girls and boys have been found to show
distinct regional developmental trajectories of GM during childhood and adolescence (Lenroot and Giedd, 2010; Rijpkema et al, 2011). The occurrence of adverse events during these periods
could therefore have gender-dependent associations with GM in specific brain regions (Tottenham and Sheridan, 2009). Moreover, the type of event could also have a different impact.
Importantly, men and women not only differ in prevalence of affective disorders, but also in their etiological pathways to depression (Kendler and Gardner, 2014). For example, men seem more
vulnerable to (sexual) abuse, life events, and instrumental problems, whereas for women, problems relating to interpersonal warmth and relationships have greater impact (Kendler and Gardner,
2014). Finally, men have often been considerably underrepresented in the majority of studies looking into childhood events, making it difficult to generalize these results to both sexes,
whereas the prevalence of CA in men is substantial (Edwards et al, 2003). We therefore hypothesized that sex could interact with the type of CA in its effect on GM structure.
The study was part of the Cognomics Initiative’s Brain Imaging Genetics (BIG) project at the Donders Institute for Brain, Cognition and Behavior of the Radboud University in Nijmegen, the
Netherlands (www.cognomics.nl). Participants were screened before participation in this study by self-reported questionnaires. They were excluded if they had a history of somatic disease
potentially affecting the brain, current or past psychiatric or neurological disorder, medication (except hormonal contraceptives) or illicit drug use during the past 6 months, history of
substance abuse, current or past alcohol dependence, pregnancy, lactation, menopause or MRI contraindications. A total of 2737 subjects was included in the BIG project database at the time
of our analysis.
Subjects were specifically selected for the current analysis based on their history of CAs. An age limit (age 18–35 years) was set to homogenize the groups. Of note, this is also the age
range of onset for most forms of CA-related psychopathology (Lupien et al, 2009). Specific types of adversities were assessed using an adapted version of the ‘List of Threatening Life
Events’ (Brugha et al, 1985). Participants were asked whether they had experienced a list of predefined events (a) before the age of 16 years and/ or (b) at or after the age of 16 years
(Supplementary Table S1). Three groups were based on CA type: an ‘abuse group’, a ‘deprivation group’, and a control group. Subjects were assigned to the abuse group if (i) they reported any
verbal, physical, and/ or sexual abuse before the age of 16 years and (ii) did not report any items indicating deprivation before 16 years. These deprivation items consisted of a history of
separation from parent, severe financial problems, health problems of a close relative, and/ or death of a close relative. The latter two are not explicitly mentioned in common definitions
of childhood deprivation (eg, (Sheridan and McLaughlin, 2014)), however are considered here as indicators that a child was deprived of expected social input (eg, death of a close relative
that most likely resulted in the absence of a caregiver). Importantly, we only included subjects who indicated a history of separation from parent under 16 years, as separation from parent
later in life does not necessarily indicate deprivation (eg, leaving home to study abroad). Subjects who reported any items indicating deprivation before the age of 16 years and did not
report any lifetime abuse were assigned to the deprivation group. Subjects in the control group did not report any items before age 16 or any lifetime abuse.
The abuse group was the smallest group (n=131) in our sample. Therefore, subjects from the two other groups were matched to the abuse group based on age, sex, and educational level to obtain
equal group sizes. This gave us a subset of 393 subjects (131 per group) for our study. After the exclusion of 11 subjects due to insufficient data quality for VBM analysis or missing data,
we had a final sample of 382 subjects (167 men and 215 women) for this analysis (Table 1). The mean (SD) age was 22.1 (3.7) years for men and 22.0 (3.4) years for women.
The negative affect scale of the Positive Affect and Negative Affect Schedule (PANAS) was used to assess negative affect at the time of filling in the life-event questionnaire (Watson et al,
1988). Negative affect was entered as a covariate in our analysis to correct for possible recollection bias due to current affective state. When not correcting for PANAS scores, the effect
of life events could be overestimated, as those individuals with currently lower mood may also be more likely to report negative life events (Chan et al, 2007). We also conducted our main
analysis without PANAS correction, which enabled us to verify whether this covariate influenced our findings.
Anatomical T1-weighted MRI data were acquired at the Donders Centre for Cognitive Neuroimaging. All scans covered the entire brain and had a voxel size of 1 × 1 × 1 mm3.
For 48% (n=182) of the subjects, images were acquired at 1.5 T Siemens Sonata or Avanto scanners (Siemens, Erlangen, Germany), using small variations to a standard T1-weighted 3D MPRAGE
sequence (repetition time (TR) 2300 ms, inversion time (TI) 1100 ms, echo time (TE) 3.03 ms, 192 sagittal slices, field of view 256 mm). These variations included a TR/TI/TE/slices of
2730/1000/2.95/176, 2250/850/2.95/176, 2250/850/3.93/176, 2250/850/3.68/176, and the use of GRAPPA parallel imaging with an acceleration factor of 2.
For all other subjects images were acquired at 3 T Siemens Trio, TimTrio, or Skyra scanners (Siemens), using small variations to a standard T1-weighted 3D MPRAGE sequence (TR 2300 ms, TI
1100 ms, TE 3.93 ms, 192 sagittal slices, field of view 256 mm). These variations included TR/TI/TE/slices of 2300/1100/3.03/192, 2300/1100/2.92/192, 2300/1100/2.96/192, 2300/1100/2.99/192,
1940/1100/3.93/176, and 1960/1100/4.58/176, and the use of GRAPPA parallel imaging with an acceleration factor of 2.
Local differences in gray and white matter volume related to group differences were studied using a voxel-based morphometry approach (Ashburner and Friston, 2000). For this analysis,
T1-images were processed with the default procedures and routines of the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/) within SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). Using a unified
model, T1-images were bias-field corrected, segmented into GM, white matter, and cerebro-spinal fluid, and registered with the standard MNI152 brain template by way of high-dimensional
DARTEL warping (Ashburner, 2007). The resulting images were modulated by the non-linear part of their DARTEL warp field and smoothed with an 10-mm FWHM Gaussian smoothing kernel, providing
for an analysis of relative differences in regional gray and white matter volume, corrected for individual brain size. A detailed description of the protocol can be found in the VBM8 manual
(http://dbm.neuro.uni-jena.de/vbm8/VBM8-Manual.pdf).
For the main analyses, group was entered as a factor and age, sex, MR scanner field strength, and PANAS-negative scale were entered as covariates. These covariates were added to ensure that
small differences between our matched groups would not influence our findings. For the interaction analyses, an additional product term (group × sex) was added to the fully adjusted model.
When significant, the interaction effects were further explored by constructing a new model per sex. Group was then entered as a factor and age, scanner, and PANAS-negative scale served as
covariates.
All statistical tests were family-wise error rate (FWE) corrected for multiple comparisons across the entire brain (pFWE0.05), hippocampus, or amygdala volume (Supplementary Results). This
suggests that the context of the abuse did not significantly influence brain structure in our sample.We also found two sex-specific effects in somatosensory integration areas. The visual
posterior precuneal region was affected in women, both after abuse and deprivation in childhood. This region has been found to have close functional connectivity with the fusiform gyrus and
also represents a transition from occipital to limbic connectivity in the precuneus (Margulies et al, 2009). It is therefore a cortical area representing the interplay of sensory input and
the processing of emotions. Notably, larger functional connectivity of the precuneus with the hippocampus and ACC in women than men could be one mechanism leading to sex-specific alterations
in precuneal GM structure after CA (Zhang and Li, 2012). One other study in women found reduced cortical thickness in this area after experiencing childhood emotional abuse (Heim et al,
2013). In addition, the precuneus has been found to have an important role in the mediation of grief in women (Gündel et al, 2003). Of note, thickness of the precuneal cortex has been shown
to be inversely correlated with sensitivity to interpersonal rejection in a large sample of healthy college students, suggesting that this anatomical difference may underlie differences in
emotion processing in a healthy population (Sun et al, 2014).The postcentral gyrus was the second region showing sex-specific changes, where males had smaller GM volumes after a history of
deprivation than male subjects with a history of abuse. However, as this effect disappeared after correction for more recent abuse, this finding may not be specific for childhood abuse. In
the light of previous findings in a female clinical sample, we had expected to find smaller GM volumes in the somatosensory cortex related to a history of sexual abuse (Heim et al, 2013).
Although these findings seem contradictory, they in fact highlight the importance of defining sex-specific pathways in the processing of stressful stimuli. Different pathways could be
involved in the processing of fearful events in men and women and therefore lead to different neural correlates (Everaerd et al, 2012). For example, in men only, the postcentral gyrus seems
to be involved in the processing of fearful faces (Weisenbach et al, 2014), which could be one potential factor of interest in the interaction we found in our sample.From a developmental
perspective, sex differences in neural correlates of CA may occur because of a number of reasons, such as the influence of gonadal hormones and different ‘sensitive’ time windows during
neural development in boys and girls (Crozier et al, 2014; Lenroot et al, 2007; Young and Korszun, 2010). For example, with respect to our data, a recent study found that conn
ectivity between amygdala sub-regions, the precuneus, and the postcentral gyrus shows an age by sex interaction in adolescents (Alarcón et al, 2015). This could be one potential mechanism
leading to sex-specific effects in these regions dependent on the timing of adverse events. In psychopathology, these sex differences may contribute to the differences in prevalence of
different psychiatric disorders, such as more depression in women and more impulse control disorders in men (Kessler et al, 1993, 2006).Remarkably, we did not find any differences in GM
structure of other areas that have often been associated with CA, such as the amygdala, hippocampus, ACC, and prefrontal cortex (Dannlowski et al, 2012; Edmiston et al, 2011; Hanson et al,
2015). One explanation for this missing finding could be that our population consisted of particularly young, highly educated, and healthy subjects with no psychiatric consequences of their
early adverse life environments. Consequently, these subjects might be a particularly resilient subset of young adults exposed to childhood stress, and differ in that aspect from patient
populations that are traditionally examined for consequences of CA (Lim et al, 2014), or from subjects in longitudinal studies involving severe adversity such as institutionalization (Zeanah
et al, 2003). Furthermore, it has been suggested that differences in hippocampal volume after CA are not yet visible in late adolescence (Tottenham and Sheridan, 2009). In addition, we
excluded subjects with threatening life events after adolescence, in contrast to most other studies. As a consequence, the differences owing to CA within our young, resilient population may
be difficult to detect. However, we expect that the differences we were able to detect are particularly robust and representative of consequences of CA in a healthy population, although our
results necessitate replication in different cohorts.Potentially limiting and maybe related to the healthy nature of our subjects, we found the most pronounced differences between the two CA
groups and not when comparing these groups with a control group. One speculative explanation could be that deprived children are significantly under-stimulated as compared with their peers,
which in turn may lead to an underdeveloped visual association cortex (Sheridan and McLaughlin, 2014). In contrast, experience with high-threat situations in childhood may increase GM
volumes in these areas, possibly leading to superior vigilance for fearful and sad facial expression after maltreatment (Leist and Dadds, 2009). Although this reasoning is highly
speculative, our findings do stress the importance of specifically assessing the nature of adverse childhood experiences, and highlight the need for future studies looking into their
mechanistic underpinnings.Importantly, we used a large sample that consisted of healthy subjects without any confounding variables such as somatic or psychiatric disease and with a
substantia
l subset of male participants. In addition, we used a questionnaire that allowed us to correct for possibly confounding contributions of more recent adverse events (Brugha et al, 1985).
Limitations are the use of a self-reported questionnaire, the absence of information on the exact timing and frequency of the events during development, and the cross-sectional design. In
addition, it is possible that not all adverse childhood events were reported by all subjects, because of the sensitive nature of this information.To conclude, we found partly sex-dependent
differences in GM volumes between three well-controlled groups of healthy, young adults that had experienced environments of either abuse or deprivation in childhood and a control group.
These differences could potentially give rise to specific changes in mental well-being or symptoms of psychopathology (Edmiston et al, 2011; Hanson et al, 2015). Future studies are needed to
expand the current findings by examining behavioral consequences of the observed structural differences in even larger, similarly well-selected populations, such as possible differences in
processing of facial expressions or other salient information, differences in parent-child attachment, somatic symptoms in psychiatric disease or somatic disease, all of which have been
associated with CA in the past (Cuijpers et al, 2011; Leist and Dadds, 2009; McGoron et al, 2012). Finding specific behavioral consequences of different types of CA could provide insight
into the emergence of distinct neurodevelopmental trajetories, and into potential development of specific psychiatric symptoms and disease in some vulnerable individuals.FUNDING AND
DISCLOSUREThe authors have no competing financial interests in relation to the work described. BF has received speaker fees from Merz and IT has received speaker fees from Servier and
Lundbeck. The remaining authors declare no conflict of interest. We wish to thank all persons who kindly participated in the BIG research and the BIG team for their help with data
processing. This work makes use of the BIG database, first established in Nijmegen, The Netherlands, in 2007. This resource is now part of the Cognomics Initiative (www.cognomics.nl), a
joint initiative by researchers of the Donders Centre for Cognitive Neuroimaging, the Human Genetics and Cognitive Neuroscience departments of the Radboud University Medical Center and the
Max Planck Institute for Psycholinguistics in Nijmegen. The Cognomics Initiative is supported by the participating departments and centers and by external grants, ie, from the Biobanking and
Biomolecular Resources Research Infrastructure (Netherlands) (BBMRI-NL), the Hersenstichting Nederland, the Netherlands Organization for Scientific Research (NWO), and from the National
Institutes of Health (NIH) Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centers of Excellence.Author informationAuthors and
AffiliationsDonders I
nstitute for Brain, Cognition and Behaviour, Radboud University, Nijmegen, The NetherlandsDaphne Everaerd, Floris Klumpers, Marcel Zwiers, Barbara Franke, Iris van Oostrom, Aart Schene,
Guillén Fernández & Indira TendolkarDepartment of Psychiatry, Radboud University Medical Center, Nijmegen, The NetherlandsDaphne Everaerd, Barbara Franke, Iris van Oostrom, Aart Schene &
Indira TendolkarDepartment for Cognitive Neuroscience, Radboud University Medical Center, Nijmegen, The NetherlandsFloris Klumpers & Guillén FernándezLanguage and Genetics Department, Max
Planck Institute for Psycholinguistics, Nijmegen, The NetherlandsTulio GuadalupeDepartment of Human Genetics, Radboud University Medical Center, Nijmegen, The NetherlandsBarbara FrankeLVR
Clinics of Psychiatry and Psychotherapy, University Medical Center, University of Essen Duisburg, Essen, GermanyIndira TendolkarAuthorsDaphne EveraerdView author publicationsYou can also
search for this author inPubMed Google ScholarFloris KlumpersView author publicationsYou can also search for this author inPubMed Google ScholarMarcel ZwiersView author publicationsYou can
also search for this author inPubMed Google ScholarTulio GuadalupeView author publicationsYou can also search for this author inPubMed Google ScholarBarbara FrankeView author publicationsYou
can also search for this author inPubMed Google ScholarIris van OostromView author publicationsYou can also search for this author inPubMed Google ScholarAart ScheneView author
publicationsYou can also search for this author inPubMed Google ScholarGuillén FernándezView author publicationsYou can also search for this author inPubMed Google ScholarIndira
TendolkarView author publicationsYou can also search for this author inPubMed Google Scholar Supplementary Information accompanies the paper on the Neuropsychopharmacology
websiteSupplementary informationSupplementary Information (DOCX 861 kb)PowerPoint slidesPowerPoint slide for Fig. 1PowerPoint slide for Fig. 2PowerPoint slide for Fig. 3Rights and
permissionsReprints and permissions Received: 11 June 2015Revised: 21 September 2015Accepted: 16 October 2015Published: 18 November 2015Issue Date: June 2016DOI:
https://doi.org/10.1038/npp.2015.344Share this articleAnyone you share the following link with will be able to read this content:Get shareable linkSorry, a shareable link is not currently
available for this article.
As 34% of the subjects in the abuse group also reported having experienced abuse after the age of 16 years, we tested whether our findings could be due to the events that occurred later in
life. To this end, we added the binary variable of later abuse (yes or no) as a covariate to our fully adjusted model. Later abuse was defined as any of the items indicating ‘abuse’ in Table
1 occurring after age 16 years. After this correction, only the sex-related differences in GM volume in the postcentral gyrus (men with deprivation vs men with abuse) were no longer
detectable. All other findings remained significant (see for all data Supplementary Table S5).
Surprisingly, we did not find any effects on hippocampus and amygdala volume using our voxel-wise, brain-wide analysis, despite previous evidence of CA-related psychopathology involving
these regions (eg, Hanson et al, 2015). Therefore, we decided to revisit these null findings by conducting a volumetric analysis using an automated segmentation technique (Supplementary
Materials and Methods). Here, we replicated our previous finding that there were no differences in bilateral hippocampus or amygdala volume between the three groups (p>0.1) (Supplementary
Table S6).
The present data suggest that even in a healthy sample, subtle CA-specific alterations in GM structure can be found. In line with the hypothesis of specific effects of CA subtypes, specific
associations were found in the fusiform gyrus and middle occipital gyrus, whereby subjects in the deprivation group revealed significantly smaller GM volumes than those belonging to the
abuse group. In addition, sex-specific differences in somatosensory integration areas were found.
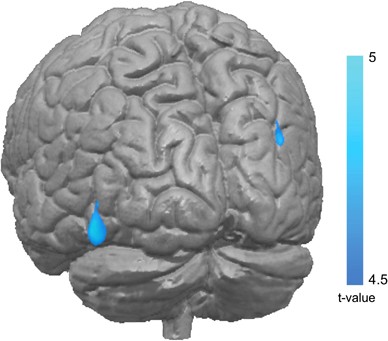
The fusiform gyrus and middle occipital gyrus are brain regions responsible for visual processing and multimodal integration (Kravitz et al, 2011). More specifically, these areas have been
associated with face perception (Kanwisher et al, 1997) and scene perception (Dilks et al, 2013), respectively. Enhanced activity in the fusiform gyrus has also been related to the
processing of personally familiar faces compared with faces of strangers, which suggests that its function may go beyond simple face perception (Gobbini et al, 2004). Previous studies
reporting structural changes in visual processing areas in relation to CA are scarce. One study showed reduced cortical thickness in V2 and the left occipital pole after witnessing domestic
violence in childhood (Tomoda et al, 2012). Another study found that subjects had smaller GM volumes in the fusiform and middle occipital gyrus after sexual abuse in childhood (Tomoda et al,
2009). Although these previous studies all report smaller GM volumes after abuse, our data revealed smaller GM volumes in subjects that had experienced deprivation in childhood. One
explanation for this inconsistency could be that, whereas high-threat environments in humans are often well defined and investigated, the amount of deprivation in these same environments is
usually unclear (Sheridan and McLaughlin, 2014), and the contribution of deprivation to the findings in abuse studies is therefore often unknown. Possibly, some of the effects that have been
attributed to abuse in the past could be in fact related to deprivation. Notably, from animal models we know that deprivation in early development can have extensive consequences on neural
development (Diamond et al, 1975). Studies in the field of perceptual neuroscience have shown that early visual deprivation in animals and humans leads to radical structural changes,
resulting from the reduction of synapses in the primary visual cortex (Leporé et al, 2010; O'Kusky, 1985). In humans, widespread reductions in cortical thickness in regions including the
fusiform gyrus and precuneus have been found in Romanian children that had experienced pronounced early-life deprivation, mediating problems with inattention and/ or impulsivity (McLaughlin
et al, 2014b; Sheridan et al, 2012).
Of note, in our subjects, experiences of deprivation all occurred within the context of the subject’s family, whereas abuse could also occur outside of the family context, which could
suggest a possible bias toward more severe experiences in the deprivation group (Edwards et al, 2003). We tested this hypothesis by dividing the abuse group into two subgroups: one with
subjects who reported abuse within the family and one with subjects who exclusively reported abuse outside their family. Importantly, only a minority (35%) of the subjects in the abuse group
exclusively experienced abuse outside their family. Moreover, additional analyses showed that the subjects within the abuse group that had either experienced abuse within or outside their
family did not significantly differ from each other in GM volume (whole-brain pFWE>0.05), hippocampus, or amygdala volume (Supplementary Results). This suggests that the context of the abuse
did not significantly influence brain structure in our sample.
We also found two sex-specific effects in somatosensory integration areas. The visual posterior precuneal region was affected in women, both after abuse and deprivation in childhood. This
region has been found to have close functional connectivity with the fusiform gyrus and also represents a transition from occipital to limbic connectivity in the precuneus (Margulies et al,
2009). It is therefore a cortical area representing the interplay of sensory input and the processing of emotions. Notably, larger functional connectivity of the precuneus with the
hippocampus and ACC in women than men could be one mechanism leading to sex-specific alterations in precuneal GM structure after CA (Zhang and Li, 2012). One other study in women found
reduced cortical thickness in this area after experiencing childhood emotional abuse (Heim et al, 2013). In addition, the precuneus has been found to have an important role in the mediation
of grief in women (Gündel et al, 2003). Of note, thickness of the precuneal cortex has been shown to be inversely correlated with sensitivity to interpersonal rejection in a large sample of
healthy college students, suggesting that this anatomical difference may underlie differences in emotion processing in a healthy population (Sun et al, 2014).
The postcentral gyrus was the second region showing sex-specific changes, where males had smaller GM volumes after a history of deprivation than male subjects with a history of abuse.
However, as this effect disappeared after correction for more recent abuse, this finding may not be specific for childhood abuse. In the light of previous findings in a female clinical
sample, we had expected to find smaller GM volumes in the somatosensory cortex related to a history of sexual abuse (Heim et al, 2013). Although these findings seem contradictory, they in
fact highlight the importance of defining sex-specific pathways in the processing of stressful stimuli. Different pathways could be involved in the processing of fearful events in men and
women and therefore lead to different neural correlates (Everaerd et al, 2012). For example, in men only, the postcentral gyrus seems to be involved in the processing of fearful faces
(Weisenbach et al, 2014), which could be one potential factor of interest in the interaction we found in our sample.
From a developmental perspective, sex differences in neural correlates of CA may occur because of a number of reasons, such as the influence of gonadal hormones and different ‘sensitive’
time windows during neural development in boys and girls (Crozier et al, 2014; Lenroot et al, 2007; Young and Korszun, 2010). For example, with respect to our data, a recent study found that
connectivity between amygdala sub-regions, the precuneus, and the postcentral gyrus shows an age by sex interaction in adolescents (Alarcón et al, 2015). This could be one potential
mechanism leading to sex-specific effects in these regions dependent on the timing of adverse events. In psychopathology, these sex differences may contribute to the differences in
prevalence of different psychiatric disorders, such as more depression in women and more impulse control disorders in men (Kessler et al, 1993, 2006).
Remarkably, we did not find any differences in GM structure of other areas that have often been associated with CA, such as the amygdala, hippocampus, ACC, and prefrontal cortex (Dannlowski
et al, 2012; Edmiston et al, 2011; Hanson et al, 2015). One explanation for this missing finding could be that our population consisted of particularly young, highly educated, and healthy
subjects with no psychiatric consequences of their early adverse life environments. Consequently, these subjects might be a particularly resilient subset of young adults exposed to childhood
stress, and differ in that aspect from patient populations that are traditionally examined for consequences of CA (Lim et al, 2014), or from subjects in longitudinal studies involving
severe adversity such as institutionalization (Zeanah et al, 2003). Furthermore, it has been suggested that differences in hippocampal volume after CA are not yet visible in late adolescence
(Tottenham and Sheridan, 2009). In addition, we excluded subjects with threatening life events after adolescence, in contrast to most other studies. As a consequence, the differences owing
to CA within our young, resilient population may be difficult to detect. However, we expect that the differences we were able to detect are particularly robust and representative of
consequences of CA in a healthy population, although our results necessitate replication in different cohorts.
Potentially limiting and maybe related to the healthy nature of our subjects, we found the most pronounced differences between the two CA groups and not when comparing these groups with a
control group. One speculative explanation could be that deprived children are significantly under-stimulated as compared with their peers, which in turn may lead to an underdeveloped visual
association cortex (Sheridan and McLaughlin, 2014). In contrast, experience with high-threat situations in childhood may increase GM volumes in these areas, possibly leading to superior
vigilance for fearful and sad facial expression after maltreatment (Leist and Dadds, 2009). Although this reasoning is highly speculative, our findings do stress the importance of
specifically assessing the nature of adverse childhood experiences, and highlight the need for future studies looking into their mechanistic underpinnings.
Importantly, we used a large sample that consisted of healthy subjects without any confounding variables such as somatic or psychiatric disease and with a substantial subset of male
participants. In addition, we used a questionnaire that allowed us to correct for possibly confounding contributions of more recent adverse events (Brugha et al, 1985). Limitations are the
use of a self-reported questionnaire, the absence of information on the exact timing and frequency of the events during development, and the cross-sectional design. In addition, it is
possible that not all adverse childhood events were reported by all subjects, because of the sensitive nature of this information.
To conclude, we found partly sex-dependent differences in GM volumes between three well-controlled groups of healthy, young adults that had experienced environments of either abuse or
deprivation in childhood and a control group. These differences could potentially give rise to specific changes in mental well-being or symptoms of psychopathology (Edmiston et al, 2011;
Hanson et al, 2015). Future studies are needed to expand the current findings by examining behavioral consequences of the observed structural differences in even larger, similarly
well-selected populations, such as possible differences in processing of facial expressions or other salient information, differences in parent-child attachment, somatic symptoms in
psychiatric disease or somatic disease, all of which have been associated with CA in the past (Cuijpers et al, 2011; Leist and Dadds, 2009; McGoron et al, 2012). Finding specific behavioral
consequences of different types of CA could provide insight into the emergence of distinct neurodevelopmental trajetories, and into potential development of specific psychiatric symptoms and
disease in some vulnerable individuals.
The authors have no competing financial interests in relation to the work described. BF has received speaker fees from Merz and IT has received speaker fees from Servier and Lundbeck. The
remaining authors declare no conflict of interest.
We wish to thank all persons who kindly participated in the BIG research and the BIG team for their help with data processing. This work makes use of the BIG database, first established in
Nijmegen, The Netherlands, in 2007. This resource is now part of the Cognomics Initiative (www.cognomics.nl), a joint initiative by researchers of the Donders Centre for Cognitive
Neuroimaging, the Human Genetics and Cognitive Neuroscience departments of the Radboud University Medical Center and the Max Planck Institute for Psycholinguistics in Nijmegen. The Cognomics
Initiative is supported by the participating departments and centers and by external grants, ie, from the Biobanking and Biomolecular Resources Research Infrastructure (Netherlands)
(BBMRI-NL), the Hersenstichting Nederland, the Netherlands Organization for Scientific Research (NWO), and from the National Institutes of Health (NIH) Consortium grant U54 EB020403,
supported by a cross-NIH alliance that funds Big Data to Knowledge Centers of Excellence.
Donders Institute for Brain, Cognition and Behaviour, Radboud University, Nijmegen, The Netherlands
Daphne Everaerd, Floris Klumpers, Marcel Zwiers, Barbara Franke, Iris van Oostrom, Aart Schene, Guillén Fernández & Indira Tendolkar
Department of Psychiatry, Radboud University Medical Center, Nijmegen, The Netherlands
Daphne Everaerd, Barbara Franke, Iris van Oostrom, Aart Schene & Indira Tendolkar
Department for Cognitive Neuroscience, Radboud University Medical Center, Nijmegen, The Netherlands
Language and Genetics Department, Max Planck Institute for Psycholinguistics, Nijmegen, The Netherlands
Department of Human Genetics, Radboud University Medical Center, Nijmegen, The Netherlands
LVR Clinics of Psychiatry and Psychotherapy, University Medical Center, University of Essen Duisburg, Essen, Germany
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Anyone you share the following link with will be able to read this content:
