Evolution and function of interleukin-4 receptor signaling in adaptive immunity and neutrophils
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The cytokines interleukin (IL)-4 and IL-13, signaling via the IL-4 receptor (IL-4R), orchestrate type 2 immunity to helminth infections and toxins. Activation of epithelial and
myeloid cells, and a transient neutrophils influx initiates type 2 immune responses, which are dominated by basophils, eosinophils, mast cells, B cell immunoglobulin E production, and type 2
T helper and T follicular helper cells. Interestingly, IL-4 and IL-13 can curtail chemotaxis and several effector functions of neutrophils in mice and humans. This inhibitory role of IL-4
and IL-13 probably developed to limit tissue damage by neutrophils during type 2 immunity where a “weep and sweep” response aims at expulsion and decreased fecundity, instead of killing, of
macroparasites. Here, we review when IL-4R signaling cytokines appeared during evolution relative to neutrophils and adaptive immunity. Neutrophil-like granular phagocytes were present in
invertebrates throughout the bilaterian clade, but we were unable to find data on IL-4, IL-13, or their receptors in invertebrates. Conversely, vertebrates had both adaptive immunity and
IL-4, IL-13, and IL-4Rs, suggesting that type 2 cytokines evolved together with adaptive immunity. Further studies are necessary to determine whether IL-4R signaling in neutrophils was
established simultaneously with the appearance of adaptive immunity or later. SIMILAR CONTENT BEING VIEWED BY OTHERS BASOPHILS PRIME GROUP 2 INNATE LYMPHOID CELLS FOR NEUROPEPTIDE-MEDIATED
INHIBITION Article 17 August 2020 ELUCIDATING DIFFERENT PATTERN OF IMMUNOREGULATION IN BALB/C AND C57BL/6 MICE AND THEIR F1 PROGENY Article Open access 15 January 2021 COOPERATION OF ILC2S
AND TH2 CELLS IN THE EXPULSION OF INTESTINAL HELMINTH PARASITES Article 05 October 2023 INTRODUCTION Interleukin (IL)-4 and IL-13 are well known for their key roles in type 2 immune
responses, which result in resistance to helminth parasites and inactivation of toxins. IL-4 and IL-13 induce differentiation of naïve T cells to type 2 T helper and T follicular helper
cells, B cell antibody production and isotype switching to immunoglobulin E (IgE), expansion of basophils and eosinophils, mast cell activation, skewing of macrophages toward the subtype of
alternatively-activated macrophages (also known as type 2 or M2 macrophages), and goblet cell hyperplasia [1, 2]. It is well established that neutrophils are present in type 1 and type 3
immune responses, which serve to fight intracellular and extracellular pathogens, respectively. However, recent evidence has revealed a role for neutrophils in protection against parasite
infections [3, 4]. Thus, it was shown that during the initial phase of type 2 responses, the presence of neutrophils was beneficial for limiting parasite survival and spreading. This was
primarily due to formation of neutrophil extracellular traps (NETs) and degranulation [5]. Accordingly, also in type 2 immune responses, neutrophils seem to be the first nonresident immune
cells to arrive to the affected site. Despite their very short lifespan, neutrophils are able to shape the immune response long after their death, for example by guiding and attracting other
immune cells or by their ability to prime macrophages to become M2 macrophages [6]. These M2 macrophages are efficient in protecting during a secondary infection. Thus, neutrophils do not
only leave a temporary mark but are able to impact future immune responses. However, there is accumulating evidence showing that IL-4- and IL-13-mediated IL-4 receptor (IL-4R) signaling in
both mouse and human neutrophils inhibits their migration and effector functions in vitro and in vivo [7, 8]. In a number of different mouse models including sterile inflammation, bacterial
infection, helminth infestation, and rheumatoid arthritis, IL-4R signaling was shown to have an inhibitory effect on neutrophils [9,10,11,12]. Human neutrophils isolated from allergic
patients, a condition dominated by the presence of IL-4 and IL-13, were less capable of migrating and producing NETs than neutrophils from healthy donors [13]. Thus, we hypothesize that
inhibition of neutrophil effector functions in type 2 immune responses constitutes a crucial effect of the IL-4/IL-13–IL-4R system. Failure of this regulatory system can cause detrimental
tissue damage, as seen with neutrophilic types of asthma. Why neutrophils are beneficial for type 2 immune responses and, simultaneously, type 2 cytokines restrict neutrophil effector
functions, can be explained when considering the timing of events. During the initiation phase of a type 2 immune response, there is little or no type 2 cytokines present, and neutrophils
are needed as a first wave of defense. Once the type 2 immune response is fully active, abundant IL-4 and IL-13 suppress neutrophil effector functions, which at this stage—via neutrophil
degranulation and NET formation—would cause excessive tissue damage. Thus, timed IL-4R signaling in neutrophils allows early influx but limits tissue damage by neutrophils during the “weep
and sweep” phase of type 2 immunity. Considering this IL-4R-mediated mechanism of neutrophil regulation, we wondered whether IL-4R signaling cytokines initially evolved to refine adaptive
immune responses against parasites or to provide timed inhibition of innate immune cells, such as neutrophils, to limit tissue damage. In order to address this question, we reviewed and
combined phylogenetic data on neutrophils, the adaptive immune system, and the IL-4/IL-13–IL-4R system. THE EVOLUTION OF NEUTROPHILS Neutrophils are the most abundant leukocytes in human
blood and are typically the first nonresident immune cells to respond to an inflammatory or infectious stimulus [14]. Thus, together with barrier epithelial cells and resident immune cells,
neutrophils form the first line of defense to limit pathogens until the adaptive immune response arrives [15]. Neutrophils are able to fight infection by phagocytosis, release of
antimicrobial effector molecules (termed degranulation), production of reactive oxygen species (ROS), and the formation of NETs, which are DNA meshes decorated with antimicrobial peptides
that neutrophils can expulse in response to pathogens that are too large to phagocytose [8, 16,17,18,19]. Phagocytosis, one of the key effector functions of mammalian neutrophils, is a
ubiquitously present process throughout nature from unicellular amoebae to multicellular organisms [20]. In basic invertebrates, such as sponges or cnidarians, specialized phagocytic cells
called amoebocytes are responsible for taking up foreign material and debris, but in some cases also food particles [21,22,23]. Protostomes and invertebrate deuterostomes all have more or
less complex innate immune systems consisting of non-granular and granular hemocytes. Hemocytes are mesoderm-derived cells that recognize and phagocytose nonself particles and release
antimicrobial granules, thus being reminiscent of monocytes, macrophages, and granulocytes of higher vertebrates [23, 24]. The demonstration of DNA extracellular trap formation not only in
mammalian neutrophils and eosinophils [25], but also in granulocytes of fish [26], crustaceans [27], molluscs [28, 29], and worms [30], provides further evidence of functional analogies
between mammalian and invertebrate granulocytes. Also the production of ROS by oxidase enzyme complexes has been shown in numerous invertebrate species [31]. Moreover, histological stainings
of invertebrate granular hemocytes show acidophilic (i.e., eosinophilic), basophilic, and neutrophilic cells with multi-lobulated nuclei [23]. All these striking morphological and
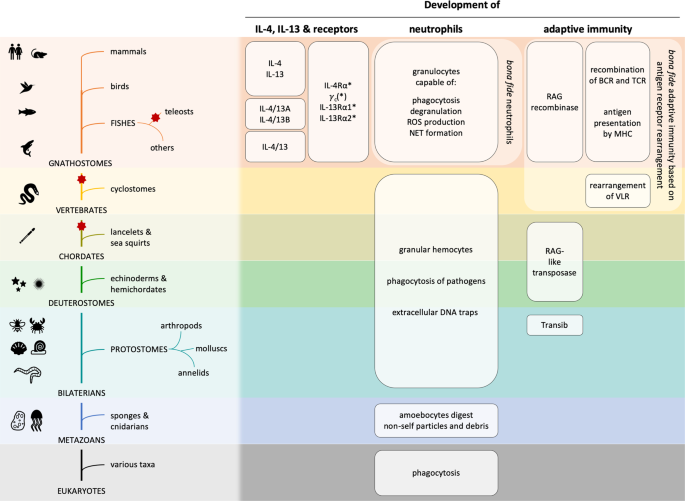
functional parallels lead to the conclusion that granular phagocytes (i.e., neutrophils) are a well-conserved and phylogenetically ancient immune cell type (Fig. 1). THE EVOLUTION OF
ADAPTIVE IMMUNITY The adaptive immune system of jawed vertebrates (gnathostomes) centers around the genes responsible for recombination of antigen receptors. The evolution of this branch of
immunity is closely linked to two major evolutionary events: the emergence of recombinase-activating gene 1 and 2 (RAG1 and RAG2) and the occurrence of several rounds of whole genome
duplication (WGD). In gnathostomes, RAG proteins are expressed in developmental stages of B and T cells, and are responsible for the random joining of one variable, one joining, and—in some
cases—one diversity gene segment of the antigen receptor gene locus. This process, also termed V(D)J recombination, allows the creation of a vast variety of different receptors from a
relatively low number of single gene segments [32]. _Rag_ or _Rag_-like genes can be found throughout the superphylum of deuterostomes, and a gene related to _Rag1_ called _Transib_ was also
found in insects (e.g., _Helicoverpa zea_). Surprisingly, Transib and RAG1 proteins have very similar enzymatic activity and specificity and the catalytic triad is conserved in both [33].
This suggests that the ancestor of modern-day _Rag_ was acquired by a common ancestor of protostomes and deuterostomes. While _Rag_-like genes are ancient and well conserved, their function
changed during evolution: _Transib_ and active _Rag_-like loci in invertebrates act as transposons, i.e., DNA segments coding for a protein that excises their own DNA segment and inserts it
at another site in the genome. RAG proteins in jawed vertebrates, however, act as recombinases; they do not excise their own gene but DNA in between variable, diversity, and joining gene
segments [34]. Interestingly, cyclostomes, the only living jawless vertebrates, do not have _Rag_ but they have an adaptive immune system based on recombination of leucine-rich repeats
leading to the generation of specific agglutinins called variable lymphocyte receptors that are membrane-bound or secreted [32]. Collectively, the presence of _Rag_-related genes is
widespread throughout the bilaterian clade, but only gnathostomes use RAG as a recombinase which enables the development of a _bona fide_ adaptive immune system (Fig. 1). It is now widely
accepted that the genome of a common vertebrate ancestor underwent two rounds of WGD, resulting in a fourfold amount of DNA [35]. This increase in accessible raw material made it possible to
refine and diversify the genome. Refinement can be achieved by subfunctionalization, a process by which the functionalities of the original gene are distributed among its daughters, which
can then evolve to become specialized genes [36]. By having multiple copies of the same gene, one of them can be freed from selective pressure and can accumulate mutations, potentially
resulting in new genes with new functions in a process called neofunctionalization, hence diversifying the genome [36]. An immunologically relevant example is the quadruplication of the
proto-major histocompatibility complex (MHC) chromosome that gave rise to four paralogous regions all coding for genes involved in antigen presentation and recognition [37, 38]. In
conclusion, the founding stones for the establishment of an adaptive immune system existed already in primitive bilaterian ancestors, but an enzyme capable of recombination rather than
translocation only occurred in jawed vertebrates. Thus, _bona fide_ adaptive immunity is solely present in gnathostomes and must have appeared first in a common ancestor. THE EVOLUTION OF
IL-4 AND IL-13 Genes encoding for proteins related to signaling in the immune system are under a constant evolutionary pressure to adapt and shape immunity toward the most favorable
protection of the host. This is nicely illustrated by the finding that among the top 25 genes showing the highest degree of evolutionary divergence between mouse and human orthologues, 7
encode for cytokines or cytokine receptors [39]. Due to their low homology even within mammals, the genes encoding for IL-4 and IL-13 are difficult to track in other species. In the
mammalian genome, _Il4_ and _Il13_ are placed side by side and researchers are therefore often searching for both _Il4_- and _Il13_-linked genes as well as flanking genes such as _Kif3a_ and
_Rad50_ that are much better conserved [40]. With the increasing number of genomes being sequenced, a substantial amount of evidence is emerging to shed light on the evolution of these and
other genes. _Il4_/_Il13_-related genes have been found in a number of both fish and bird species and even in amphibians although the latter has not been confirmed by functional studies
(Fig. 1) [40,41,42]. A single _Il4_/_Il13_-related gene was found in spotted gar (_Lepisosteus oculatus_), an example of a bony fish that only went through two rounds of WGD, whereas two
_Il4_/_Il13_-related genes (_Il4/13a_ and _Il4/13b_) have been found in pufferfish (_Tetraodon nigroviridis_) and in zebrafish (_Danio rerio_) as a result of a third round of WGD that
teleost fish went through [43]. Based on these findings, it is believed that a single _Il4_/_Il13_ gene existed in ancestral gnathostomes, which has duplicated during WGD and/or tandem
duplication during vertebrate evolution and thereafter evolved into the so-called type 2 cytokine locus including _Il4_, _Il5_, and _Il13_ [40]. THE EVOLUTION OF THE IL-4 RECEPTOR SYSTEM
IL-4 and IL-13 signal via heterodimeric IL-4Rs composed of three receptor subunits: IL-4Rα, the common gamma chain cytokine receptor (_γ_c), and IL-13Rα1 [8]. IL-4 signals via both the type
1 IL-4R composed of IL-4Rα and _γ_c and the type 2 IL-4R composed of IL-4Rα and IL-13Rα1. IL-13 only signals via the type 2 IL-4R. In addition, IL-13 can interact with IL-13Rα2, which is
thought to be a decoy receptor without signaling function. IL-4R subunits are found in all jawed vertebrates (Fig. 1) [40]. All of the IL-4R and IL-13R genes belong to the class I cytokine
receptors, which most likely originated from glycoprotein 130-like receptors present in invertebrates [44]. Although some class I cytokine receptor genes seem to have arisen from the two
WGD, others have likely been created by tandem or _en bloc_ gene duplication. The extra WGD the teleost lineage went through is possibly responsible for the unique teleost IL-4Rs and
IL-13Rs. There is very sparse information available on cross-reactivity of the IL-4/IL-13–IL-4R system in different species [45]. IL-4Rα (also termed CD124), the shared receptor subunit of
the type 1 and type 2 IL-4Rs, was identified in a large number of sequenced bird genomes [46]. Based on this, the gene was found to have an enhanced rate of nonsynonymous substitutions, and
certain sites were classified as being under particularly high positive selection pressure. Interestingly, this might relate to a finding in the human _Il4ra_ gene where some polymorphisms
led to a higher susceptibility to asthma [47], and in mice where a single amino acid substitution in the _Il4ra_ gene favored the development of asthma-like lung disease [48]. Again, the
fishes undergoing a third round of WGD have two genes encoding for IL-4Rα (IL-4Rα1 and IL-4Rα2), and the _Il4ra_ gene variants differ considerably between species. In zebrafish, alternative
splicing results in a secreted IL-4Rα isoform found in liver, brain, and muscle tissue. Administration of zebrafish recombinant IL-4/13A showed in vivo effects including antibody production
by B cells and CD40 expression, which is important for induction of type 2 immunity [49]. This serves as further proof that a well-developed adaptive immune system is already established in
fishes. _γ_c (also known as CD132, encoded by the _Il2rg_ gene) is a shared receptor subunit of the cytokines IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 [50]. _Il2rg_ was found in both fishes
and birds. Although the gene in birds is very similar to that of mammals, alternative splice variants exist [51]. _γ_c-expressing T cells in chicken have been shown to be important in
fighting virus infections [52], thus demonstrating a genetic and functional similarity of _γ_c between chickens and humans. In fish, _Il2rg_ was initially identified in rainbow trout
(_Oncorhynchus mykiss)_ and was later found in zebrafish (_Danio rerio_), elephant shark (_Callorhinchus milii_), spotted gar (_Lepisosteus oculatus)_, and in several species of the
_Tilapia_ genus. Similar to _Il4ra_, some fishes have two paralogues of _Il2rg_ [40]. Here, however, the mechanism giving rise to the duplication might not be solely due to a third round of
WGD, as some species have the two genes on the same chromosome. Less research has gone into investigating _Il13ra1_ and _Il13ra2_ (also termed CD213a1 and CD213a2, respectively), but also
these two genes exist in all jawed vertebrates. From a functional perspective, both IL-13Rα1 and IL-13Rα2 are upregulated upon infectious stimuli in chicken [53, 54]. In trout, two
paralogues of both _Il13ra1_ and _Il13ra2_ are present due to a third WGD; whereas the IL-13Rα2-related proteins (IL-13Rα2a/IL-13Rα2b) show 79% amino acid similarity, IL-13Rα1a and IL-13Rα1b
have an amino acid identity of only 34% [55]. A distinctive expression pattern also applies to all of the subunits in trout: thus, IL-13Rα1b and IL-13Rα2b are primarily expressed in the
ovaries, whereas IL-13Rα2a is expressed in spleen, head kidney, and mucosal tissue and IL-13Rα1a in scales, gills, and skin [56]. This suggests that the paralogues specialized to become
tissue-specific. Based on these findings, the expansion and development of the class I cytokine receptors and thereby the IL-4R and IL-13R subunits correlated with the appearance of adaptive
immunity, which occurred together with a refinement of the innate immune system. CONCLUSION Whereas neutrophil-like granular phagocytes were present in invertebrates throughout the
bilaterian clade, we were unable to find data on IL-4, IL-13, IL-4Rα, IL-13Rα1, IL-13Rα2, and _γ_c in invertebrates. Rather, IL-4, IL-13, and their receptors are found in vertebrates, thus
coinciding with the phylogenetic development of a _bona fide_ adaptive immune system. Notably, we did not find any evidence of type 2 cytokines in invertebrates, which could either indicate
that these cytokines evolved later or could be due to a lack of data. The presence of eosinophilic and basophilic granular hemocytes in invertebrates could indicate a primal form of type 2
immunity, possibly harnessing factors that are upregulated during early phases of helminth infestations, such as arginase-1, chitinase-like protein 3, and resistin-like molecule α. However,
how exactly these cells recognize and fight parasites will need to be further investigated. Future studies are necessary to determine whether IL-4R signaling in neutrophils always served a
dual function in adaptive immunity and in curtailing neutrophil effector functions, or whether the neutrophil-specific function of IL-4R signaling evolved later. Moreover, the primary
evolutionary source of IL-4/IL-13 production is still unknown and remains to be assessed in the future. Functional assays in phylogenetically older taxa, such as fishes, are needed to
explore these questions. REFERENCES * Lloyd CM, Snelgrove RJ. Type 2 immunity: expanding our view. Sci Immunol. 2018;3:1–12. Google Scholar * Allen JE, Maizels RM. Diversity and dialogue in
immunity to helminths. Nat Rev Immunol. 2011;11:375–88. CAS PubMed Google Scholar * Bonne-Année S, Kerepesi LA, Hess JA, O’Connell AE, Lok JB, Nolan TJ, et al. Human and mouse
macrophages collaborate with neutrophils to kill larval strongyloides stercoralis. Infect Immun. 2013;81:3346–55. PubMed PubMed Central Google Scholar * Sutherland TE, Logan N, Rückerl D,
Humbles AA, Allan SM, Papayannopoulos V, et al. Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat Immunol.
2014;15:1116–25. CAS PubMed PubMed Central Google Scholar * Bonne-Année S, Kerepesi LA, Hess JA, Wesolowski J, Paumet F, Lok JB, et al. Extracellular traps are associated with human and
mouse neutrophil and macrophage mediated killing of larval Strongyloides stercoralis. Microbes Infect. 2014;16:502–11. PubMed PubMed Central Google Scholar * Chen F, Wu W, Millman A,
Craft JF, Chen E, Patel N, et al. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat Immunol. 2014;15:938–46. CAS PubMed PubMed
Central Google Scholar * Heeb LEM, Egholm C, Impellizzieri D, Ridder F, Boyman O. Regulation of neutrophils in type 2 immune responses. Curr Opin Immunol. 2018;54:115–22. CAS PubMed
Google Scholar * Egholm C, Heeb LEM, Impellizzieri D, Boyman O. The regulatory effects of interleukin-4 receptor signaling on neutrophils in type 2 immune responses. Front Immunol. 2019;10.
https://doi.org/10.3389/fimmu.2019.02507. * Woytschak J, Keller N, Krieg C, Impellizzieri D, Thompson RW, Wynn TA, et al. Type 2 interleukin-4 receptor signaling in neutrophils antagonizes
their expansion and migration during infection and inflammation. Immunity. 2016;45:172–84. CAS PubMed Google Scholar * Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, et al. An
essential role for T H 2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260–6. CAS PubMed PubMed Central Google Scholar * Chen Z,
Andreev D, Oeser K, Krljanac B, Hueber A, Kleyer A, et al. Th2 and eosinophil responses suppress inflammatory arthritis. Nat Commun. 2016;7:1–12. Google Scholar * Panda SK, Wigerblad G,
Jiang L, Jiménez-Andrade Y, Iyer VS, Shen Y, et al. IL-4 controls activated neutrophil FcγR2b expression and migration into inflamed joints. Proc Natl Acad Sci USA. 2020;117:3103–13. CAS
PubMed Google Scholar * Impellizzieri D, Ridder F, Raeber ME, Egholm C, Woytschak J, Kolios AGA, et al. IL-4 receptor engagement in human neutrophils impairs their migration and
extracellular trap formation. J Allergy Clin Immunol. 2019;144:267–.e4. CAS PubMed Google Scholar * Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from
mechanisms to disease. Annu Rev Immunol. 2012;30:459–89. CAS PubMed Google Scholar * Deniset JF, Kubes P. Recent advances in understanding neutrophils. F1000Res. 2016;5:2912. PubMed
PubMed Central Google Scholar * Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. CAS PubMed PubMed Central Google Scholar * Brinkmann V, Reichard U, Goosmann
C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. CAS PubMed Google Scholar * Uribe-Querol E, Rosales C. Control of
phagocytosis by microbial pathogens. Front Immunol. 2017;8. https://doi.org/10.3389/fimmu.2017.01368. * Gazendam RP, van de Geer A, Roos D, van den Berg TK, Kuijpers TW. How neutrophils kill
fungi. Immunol Rev. 2016;273:299–311. CAS PubMed Google Scholar * Barreda DR, Neely HR, Flajnik MF. Evolution of myeloid cells. Microbiol Spectr. 2016;4.
https://doi.org/10.1128/microbiolspec.MCHD-0007-2015. * Gold DA, Jacobs DK. Stem cell dynamics in Cnidaria: are there unifying principles? Dev Genes Evol. 2013;223:53–66. PubMed Google
Scholar * Ricci CA, Kamal AHM, Chakrabarty JK, Fuess LE, Mann WT, Jinks LR, et al. Proteomic investigation of a diseased gorgonian coral indicates disruption of essential cell function and
investment in inflammatory and other immune processes. Integr Comp Biol. 2019;59:830–44. PubMed Google Scholar * Hartenstein V. Blood cells and blood cell development in the animal
kingdom. Annu Rev Cell Dev Biol. 2006;22:677–712. CAS PubMed Google Scholar * Leiding JW. Neutrophil evolution and their diseases in humans. Front Immunol. 2017;8.
https://doi.org/10.3389/fimmu.2017.01009. * Boeltz S, Amini P, Anders H-J, Andrade F, Bilyy R, Chatfield S, et al. To NET or not to NET:current opinions and state of the science regarding
the formation of neutrophil extracellular traps. Cell Death Differ. 2019;26:395–408. PubMed PubMed Central Google Scholar * Pijanowski L, Golbach L, Kolaczkowska E, Scheer M, Verburg-van
Kemenade BML, Chadzinska M. Carp neutrophilic granulocytes form extracellular traps via ROS-dependent and independent pathways. Fish Shellfish Immunol. 2013;34:1244–52. CAS PubMed Google
Scholar * Ng TH, Chang SH, Wu MH, Wang HC. Shrimp hemocytes release extracellular traps that kill bacteria. Dev Comp Immunol. 2013;41:644–51. CAS PubMed Google Scholar * Poirier AC,
Schmitt P, Rosa RD, Vanhove AS, Kieffer-Jaquinod S, Rubio TP, et al. Antimicrobial histones and DNA traps in invertebrate immunity: evidences in Crassostrea gigas. J Biol Chem.
2014;289:24821–31. CAS PubMed PubMed Central Google Scholar * Lange MK, Penagos-Tabares F, Muñoz-Caro T, Gärtner U, Mejer H, Schaper R, et al. Gastropod-derived haemocyte extracellular
traps entrap metastrongyloid larval stages of Angiostrongylus vasorum, Aelurostrongylus abstrusus and Troglostrongylus brevior. Parasites Vectors. 2017;10:1–12. Google Scholar * Homa J.
Earthworm coelomocyte extracellular traps: structural and functional similarities with neutrophil NETs. Cell Tissue Res. 2018;371:407–14. PubMed PubMed Central Google Scholar *
Siauciunaite R, Foulkes NS, Calabrò V, Vallone D. Evolution shapes the gene expression response to oxidative stress. Int J Mol Sci. 2019;20. https://doi.org/10.3390/ijms20123040. * Flajnik
MF, Kasahara M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat Rev Genet. 2010;11:47–59. CAS PubMed Google Scholar * Carmona LM, Schatz
DG. New insights into the evolutionary origins of the recombination-activating gene proteins and V(D)J recombination. FEBS J. 2017;284:1590–605. CAS PubMed PubMed Central Google Scholar
* Morales Poole JR, Huang SF, Xu A, Bayet J, Pontarotti P. The RAG transposon is active through the deuterostome evolution and domesticated in jawed vertebrates. Immunogenetics.
2017;69:391–400. CAS PubMed Google Scholar * Kasahara M. The 2R hypothesis: an update. Curr Opin Immunol. 2007;19:547–52. CAS PubMed Google Scholar * Moriyama Y, Koshiba-Takeuchi K.
Significance of whole-genome duplications on the emergence of evolutionary novelties. Brief Funct Genomics. 2018;17:329–38. CAS PubMed Google Scholar * Ohta Y, Kasahara M, O’Connor TD,
Flajnik MF. Inferring the “Primordial Immune Complex”: origins of mhc class I and antigen receptors revealed by comparative genomics. J Immunol. 2019;203:1882–96. CAS PubMed Google Scholar
* Ohta Y, Flajnik MF. Coevolution of MHC genes (LMP/TAP/class Ia, NKT-class Ib, NKp30-B7H6): lessons from cold-blooded vertebrates. Immunol Rev. 2015;267:6–15. CAS PubMed PubMed Central
Google Scholar * Scapigliati G, Buonocore F, Mazzini M. Biological activity of cytokines: an evolutionary perspective. Curr Pharm Des. 2006;12:3071–81. CAS PubMed Google Scholar * Wang
T, Secombes CJ. The evolution of IL-4 and IL-13 and their receptor subunits. Cytokine. 2015;75:8–13. PubMed Google Scholar * Kaiser P, Poh TY, Rothwell L, Avery S, Balu S, Pathania US, et
al. A genomic analysis of chicken cytokines and chemokines. J Inter Cytokine Res. 2005;25:467–84. CAS Google Scholar * Stocchi V, Wang T, Randelli E, Mazzini M, Gerdol M, Pallavicini A,
et al. Evolution of Th2 responses: characterization of IL-4/13 in sea bass (Dicentrarchus labrax L.) and studies of expression and biological activity. Sci Rep. 2017;7.
https://doi.org/10.1038/s41598-017-02472-y. * Sequeida A, Maisey K, Imarai M. Interleukin 4/13 receptors: an overview of genes, expression and functional role in teleost fish. Cytokine
Growth Factor Rev. 2017;38:66–72. CAS PubMed Google Scholar * Liongue C, Ward AC. Evolution of Class I cytokine receptors. BMC Evol Biol. 2007;7:120. PubMed PubMed Central Google
Scholar * Andrews RP, Rosa LR, Daines MO, Khurana Hershey GK. Reconstitution of a functional human type II IL-4/IL-13 receptor in Mouse B cells: demonstration of species specificity. J
Immunol. 2001;166:1716–22. CAS PubMed Google Scholar * Downing T, Lynn DJ, Connell S, Lloyd AT, Bhuiyan AK, Silva P, et al. Evidence of balanced diversity at the chicken interleukin 4
receptor alpha chain locus. BMC Evol Biol. 2009;9:1–13. Google Scholar * Amirzargar AA, Movahedi M, Rezaei N, Moradi B, Dorkhosh S, Mahloji M, et al. Polymorphisms in IL4 and IL4RA confer
susceptibility to asthma. J Investig Allergol Clin Immunol. 2009;19:433–8. CAS PubMed Google Scholar * Tachdjian R, Mathias C, Khatib SAl, Bryce PJ, Kim HS, Blaeser F, et al.
Pathogenicity of a disease-associated human IL-4 receptor allele in experimental asthma. J Exp Med. 2009;206:2191–204. CAS PubMed PubMed Central Google Scholar * Zhu L, Pan P, Fang W,
Shao J, Xiang L. Essential role of IL-4 and IL-4Rα interaction in adaptive immunity of zebrafish: insight into the origin of Th2-like regulatory mechanism in ancient vertebrates. J Immunol.
2012;188:5571–84. CAS PubMed Google Scholar * Raeber ME, Zurbuchen Y, Impellizzieri D, Boyman O. The role of cytokines in T-cell memory in health and disease. Immunol Rev.
2018;283:176–93. CAS PubMed Google Scholar * Min W, Lillehoj HS, Fetterer RH. Identification of an alternatively spliced isoform of the common cytokine receptor γ chain in chickens.
Biochem Biophys Res Commun. 2002;299:321–7. CAS PubMed Google Scholar * Wang S, Teng Q, Jia L, Sun X, Wu Y, Zhou J. Infectious bursal disease virus influences the transcription of chicken
γ c and γ c family cytokines during infection. PLoS ONE. 2014;9. https://doi.org/10.1371/journal.pone.0084503. * Miyoshi M, Horiuchi H, Fukushima Y, Matsuda H, Furusawa S. Cloning of the
chicken interleukin-13 receptor α2 gene and production of a specific monoclonal antibody. Dev Comp Immunol. 2007;31:394–406. CAS PubMed Google Scholar * Morgan RW, Sofer L, Anderson AS,
Bernberg EL, Cui J, Burnside J. Induction of host gene expression following Infection of chicken embryo fibroblasts with oncogenic marek’s disease virus. J Virol. 2001;75:533–9. CAS PubMed
PubMed Central Google Scholar * Lockyer A, Jones C, Noble L, Verspoor E, Holland J, Secombes C. Isolation and characterisation of a putative interleukin 13 receptor a2 sequence from
rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2001;11:541–6. CAS PubMed Google Scholar * Wang T, Huang W, Costa MM, Martin SAM, Secombes CJ. Two copies of the genes
encoding the subunits of putative interleukin (IL)-4/IL-13 receptors, IL-4Rα, IL-13Rα1 and IL-13Rα2, have been identified in rainbow trout (Oncorhynchus mykiss) and have complex patterns of
expression and modulation. Immunogenetics. 2011;63:235–53. CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank the current and former members of the
Boyman laboratory for helpful discussions, especially Janine Woytschak and Yulia Butscheid. This work was supported by the Swiss National Science Foundation (310030–172978), the
Hochspezialisierte Medizin Schwerpunkt Immunologie (HSM-2-Immunologie), and the Clinical Research Priority Program CYTIMM-Z of the University of Zurich (all to OB). AUTHOR INFORMATION Author
notes * These authors contributed equally: Lukas E. M. Heeb, Cecilie Egholm AUTHORS AND AFFILIATIONS * Department of Immunology, University Hospital Zurich, CH-8091, Zurich, Switzerland
Lukas E. M. Heeb, Cecilie Egholm & Onur Boyman * Faculty of Medicine, University of Zurich, CH-8006, Zurich, Switzerland Onur Boyman Authors * Lukas E. M. Heeb View author publications
You can also search for this author inPubMed Google Scholar * Cecilie Egholm View author publications You can also search for this author inPubMed Google Scholar * Onur Boyman View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Onur Boyman. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that
they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution
and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if
changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the
material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to
obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Heeb, L.E.M., Egholm, C. & Boyman, O. Evolution and function of interleukin-4 receptor signaling in adaptive immunity and neutrophils. _Genes Immun_ 21, 143–149 (2020).
https://doi.org/10.1038/s41435-020-0095-7 Download citation * Received: 06 December 2019 * Revised: 18 February 2020 * Accepted: 21 February 2020 * Published: 06 March 2020 * Issue Date: May
2020 * DOI: https://doi.org/10.1038/s41435-020-0095-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
