Oxygen consumption in platelets as an adjunct diagnostic method for pediatric mitochondrial disease
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT BACKGROUND Diagnosing mitochondrial disease (MD) is a challenge. In addition to genetic analyses, clinical practice is to perform invasive procedures such as muscle biopsy for
biochemical and histochemical analyses. Blood cell respirometry is rapid and noninvasive. Our aim was to explore its possible role in diagnosing MD. METHODS Blood samples were collected from
113 pediatric patients, for whom MD was a differential diagnosis. A respiratory analysis model based on ratios (independent of mitochondrial specific content) was derived from a group of
healthy controls and tested on the patients. The diagnostic accuracy of platelet respirometry was evaluated against routine diagnostic investigation. RESULTS MD prevalence in the cohort was
16%. A ratio based on the respiratory response to adenosine diphosphate in the presence of complex I substrates had 96% specificity for disease and a positive likelihood ratio of 5.3. None
of the individual ratios had sensitivity above 50%, but a combined model had 72% sensitivity. CONCLUSION Normal findings of platelet respirometry are not able to rule out MD, but
pathological results make the diagnosis more likely and could strengthen the clinical decision to perform further invasive analyses. Our results encourage further study into the role of
blood respirometry as an adjunct diagnostic tool for MD. SIMILAR CONTENT BEING VIEWED BY OTHERS MITOCHONDRIAL FUNCTION IN PERIPHERAL BLOOD CELLS ACROSS THE HUMAN LIFESPAN Article Open access
07 February 2024 GENETIC TESTING FOR MITOCHONDRIAL DISEASE: THE UNITED KINGDOM BEST PRACTICE GUIDELINES Article Open access 13 December 2022 USE OF DUAL GENOMIC SEQUENCING TO SCREEN
MITOCHONDRIAL DISEASES IN PEDIATRICS: A RETROSPECTIVE ANALYSIS Article Open access 14 March 2023 MAIN For the clinician facing a severely sick child with indistinct but serious symptoms such
as seizures, hypotonia, or liver failure, mitochondrial disease (MD) is one of the conditions that need to be considered. While certain diagnoses have a characteristic clinical picture, and
some cases can be rapidly confirmed by targeted genetic testing, diagnosing MD is often a challenge (1, 2). Standardized clinical criteria have been proposed to facilitate a general
mitochondrial diagnosis and rely on a combination of symptoms and tests of varying difficulty (3, 4). Elevated blood lactate is an important indicator for MD, but is nonspecific and may be
caused by numerous systemic conditions such as hypoxia or sepsis (5). Cerebrospinal fluid lactate is more specific in diagnosing mitochondrial encephalopathy, but requires an invasive
procedure (6). Magnetic resonance imaging of the brain may show typical patterns (as in Leigh syndrome) or less specific pathology. Analysis of organic acids in urine, like lactate, is
noninvasive but may have low sensitivity if the patient is clinically stable (7). A muscle biopsy is often necessary to confirm the diagnosis. It is used for biochemical, histochemical, and,
in some cases, genetic analyses (1, 8, 9). Muscle biopsy is an invasive procedure requiring sedation or most often general anesthesia in children. Fresh muscle tissue is preferred over
frozen and patients need to be transferred to centers with accredited laboratories for such analyses (7). Physicians can obviously not order this investigation on too wide a suspicion, nor
expect expeditious results. While new methods of genetic testing facilitate large nuclear gene panels and whole-exome sequencing, and will likely play an increasingly important role in the
future, they are not considered first-line diagnostic tools. An accessible mitochondrial test derived from a normal blood sample would be of value to direct the early stages of diagnostic
investigations. Mitochondrial function in platelets has been suggested as a marker of systemic mitochondrial function and has been studied in a variety of conditions where mitochondrial
dysfunction is part of the pathology, such as amyotrophic lateral sclerosis, Alzheimer’s disease, and sepsis (10, 11, 12). Only a few studies have investigated blood cells from patients with
primary MD, mostly in lymphocytes. Results have indicated that blood cell mitochondria are affected in some primary MDs, but study populations have often been small (13, 14, 15, 16). Two
studies on lymphocyte respirometry suggest candidate measurements for diagnostic use (14, 16). To our knowledge, no published study has evaluated respirometry in blood cells as a diagnostic
method on a larger group of patients where MD was a differential diagnosis. The aim of this study was to investigate the value of platelet respirometry as a complement to existing diagnostic
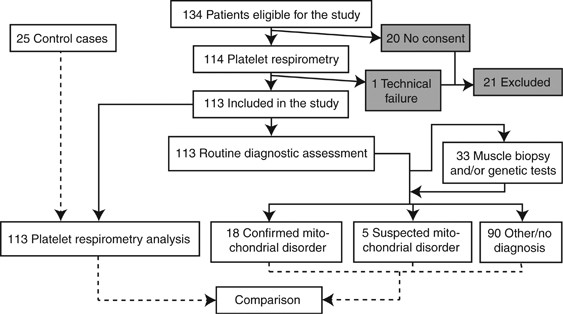
tests for MD. The method is rapid and noninvasive and could be performed in the early stages of investigation. RESULTS CLINICAL DATA The study included 113 patients where MD was considered
a differential diagnosis. A wide pediatric age span was represented in the material, but most patients were under 5 years (Table 1). Central nervous system symptoms were heavily featured
among reported symptoms (Table 2). Thirty-three patients went through a more extensive clinical investigation including muscle biopsy, genetic testing, or both. Eighteen patients were
confirmed to have MD and the basis for these diagnoses is presented in Table 3. Five patients still had suspected but unconfirmed MD after extended investigation. These patients were
considered as not having MD for all calculations in the study. In the remaining 90 cases, routine clinical investigation did not indicate MD (Figure 1). EVALUATION OF DIAGNOSTIC RESPIRATORY
RATIOS Absolute values for platelet respirometry in the reference group and established reference bounds for respiratory ratios are shown in Table 4. Diagnostic evaluation of the six
separate tests (4 one-sided ratios and 1 two-sided ratio) and the combined model is presented in Table 5. Oxidative phosphorylation (OXPHOS) CImalate and pyruvate/digitonin, malate and
pyruvate successfully identified 4 out of 18 MD patients, with few false positives resulting in 96% specificity and a positive-likelihood ratio (PLR) of 5.3. Low values of Log(ETS
CI+II/Routine) and ΔADP (adenosine diphosphate)/ΔSuccinate identified 9 and 6 MD patients, respectively. The remaining two tests had no true positives. The sensitivity for the combined model
(positive here being defined as testing positive in any of the six tests) was 72% and the specificity 56%. No single test had higher sensitivity than 50%. Table 6 shows an overview of the
18 MD patients’ individual respirometry results in relation to other clinical tests. Thirteen out of the 18 MD patients were identified by at least one respiratory ratio. EXPLORATIVE
ANALYSES A correlation between high blood lactate and pathologic respirometry results has previously been described (14). We plotted respiratory ratios in relation to lactate values (Figure
2), and while no linear correlation was seen, selected tests in combination with high lactate improved specificity (Table 7). A low OXPHOS CIMP/DMP ratio in combination with high lactate had
99% specificity and a PLR of 21. Also, Log(ETS CI+II/Routine) and ΔADP/ΔSuccinate, respectively, combined with high lactate raised specificity and PLR. Positive result in all of the three
best tests had 100% specificity for MD, but this model only identified three of the patients. A scaled down version of the main model, using only the two most sensitive tests and counting a
positive result on either test or on both tests as positive, retained a sensitivity of 67% and raised specificity to 73% (Table 7). Finally, when applying stricter criteria for true
positive, defining confirmed diagnosis as confirmed by genetic testing, sensitivity and specificity for the three best tests and the combined model did not change conspicuously (Table 8).
DISCUSSION We show that platelet respirometry provides useful diagnostic information through a rapid and noninvasive procedure in pediatric patients, in whom MD was clinically considered a
differential diagnosis. Positive results substantially increase the likelihood of MD. Diagnosis of MD is a clinical challenge. MD is the most common group of inherited metabolic disorders,
but their diverse presentation and complex pathophysiology make them hard to investigate. Many of the patients are young children with severe symptoms and the invasiveness or inaccessibility
of the best diagnostic methods may delay or even prevent confirmation of diagnosis. The study population is a representative cohort of pediatric patients with clinically suspected MD at a
tertiary hospital, making the study highly relevant to clinicians investigating these often severely sick children. The prevalence of MD in the study population was 16%, in line with
previous reports from patient cohorts with similar clinical presentation (6, 14, 23). Oxygen consumption in platelets was assessed to evaluate mitochondrial function. We used ratios rather
than absolute values of oxygen consumption, circumventing the need to calculate and adjust for mitochondrial content in the samples. A low OXPHOS CIMP/DMP ratio had high specificity (96%)
and a PLR of 5.3, meaning that in this sample the likelihood of disease rose from 16 to 50% after testing positive. The ratio was constructed to be sensitive to CI dysfunction, as inadequate
response to ADP in the presence of CI substrates would lower the ratio (14, 16). While all true-positive findings had confirmed CI dysfunction according to the current standard
investigation, some patients with CI dysfunction were not caught by the test. The ratio OXPHOS CIMP/DMP had a low sensitivity, possibly only detecting severe dysfunction. Log(ETS
CI+II/Routine) and ΔADP/ΔSuccinate appear to better detect mild dysfunction; they were more sensitive and their true positives overlap those of OXPHOS CIMP/DMP. Pathological results in all
three best tests made the diagnosis of mitochondrial disorder very likely (in this material 100% specificity). Each ratio was chosen to test a different aspect of the ETS (electron transport
system) and thus a relatively low sensitivity for MD of any kind is expected when looking at the ratios individually. The combined model had a higher sensitivity at 72% but suffered from
many false positives. Although not enough to rule out disease on its own (negative likelihood ratio (NLR) of 0.5), platelet respirometry may be a useful part of an investigation. With the
currently used diagnostic procedures, each individual test is in most cases insufficient to rule out MD, and that is also the case with this method. Blood respirometry has the advantage of
swiftness, low invasiveness, and potential high availability and could be performed early in an investigation. In this material, two patients with normal muscle biopsy that later were
confirmed to have MD by genetic testing had pathologic respirometry. Plasma lactate is known to be a sensitive indicator of MD and can be obtained easily. A combination of high lactate and a
low OXPHOS CIMP/DMP ratio had 99% specificity (Table 7), comparable to the highest estimates of cerebrospinal fluid lactate, which is a markedly more invasive method (6). The likelihood for
disease in a patient testing positive rose from 16 to 80%. Cerebrospinal fluid lactate is primarily suitable to detect MD affecting the central nervous system and has lower specificity in
acutely ill patients (24, 25). Blood lactate values, in contrast to platelet respirometry results, were technically not independent from the clinical findings that informed the final
diagnosis. However, elevated lactate did not have a pivotal role for any of the MD diagnoses (Table 3). We hypothesized that a limitation of OXPHOS at complex V (CV) would yield higher ETS
than OXPHOS and result in a low OXPHOS CI+II/ETS CI+II ratio, indicating CV dysfunction. No patient had biochemically or genetically confirmed dysfunction in CV, so this hypothesis could not
be tested. A low OXPHOS CIMP/OXPHOS CIMPG ratio failed to single out any of the three patients with pyruvate dehydrogenase (PDH) deficiency, despite them being identified by other ratios.
Study limitations include insufficient information on any ongoing, potentially confounding medical treatment at the time of sampling (14). Further, the diversity of MD makes categorizing at
all levels problematic. Although a clinical definition of confirmed MD was thought to best serve the aims of this study, a strict biochemical or genetic definition may have facilitated more
detailed comparisons. (A strict genetic definition of true positive would arguably have had the benefit of less ambiguity. We included a _post hoc_ analysis with such a definition, in which
the main findings largely remains the same.) Also, the number of MD patients in the study was limited and many of the subdiagnoses were only represented by one patient each. The results
therefore need to be confirmed by further studies. Another reason such studies are warranted is that the circumstances of data collection and analysis in this pilot project did not allow for
strict blinding, something that would have to be readdressed in a follow-up investigation. Lastly, the inherent problem of tests with significant false-negative rates needs to be taken into
account (if future models do not improve sensitivity), making sure a negative result in platelet testing alone does not unintentionally refrain the diagnostician from pursuing further
investigations when needed. We do not expect blood cell respirometry to replace any part of the current diagnostic workup, but believe that the addition of this rapid test could potentially
reduce time to diagnosis for certain patients. When discussing the possible role of blood respirometry, it is important to consider not just the current clinical practices but also how they
are projected to evolve. As mentioned above, genetic methods such as next-generation sequencing, either in the form of large-scale gene panels, whole-exome sequencing or in some settings
whole-genome sequencing, are currently gaining traction (2, 26). Interestingly, as one recent review pointed out, improved genetic testing may actually place greater emphasis on less
invasive tests and methods to confirm pathogenicity (26). Blood cell respirometry may potentially be a part of the first-line screening arsenal, and as such it would not likely be replaced
by large-scale genetic testing any time soon. Additionally, respirometry being a functional analysis, we see it as a complement rather than a competitor to genetic tests. CONCLUSION We have
shown that pathologic platelet respirometry, as defined in this study, increased the likelihood of MD in a clinically relevant situation. Combining blood respirometry with blood lactate
further increased its diagnostic yield. As it is a fast method with low invasiveness and potentially high availability, these results encourage further study into the method’s possible role
as an adjunct diagnostic tool for MD. METHODS PATIENTS AND CONTROL SUBJECTS Patient samples were collected at the Skåne University Hospital (Lund, Sweden). Patients under the age of 18 years
presenting from July 2008 through December 2013, where MD was clinically considered as a differential diagnosis by the attending physician, were eligible for the study. Patients with
malignant disease were excluded. The pediatric control group comprised samples from patients undergoing anesthesia for minor elective surgery (inguinal hernia repair or phimosis surgery).
Control group samples were drawn before induction of anesthesia. Written informed consent was obtained from parents or guardians. The regional ethical review board of Lund, Sweden, approved
this study (59/2009 and 97/2009). All patients were given standard care and the study was conducted according to the Declaration of Helsinki. CLINICAL DATA AND ROUTINE DIAGNOSTIC ASSESSMENT
The standard care of patients included in the trial was not affected by the results of this study, as no patient was excluded from further clinical investigation based on a negative blood
cell respirometry. Clinical data were reported by clinicians and supplemented with review of medical records in Lund and at Sahlgrenska University Hospital, Gothenburg (where a majority of
the muscle biopsies and genetic analyses were performed). Muscle biopsies were analyzed according to established practices (including histology, spectrophotometry, and oximetry/respirometry,
normally all three) (7, 8). Genetic analyses included screening for suspected mutations with or without the addition of whole-exome sequencing. Patients were considered to have confirmed
disease when a specialist in pediatric neurology had documented the diagnosis of MD as confirmed in the patient journal, after compiling the results of the investigation. This definition
was, as with the inclusion criteria, chosen to most closely adhere to the clinical reality. Although in practice the Bernier criteria are generally used at the participating hospitals in
this study, there are neither national nor regional guidelines for diagnosing MD. A suspected case of MD not disproven during early investigation (due to, for instance, confirmation of an
alternative diagnosis or clinical recovery), was usually further evaluated with muscle biopsy, genetic testing, or both. The final diagnosis was preceded by discussion among several
specialists and was made independently of the experimental platelet model. Patients were defined as having “still suspected” MD if routine diagnostics could neither confirm nor rule out a
diagnosis. Patients where disease was “still suspected” were considered as not having MD in all calculations of diagnostic accury in platelets, unless specified otherwise. PLATELET
PREPARATION Depending on age and weight of the patient, 6–12 ml venous blood was drawn to EDTA vials. Blood samples were analyzed within 3–5 h as described previously (11, 17).
HIGH-RESOLUTION RESPIROMETRY Mitochondrial respiration was measured with an Oxygraph-2k (Oroboros Instruments, Innsbruck, Austria) following a previously described protocol (11, 17). The
platelet pellet was dissolved in a mitochondrial respiration medium (MiR05) containing sucrose 110 mM, HEPES 20 mM, taurine 20 mM, K-lactobionate 60 mM, MgCl2 3 mM, KH2PO4 10 mM, EGTA 0.5
mM, bovine serum albumin 1 g/l, at pH 7.1 (ref. 18). The final cell concentration in the chamber was 19–200 × 106 cells per ml. Data were recorded with the DatLab Software 4.3 (Oroboros
Instruments, Innsbruck, Austria). EXPERIMENTAL PROTOCOL FOR PERMEABILIZED PLATELETS The analytic protocol is described in Figure 3. At the onset, the complexes of the respiratory system are
coupled to the process of OXPHOS, phosphorylating ADP to adenosine triphosphate (ATP) as oxygen is consumed. The respiratory state in which the respiratory system is active but artificially
uncoupled from OXPHOS is termed ETS (electron transport system). If neither OXPHOS nor artificial uncoupling drives the respiratory system, a certain amount of protons leaking over the inner
membrane may still drive the respiratory system to some extent, as the mitochondria pump the protons back to maintain the electrochemical potential over the inner mitochondrial membrane.
This state is called LEAK. First, Routine respiration was established. Subsequently, the detergent digitonin was added to permeabilize the plasma membrane and allow the mitochondria to
access exogenous (added) substrates. Malate (5 mM) and pyruvate (5 mM) were added simultaneously (respiratory state DMP). ADP (1 mM) was then added to induce OXPHOS with these substrates
(OXPHOS CIMP). Further, addition of glutamate (5 mM) provided additional electrons for complex I (CI), also via NADH (OXPHOS CIMPG). Convergent input of electrons through both CI and complex
II (CII) was achieved by the addition of succinate (10 mM), which is oxidized by CII. This achieves maximum OXPHOS capacity through CI and CII (OXPHOS CI+II). Next, proton leak over the
mitochondrial membrane was measured by adding the ATP synthase inhibitor oligomycin (1 μg/ml), blocking OXPHOS (LEAK CI+II). This was followed by titration of the uncoupler protonophore
carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone creating a chemically induced leak of protons over the inner mitochondrial membrane, thereby stimulating the ETS to maintain the
chemiosmotic proton gradient (ETS CI+CII). Subsequently, by adding CI inhibitor rotenone, the CII-dependent uncoupled respiration was measured (ETS CII). To adjust for nonmitochondrial
oxygen consumption by the cells, the complex III (CIII) inhibitor antimycin-A was added and this value was subtracted from each of the other parameters. Finally, tetramethylphenylenediamine
(0.5 mM) was added, driving complex IV (CIV) activity through the reduction of cytochrome _c_. To control oxygen consumed through the autoxidation of tetramethylphenylenediamine, CIV is
inhibited by azide (10 mM) and the remaining respiration is subtracted from the former value (CIV activity). From the measured parameters we constructed five ratios to cover different
aspects of mitochondrial dysfunction. We used ratios of respirometry measurements, as opposed to absolute values, thus circumventing the need to adjust for mitochondrial content in the
sample. This keeps the protocol simple, rapid, and more compatible with routine clinical use. A low OXPHOS CIMP/DMP ratio (in the absence of other findings indicating CII–V dysfunction) was
assumed to reflect dysfunction in CI or upstream (of CI) processes, such as pyruvate dehydrogenase deficiency, as only CI substrates are present in this state (13, 16). A low ETS
CI+CII/Routine ratio was assumed to reflect general dysfunction for all complexes except CV and a reduced reserve capacity of the respiratory system. The OXPHOS CI+II/ETS CI+II was assumed
to reflect CV dysfunction as respiration in the numerator but not in the denominator is limited by CV capacity. The ΔADP/ΔSuccinate ratio was defined to reflect either CI or CII dysfunction.
A high response to ADP (in the presence of CI substrates) in comparison with the sequential response to succinate would indicate CII dysfunction and raise the ratio. The opposite would
imply CI dysfunction. Finally, we assumed that a low OXPHOS CIMP/OXPHOS CIMPG ratio would indicate PDH deficiency. Transaminases in the malate-aspartate shuttle convert glutamate and
oxaloacetate to α-ketoglutarate and aspartate, replenishing the tricarboxylic acid cycle downstream of PDH. Increased oxygen consumption with the addition of glutamate could thus indicate a
limitation to mitochondrial respiration by PDH (19). STATISTICAL METHODS Respiratory parameters from a control group of 25 pediatric patients were used to establish reference intervals for
the respiratory ratios. The method for establishing reference intervals was adapted from the Clinical and Laboratory Standards Institute (CLSI) guidelines (20). Outliers were detected with
Tukey’s outlier labeling rule and two ratios had one outlier each removed (21). The five chosen ratios were tested for normal distribution with D’Agostinos test for skewness and the
Anscombe–Glynn test of kurtosis. All were found to have normal distribution except ETS CI+II/Routine. Logarithmic transformation (base 10) of that ratio produced normal distribution and was
used. The reference interval for each ratio was defined as the mean±1.96 standard deviations. With one-sided hypotheses for four ratios and a two-sided hypothesis for one, ratios were
treated as six separate (but not independent) tests with binary outcomes. Diagnostic evaluation was performed for each of the tests separately and for a combined model where positive test
result was defined as a positive result in one or more out of the six tests. The relation between test ratios and lactate value was examined with scatter plots. Diagnostic accuracy was
presented as sensitivity, specificity, positive and negative predictive value, and PLR and NLR. Likelihood ratios describe how much information a given test result adds compared with pretest
knowledge (22). High PLR indicates that the probability of disease rises after testing positive. A low NLR means a negative result lowers probability of disease. These ratios are
independent of disease prevalence. Statistical analyses were mainly performed with SPSS (IBM SPSS Statistics Software, version 23, IBM Corp., Armonk, NY) and GraphPad PRISM (GraphPad
Software, version 6.0d, GraphPad Software, Inc., La Jolla, CA). Tests of normality were performed using Free Statistics Software (Wessa, P. 2015, Office for Research Development and
Education, version 1.1.23-r7, URL http://www.wessa.net/) and confidence intervals for the diagnostic evaluation results were calculated with MedCalc (online version 15.11.4, MedCalc
Software, Ostend, Belgium). Statistical figures were constructed with GraphPad PRISM. REFERENCES * McFarland R, Taylor RW, Turnbull DM . A neurological perspective on mitochondrial disease.
_Lancet Neurol_ 2010;9:829–840. Article CAS PubMed Google Scholar * Parikh S, Goldstein A, Koenig MK _et al_. Diagnosis and management of mitochondrial disease: a consensus statement
from the Mitochondrial Medicine Society. _Genet Med_ 2015;17:689–701. Article CAS PubMed Google Scholar * Bernier FP, Boneh A, Dennett X, Chow CW, Cleary MA, Thorburn DR . Diagnostic
criteria for respiratory chain disorders in adults and children. _Neurology_ 2002;59:1406–1411. Article CAS PubMed Google Scholar * Wolf NI, Smeitink JA . Mitochondrial disorders: a
proposal for consensus diagnostic criteria in infants and children. _Neurology_ 2002;59:1402–1405. Article PubMed Google Scholar * Haas RH, Parikh S, Falk MJ _et al_. Mitochondrial
disease: a practical approach for primary care physicians. _Pediatrics_ 2007;120:1326–1333. Article PubMed Google Scholar * Yamada K, Toribe Y, Yanagihara K, Mano T, Akagi M, Suzuki Y .
Diagnostic accuracy of blood and CSF lactate in identifying children with mitochondrial diseases affecting the central nervous system. _Brain Dev_ 2012;34:92–97. Article PubMed Google
Scholar * Haas RH, Parikh S, Falk MJ _et al_. The in-depth evaluation of suspected mitochondrial disease. _Mol Genet Metab_ 2008;94:16–37. Article CAS PubMed PubMed Central Google
Scholar * Tulinius MH, Holme E, Kristiansson B, Larsson NG, Oldfors A . Mitochondrial encephalomyopathies in childhood. I. Biochemical and morphologic investigations. _J Pediatr_
1991;119:242–250. Article CAS PubMed Google Scholar * Dinopoulos A, Smeitink J, ter Laak H . Unusual features of mitochondrial degeneration in skeletal muscle of patients with nuclear
complex I mutation. _Acta Neuropathol_ 2005;110:199–202. Article PubMed Google Scholar * Mancuso M, Calsolaro V, Orsucci D _et al_. Mitochondria, cognitive impairment, and
Alzheimer's disease. _Int J Alzheimers Dis_ 2009: 2009. * Sjovall F, Morota S, Hansson MJ, Friberg H, Gnaiger E, Elmer E . Temporal increase of platelet mitochondrial respiration is
negatively associated with clinical outcome in patients with sepsis. _Crit Care_ 2010;14:R214. Article PubMed PubMed Central Google Scholar * Ehinger JK, Morota S, Hansson MJ, Paul G,
Elmer E . Mitochondrial dysfunction in blood cells from amyotrophic lateral sclerosis patients. _J Neurol_ 2015;262:1493–1503. Article CAS PubMed Google Scholar * Kunz D, Luley C, Fritz
S _et al_. Oxygraphic evaluation of mitochondrial function in digitonin-permeabilized mononuclear cells and cultured skin fibroblasts of patients with chronic progressive external
ophthalmoplegia. _Biochem Mol Med_ 1995;54:105–111. Article CAS PubMed Google Scholar * Artuch R, Colome C, Playan A _et al_. Oxygen consumption measurement in lymphocytes for the
diagnosis of pediatric patients with oxidative phosphorylation diseases. _Clin Biochem_ 2000;33:481–485. Article CAS PubMed Google Scholar * Pecina P, Gnaiger E, Zeman J, Pronicka E,
Houstek J . Decreased affinity for oxygen of cytochrome-_c_ oxidase in Leigh syndrome caused by SURF1 mutations. _Am J Physiol Cell Physiol_ 2004;287:C1384–C1388. Article CAS PubMed
Google Scholar * Pecina P, Houšťková H, Mráček T _et al_. Noninvasive diagnostics of mitochondrial disorders in isolated lymphocytes with high resolution respirometry. _BBA Clin_
2014;2:62–71. Article PubMed PubMed Central Google Scholar * Sjovall F, Ehinger JK, Marelsson SE _et al_. Mitochondrial respiration in human viable platelets—methodology and influence of
gender, age and storage. _Mitochondrion_ 2013;13:7–14. Article PubMed Google Scholar * Gnaiger E, Kuznetsov AV, Schneeberger S _et al_. Mitochondria in the cold In: Heldmaier G,
Klingenspor M eds. _Life in the Cold_. Heidelberg, Berlin, Germany and New York: Springer, 2000: 431–442. Book Google Scholar * Ehinger JK, Morota S, Hansson MJ, Paul G, Elmér E .
Mitochondrial respiratory function in peripheral blood cells from Huntington's disease patients. _Mov Disord Clin Pract_ 2016;3:472–482. Article PubMed PubMed Central Google Scholar
* Clinical and Laboratory Standards Institute (CLSI). _Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory: Approved Guideline_ 3rd ednCLSI Document C28-A3
Wayne, PA: Clinical and Laboratory Standards Institute, 2008. * Hoaglin DC, Iglewicz B . Fine-tuning some resistant rules for outlier labeling. _J Am Stat Assoc_ 1987;82:1147–1149. Article
Google Scholar * McGee S . Simplifying likelihood ratios. _J Gen Intern Med_ 2002;17:646–649. Article PubMed Google Scholar * Lieber DS, Calvo SE, Shanahan K _et al_. Targeted exome
sequencing of suspected mitochondrial disorders. _Neurology_ 2013;80:1762–1770. Article CAS PubMed PubMed Central Google Scholar * Hutchesson A, Preece MA, Gray G, Green A . Measurement
of lactate in cerebrospinal fluid in investigation of inherited metabolic disease. _Clin Chem_ 1997;43:158–161. CAS PubMed Google Scholar * Chow SL, Rooney ZJ, Cleary MA, Clayton PT,
Leonard JV . The significance of elevated CSF lactate. _Arch Dis Child_ 2005;90:1188–1189. Article CAS PubMed PubMed Central Google Scholar * Davison JE, Rahman S . Recognition,
investigation and management of mitochondrial disease. _Arch Dis Child_ 2017 (doi:10.1136/archdischild-2016-311370). Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS We
thank Albana Shahini for technical support, Marie Palmquist and Ann-Cathrine Berg for patient identification and recruitment, and Michael Karlsson, Sarah Piel, and Imen Chamkha for
constructive input. We thank Erich Gnaiger for participating in protocol design for the Oroboros 02k Oxygraph. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Clinical Sciences,
Mitochondrial Medicine, Lund University, Lund, Sweden Emil Westerlund, Sigurður E Marelsson, Johannes K Ehinger, Fredrik Sjövall, Saori Morota, Eleonor Åsander Frostner, Magnus J Hansson
& Eskil Elmér * Department of Pathology, Institute of Biomedicine, University of Gothenburg, Gothenburg, Sweden Anders Oldfors * Department of Pediatrics, The Queen Silvia Children’s
Hospital, University of Gothenburg, Gothenburg, Sweden Niklas Darin * Department of Pediatrics, Skåne University Hospital, Lund University, Lund, Sweden Johan Lundgren & Vineta Fellman
Authors * Emil Westerlund View author publications You can also search for this author inPubMed Google Scholar * Sigurður E Marelsson View author publications You can also search for this
author inPubMed Google Scholar * Johannes K Ehinger View author publications You can also search for this author inPubMed Google Scholar * Fredrik Sjövall View author publications You can
also search for this author inPubMed Google Scholar * Saori Morota View author publications You can also search for this author inPubMed Google Scholar * Eleonor Åsander Frostner View author
publications You can also search for this author inPubMed Google Scholar * Anders Oldfors View author publications You can also search for this author inPubMed Google Scholar * Niklas Darin
View author publications You can also search for this author inPubMed Google Scholar * Johan Lundgren View author publications You can also search for this author inPubMed Google Scholar *
Magnus J Hansson View author publications You can also search for this author inPubMed Google Scholar * Vineta Fellman View author publications You can also search for this author inPubMed
Google Scholar * Eskil Elmér View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Emil Westerlund. ETHICS DECLARATIONS
COMPETING INTERESTS Drs Ehinger, Hansson, and Elmér and Åsander Frostner have equity interests in, and/or have received salary support from NeuroVive Pharmaceutical AB, a public company
developing pharmaceuticals in the field of mitochondrial medicine. The other authors declare no financial or commercial conflict of interest. ADDITIONAL INFORMATION STATEMENT OF FINANCIAL
SUPPORT This work was supported by the Swedish Research Council (Dr Elmér: 2011-3470 and Dr Fellman: 2011-3877), The Crafoord Foundation, Linnéa and Josef Carlsson’s Foundation, Swedish
government project and salary funding for clinically oriented medical research (ALF Grants), Skåne University Hospital funds, and regional research and development grants (Southern
healthcare region, Sweden). RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Westerlund, E., Marelsson, S., Ehinger, J. _et al._ Oxygen consumption in
platelets as an adjunct diagnostic method for pediatric mitochondrial disease. _Pediatr Res_ 83, 455–465 (2018). https://doi.org/10.1038/pr.2017.250 Download citation * Received: 03 May 2017
* Accepted: 19 September 2017 * Published: 15 November 2017 * Issue Date: February 2018 * DOI: https://doi.org/10.1038/pr.2017.250 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
