Mixed-state electron ptychography enables sub-angstrom resolution imaging with picometer precision at low dose
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Both high resolution and high precision are required to quantitatively determine the atomic structure of complex nanostructured materials. However, for conventional imaging methods
in scanning transmission electron microscopy (STEM), atomic resolution with picometer precision cannot usually be achieved for weakly-scattering samples or radiation-sensitive materials,
such as 2D materials. Here, we demonstrate low-dose, sub-angstrom resolution imaging with picometer precision using mixed-state electron ptychography. We show that correctly accounting for
the partial coherence of the electron beam is a prerequisite for high-quality structural reconstructions due to the intrinsic partial coherence of the electron beam. The mixed-state
reconstruction gains importance especially when simultaneously pursuing high resolution, high precision and large field-of-view imaging. Compared with conventional atomic-resolution STEM
imaging techniques, the mixed-state ptychographic approach simultaneously provides a four-times-faster acquisition, with double the information limit at the same dose, or up to a fifty-fold
reduction in dose at the same resolution. SIMILAR CONTENT BEING VIEWED BY OTHERS SUB-NANOMETER DEPTH RESOLUTION AND SINGLE DOPANT VISUALIZATION ACHIEVED BY TILT-COUPLED MULTISLICE ELECTRON
PTYCHOGRAPHY Article Open access 31 January 2025 INFLUENCE OF PLASMON EXCITATIONS ON ATOMIC-RESOLUTION QUANTITATIVE 4D SCANNING TRANSMISSION ELECTRON MICROSCOPY Article Open access 21
October 2020 LOCAL-ORBITAL PTYCHOGRAPHY FOR ULTRAHIGH-RESOLUTION IMAGING Article 29 January 2024 INTRODUCTION Determining the local atomic arrangement of complex nanostructures can provide
fundamental insights into the properties of materials1,2. Compared to traditional metals and semiconductors, newer materials systems as metal-organic frameworks and organic perovskites are
more radiation sensitive3,4,5, requiring more dose-efficient imaging techniques in order to allow high-resolution imaging with comparable level of detail. Solving the structure of biological
macromolecules or small molecular at atomic level is even more challenging6. The main problem is that, as a consequence of Poisson statistics, the required illumination dose is inversely
proportional to the square of the spatial resolution7, and thus improving spatial resolution means quadratically higher doses. The increased dose may destroy the structure of the sample
before sufficient image signal-to-noise is reached. The widely adopted atomic-resolution imaging methods in scanning transmission electron microscopy (STEM), such as annular dark-field (ADF)
or coherent bright-field (cBF) imaging, are intrinsically dose inefficient as they use only a small fraction of the scattered electrons, being constructed via a simple integration of a
limited portion of phase space. Therefore, conventional STEM imaging methods usually cannot achieve sub-angstrom resolution or even atomic-resolution for electron radiation-sensitive
materials8. Meanwhile, high-precision measurement of local atomic positions is also fundamentally hindered by the poor signal-to-noise ratio of ADF images from electron-radiation sensitive
or weakly scattering samples, such as monolayer 2D materials. Picometer precision via ADF imaging can only be achieved in electron-radiation-robust and strongly scattering bulk
samples9,10,11. Electron ptychography, however, can potentially use the entire diffraction patterns either via a Wigner-distribution deconvolution (WDD)12,13 or iterative algorithms14,15 in
a way that can account for the probe damping effect and extract the electrostatic potential of the sample. Electron ptychography has been demonstrated as a promising phase-contrast imaging
technique with advantages such as high dose efficiency13,16, high resolution5,17,18, and high contrast5,13. In particular, ptychography has now surpassed the resolution of best physical
lenses, reaching deep sub-angstrom resolution5. Simulations have suggested the possibility of extremely low-dose imaging by electron ptychography, in principle beyond that of all other
electron imaging approaches to date including high-resolution TEM imaging widely used in Cryo-EM community—a potential outcome of considerable importance for the study of electron
radiation-sensitive materials including biological macromolecules. Imperfections of the STEM imaging system especially the partial coherence of the illumination probe reduces the image
resolution and contrast of conventional STEM imaging methods19. Partial coherence also limits the performance of ptychography, although in a more indirect manner, impacting the
signal-to-noise ratio of the reconstruction5,20. WDD-based electron ptychographic phase-contrast imaging has been demonstrated to outperform the commonly adopted phase-contrast conventional
high-resolution TEM imaging by retaining resolution beyond the temporal incoherent limit16,20. However, the partial coherence in the probe can be modeled more explicitly in iterative
ptychography algorithms by decomposing the probe wavefunction into a linear combination of pure states, i.e., a mixed quantum state21. As first demonstrated in coherent diffractive imaging22
and X-ray ptychography23, partial coherence of the probe can be well accounted for by introducing such state mixtures into the reconstruction method21,24,25. High coherent field-emission
guns are widely used as the electron sources in modern electron microscopes and so electron ptychography usually assumes only a pure coherent state of the illumination probe5,26,27. This
assumption is often sufficient when an in-focus-illumination probe is adopted5,27 or when a nanometer spatial resolution is targeted26. The effects of incoherence, which are inevitably
present in electron ptychography, on the reconstruction quality remains underexplored. There are only a few proof-of-principle demonstrations of electron ptychography considering the partial
coherence via approaches either Gaussian blind deconvolution18 or modal decomposition28,29, and while these suggest the promise of the approach, to date no sub-angstrom resolution
reconstructions have been achieved, even on instruments capable of sub-angstrom resolution in conventional imaging modes. Here, we demonstrate the capability of fast, sub-angstrom resolution
and picometer-precision imaging with a large field-of-view (FOV) by mixed-state electron ptychography. We find that a complete description of the partial coherence of the electron
wavefunction using a mixed quantum state is required to accomplish reconstructions with a sub-angstrom resolution, high contrast, high precision and a large FOV via defocused probe electron
ptychography. We also demonstrate low-dose atomic-resolution ptychographic imaging using dose levels up to 50 times lower than conventional STEM imaging techniques. RESULTS EXPERIMENTAL
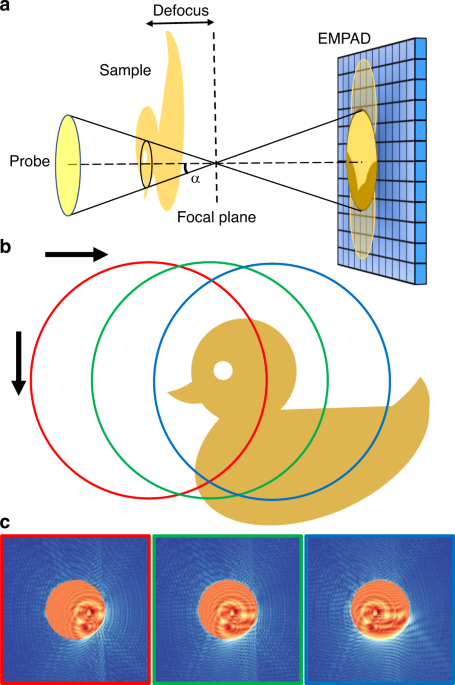
SETUP OF ELECTRON PTYCHOGRAPHY Figure 1a shows a schematic workflow of the defocused electron ptychography. Instead of focusing the electron probe on the sample, the focal plane is set to a
distance away from the sample. Because the illumination area is broadened, a larger scan step can be used given that there are sufficient overlaps between adjacent scan positions required by
ptychographic reconstruction algorithms. The sample was raster scanned along two perpendicular directions as indicated in Fig. 1b. A high-dynamic-range electron microscope pixel-array
detector (EMPAD)30 used for diffraction pattern acquisition enables capture of both the shadow image31 in the center disk formed by the large defocused probe and the much-weaker high
scattering-angle dark-field signal, as illustrated by three simulated diffraction patterns in Fig. 1c. Similar setups with a defocused probe have been adopted previously17,18,26, which has
shown benefits for overcoming the slow readout speed of conventional CCD cameras and limited stability of the imaging systems. However, usually only a few dozen diffraction patterns were
acquired, whereas fast pixel-array detectors like the EMPAD30 enables the acquisition of more than 50,000 diffraction patterns per minute. Conventional iterative ptychographic
algorithms14,15 assume a coherent illumination. The diffraction pattern is considered as the square of the amplitude of a single probe state multiplied by a sample transmission function
within the multiplicative approximation (an extension of the strong phase approximation, allowing for both phase and amplitude variations)12. However, due to experimentally unavoidable
partial coherence of the imaging system, single pure-state coherent probe illumination is never achieved in real experiments. To account for the partial coherence, the illumination is
represented by several mutually incoherent probe modes instead of using only a single coherent probe mode21. Each mode is then propagated independently to the detector as the measured
diffraction is an incoherent superposition of the contributions from all probe modes21,28,32. For practical implementations, we chose the modal decomposition approach21,28 and the probe is
expanded into several eigenmodes of the density matrix formed by a mixed state. The total intensity of all eigenmodes are normalized to the measured intensity of the diffraction patterns. A
flowchart of the algorithms showing the basic principle is given in Supplementary Fig. 1. Details of the reconstruction algorithms are summarized in the Methods section. MIXED-STATE
PTYCHOGRAPHIC IMAGING TO SUB-ANGSTROM RESOLUTION We performed a scanning diffraction experiment on a sample of a monolayer WS2 with bilayer islands of WS2/MoSe2. The MoSe2 and WS2 layers
have the same crystalline orientation, which is verified by the diffractogram of the ADF image, as shown in Supplementary Fig. 2. Owing to the 4% lattice mismatch between WS2 and MoSe2, the
bilayer regions have a complex projected atomic structure with a continuous transition from a full Mo-Se (or W–S) bond length to intermediate misaligned projected distances (a structural
model is given in Supplementary Fig. 2c). The resulting Moiré pattern serves as a good resolution test for our method. Figure 2a shows a mixed-state ptychographic reconstruction of a dataset
with an FOV of 30 × 30 nm2 corresponding to 1500 × 1500 pixels. The selected sample region contains various structural features, such as a monolayer of WS2 and both well-aligned and
misaligned stacking bilayer MoSe2/WS2 regions. An enlarged view of the bilayer region shown in Fig. 2b from the position marked on Fig. 2a shows the sliding structure of bilayer WS2/MoSe2.
The Moiré-like pattern changes continuously from hexagonal rings in well-aligned stacking regions to stripe features in the misaligned regions (see the structural model in Supplementary Fig.
2c). Unambiguous sub-Ångstrom resolution can be illustrated in both Fourier space and real space. First, the isotropic information limit shown in the diffractogram of the reconstruction in
Fig. 2d better than 1.4 Å−1 and the diffraction spots circled on Fig. 2d correspond to a real-space distance of 0.69 Å. Second, the line profiles in Fig. 2e from atomic pairs marked on Fig.
2b demonstrate real-space peak separations down to 0.6 Å. In addition, the Fourier ring correlation (FRC) analysis33, widely adopted for resolution estimation in cryo-electron microscopy and
X-ray ptychography, also demonstrates spatial resolution down to 0.66 Å. This is shown in Supplementary Fig. 3, which is very close to the 0.69 Å resolution determined from the
diffractogram in Fig. 2d. Figure 2c shows a conventional ADF image acquired with the same aperture size and a similar dose using an in-focused probe, limiting the resolution to worse than 1
Å. Compared with the ADF image in Fig. 2c, the ptychographic reconstruction in Fig. 2b demonstrates a significant and simultaneous improvement in contrast, signal-to-noise, and resolution.
What is more important is that the large FOV and sub-angstrom resolution ptychographic reconstruction are both achieved using a low-dose illumination. Typical doses used for
atomic-resolution (1.5–2 Å) STEM ADF images of monolayer transition-metal dichalcogenides at 80 keV are ~105 e Å−2 34,35. The dose for the dataset used in Fig. 2a is 1.6 × 104 e Å−2. The
ptychographic reconstruction has a 0.69 Å Abbe resolution, which doubles the resolution of ADF images achievable from the same imaging condition, ~1.37 Å (e.g., in Fig. 3). Furthermore,
sub-angstrom resolution was reached for a dataset with doses down to 4.0 × 103 e Å−2 (e.g., in Supplementary Fig. 4)—50 times lower than the dose for ADF images for the same resolution, with
lower dose lattice images discussed below. In addition, ptychography with a large defocused probe allows us to use a large scan step size of about 30% of the probe diameter15,36, because
the real-space pixel size of ptychographic reconstruction is determined by the maximum scattering angle of the diffraction pattern instead of scan step size in the conventional STEM ADF or
BF images. This decouples the scan step size from the real-space sampling required by Nyquist–Shannon sampling theorem37 for a certain resolution. This option enables a fast acquisition
despite the frame-rate of current 2D array detectors being usually two to three orders slower than the acquisition rate of point detectors30,38,39. The dataset used for the ptychographic
reconstruction shown in Supplementary Fig. 4a contains 64 × 64 diffraction patterns and the total acquisition time was 7.6 s. Assuming the same FOV and the typical acquisition conditions for
ADF imaging—a step size of 0.2 Å with 1500 × 1500 pixels, a beam current of 10–40 pA, which requires 64–16 μs per pixel dwell time to achieve 105 e Å−2 dose34,35—the total acquisition time
would be 144–36 s. Therefore, the high-quality ptychographic reconstruction with the same FOV is more than four times faster in acquisition while still using one order of magnitude lower
dose compared with conventional ADF imaging. Further tests of the practical experimental conditions show that much relaxed real-space overlap constraints, defined as (1 _−_ _r_/_D)_36, where
_r_ is the scan step size and _D_ is the diameter of probe, is required by mixed-state ptychography. As shown in Supplementary Fig. 5, mixed-state ptychography with only two probe modes
provides a stable high-quality reconstruction for a much larger scan step size up to 5.08 Å, corresponding to only 72% probe overlap, whereas artifact free reconstruction by single-state
ptychography can only be achieved with a very small step size, 0.85 Å, corresponding to a 95% probe overlap. For larger scan step sizes, shown in Supplementary Fig. 5b, c, the single-state
reconstructions fail to converge to the correct structure and generate artificial periodicities of the sample. At large step sizes, the errors in modeling of the probe partial coherence by
the single-state reconstruction accumulate, leading to a solution that is not able to describe the measured data well and therefore, in combination with the weaker constrains due to larger
scanning step, it leads to nonphysical reconstructions. Even in the case of a large probe overlap where the single-state reconstruction works, 95%, the mixed-state reconstruction has about
two times better resolution and enhanced contrast than that from the single-state (Supplementary Fig. 5). We also find that four eigenmodes are sufficient to capture the incoherence
properties of our probe, and additional modes do not lead to improvement of the reconstruction quality. A comparison of reconstructions with different numbers of modes is presented in
Supplementary Fig. 6. Therefore, under practical conditions of partially coherent illumination, mixed-state ptychography significantly relaxes the requirement of the real-space constraint,
i.e., scan step, and makes large FOV atomic-resolution imaging feasible. LOW-DOSE ATOMIC-RESOLUTION IMAGING Low-dose electron ptychography was first suggested via the Wigner-distribution
deconvolution (WDD) approach13, but only 104 e Å−2 or higher dose for atomic-resolution imaging has been realized experimentally by this approach16. In principle, the low-dose performance
should be comparable for WDD and iterative ptychography algorithms5, but the high sampling density required and currently slow detector readouts make working at low dose more challenging for
WDD40. For iterative electron ptychography, the dose-limit has been explored previously via simulations5,18,41,42 and some experimental attempts40. Simulations usually show one to two
orders lower dose than what has been realized by experiments5. This discrepancy may be caused by experimental limitations, such as sample stability, limited beam stability, and partial
coherence, which were neglected in simulations. Recent work using a highly coherent defocused probe and single-state electron ptychography has demonstrated a lattice resolution image from a
monolayer MoS2 using low-dose illumination40. However, only resolution worse than 1 Å was achieved and partial coherence of the illumination was not considered in the ptychographic
reconstruction. To explore the impact of partial coherence on the practical limit of the low-dose imaging, we show the dose dependence of ptychographic reconstructions using experimental
datasets and find benefits for both resolution and required dose after accounting for partial coherence. Figure 4 shows a series of reconstructions using datasets acquired from monolayer WS2
by varying the illumination dose via changing the beam current. For a dose higher than 3300 e Å−2 (Fig. 4a, b), both the W and S sublattice can be resolved unambiguously and the isotropic
information transfer is higher than 1.1 Å−1, corresponding to a real-space resolution better than 0.9 Å as shown in Fig. 4f, g. For doses of 1500 and 790 e Å−2 shown in Fig. 4c, d, the
reconstruction quality is slightly reduced but both sublattices are still resolvable and the information transfer is higher than 0.73 Å−1, corresponding to a real-space resolution of 1.37 Å.
Even for a dose of 375 e Å−2, shown in Fig. 4e, the lattice can still be recognized, and the information transfer is up to ~0.63 Å−1, corresponding to a real-space resolution of 1.59 Å. As
mentioned above, the dose commonly used for atomic-resolution (~1.5–2.0 Å) ADF images is about 105 e Å−2 34,35. Therefore, the minimum dose in the ptychographic reconstruction with
atomic-resolution demonstrated here is more than two orders smaller. We also compared the reconstructions from mixed-state and single-state ptychography in the low-dose conditions. As
demonstrated in Fig. 4a–e, k–o, both the resolution and contrast of the reconstructions from mixed-state ptychography are enhanced compared with single-state ptychography using the same
dose. The mixed-state ptychographic reconstruction has a better resolution at half the dose than that for single-state reconstruction; see for example, Figs. 4m and 4d. Similarly, the
mixed-state reconstructions in Fig. 4 show better quality and higher information limit compared with single-state reconstructions at similar doses reported recently40. A summary of the
dose-resolution dependence including the results in Fig. 4 and previous reported ptychographic results5,40,41 is given in Fig. 3. Some general trends and scaling behaviors can be noted, even
though the samples and experimental conditions for all the data are not identical, which may slightly affect the absolute resolution value at a certain dose. In the low dose-limit where
performance is limited by counting statistics, the resolution is well-aligned along a family of dose-limit lines, \(k/\sqrt N\). _N_ is the dose, and _k_ is a constant that depends on the
imaging method and the scattering power of the diagnosed samples43. Compared with conventional ADF images, for a certain information limit, the dose required for the mixed-state
ptychographic reconstructions is 10–50 times lower. At higher doses, the resolution is limited by the largest angle of information transfer. Ptychography is limited by the cut-off angle of
the diffraction patterns used in the reconstruction and can demonstrate a much better resolution limit than ADF imaging, which is limited by the probe-forming aperture. The negligible
readout noise (1/40 e−) and single-electron sensitivity of detector used30 is critical for approaching the limits of the low-dose ptychographic reconstruction. As shown in Supplementary Fig.
7a–d, a single diffraction pattern with dose smaller than 3300 e Å−2 contains only a few electrons per pixel in the center disk and a very large fraction of pixels with fewer than single
electron in dark-field region. Despite the very low dose, which provides only ~500 electrons per scan position for a dose of 790 e Å−2, the averaged diffraction patterns shown in
Supplementary Fig. 7e–h still retain sharp edges of the diffraction disks. This demonstrates that the interference between different diffraction paths has been well encoded in these
sparse-signal diffraction patterns. The lattice fringes in Fig. 4d reveal that the sample phase information contained in the low-dose diffraction patterns have been retrieved via electron
ptychography. HIGH-PRECISION ATOMIC POSITION MEASUREMENTS One of the advantages of ptychography is the ability to correct for positioning errors44,45,46 by maximization of mutual consistency
between the adjacent regions. These methods can serve as an independent estimation of the position measurements, which can in principle correct for sample drifts and improve the precision
of atomic position measurement. We have selected an area of monolayer WS2 (4.0 × 5.2 nm2) shown in Fig. 5a. The contrast from light sulfur elements in the ptychographic reconstruction is
very strong, because the contrast of the phase image is roughly linearly proportional to _Z_, the atomic number47, whereas the contrast in ADF is close to a _Z_2 dependence8,48. Therefore,
atomic positions of sulfur atoms can be readily obtained with high precision even in the vicinity of heavy W atoms. In conventional ADF images, the sulfur atoms are shadowed by the strong
scattering from W atoms, which hinders the estimation of the sulfur positions. The recorded EMPAD dataset shows sample drift during the data acquisition. However, the relative probe position
errors can be very well estimated via the gradient-based probe position refinement algorithm49. At first, the global geometry errors, such as scan step size scaling, axis rotation or
skewness, are fitted and corrected via an affine transformation between the nominal probe positions and estimated probe positions. Owing to the raster position scanning, a constant velocity
drift of the sample will result in a skewness of up to a few degrees for long acquisition datasets. When the geometry model is refined, the residual position errors are estimated. Figure 5b,
c shows the residual position errors along the horizontal (fast scan) and vertical (slow scan) direction, respectively. The standard deviation (st.d.) of the residual errors along both
directions shown in Fig. 5b, c is ~0.16 Å with a maximum error of ~0.6 Å. The atomic column positions were estimated using a 2D Gaussian function fit of the reconstructed phase. The
positions were used to calculate the nearest neighbor W–W and S–S atomic distances. The distributions of the S–S and W–W distances are plotted in Fig. 5d, e. The st.d. of the S–S distance is
5.8 pm and the st.d. of W–W distance is 5.2 pm, which is a precision measurement of the atomic positions as the monolayer WS2 region shown in Fig. 5a has negligible structural distortions.
The intensity variations in Fig. 5a are from residual polymer residue, and likely degrade the precision of the bond-length measurements. Therefore, the reported precision of the ptychography
method should be viewed as an upper bound. For transition-metal dichalcogenides imaged by 80 keV electrons, the dose must be kept below ~106 e Å−2 to avoid significant structural damage50.
An even lower dose beneath 104 e Å−2 is required to avoid the formation of sulfur point vacancies or structural alterations around deficient regions51. Therefore, it is important to see the
performance of ptychography for estimating atomic positions at low dose. Figure 5f shows the dose-dependent precision estimation of W–W and S–S distances. Precisions of about 10 pm for both
W–W and S–S distances can still be achieved using doses as low as 104 e Å−2. However, from a conventional ADF image, the precision of W–W atomic distance with a single fast scan (6 μs per
pixel chosen to match dose and pixel sampling) is only 13.6 pm as shown in Supplementary Fig. 8, which is about twice as large as that from ptychography with a similar dose. Using a more
stable imaging system, multiple scans and drift correction algorithms, precision for both ADF imaging and ptychography can be further improved10,11. As detector speeds increase, the multiple
scan strategy becomes more practical for ptychography. Denoising and deconvolution algorithms can also help with peak location41,52. However, sulfur atoms in the ADF image are not visible
due to their much-weaker scattering, shown in Supplementary Fig. 8a. Although sulfur atoms can be seen by using a lower collection angle for ADF35, the precision of S atomic positions
determined from low-dose ADF image is much worse than that from W. Therefore, the low-dose and high contrast capabilities of ptychography provide a picometer-precision technique for atomic
position determination of single atoms including light sulfur atoms in 2D materials. DISCUSSION We have demonstrated that mixed-state electron ptychography provides simultaneously improved
imaging capabilities, including high resolution, large FOV, low dose, high contrast/signal-to-noise ratio, and high precision. A mixed-state model is beneficial for ptychographic
reconstructions using the data from current electron microscopes, as it is able to account for many of the different factors that result in decoherence-like effects in measured diffraction
patterns20,21,42,53, such as the finite electron source size, chromatic aberration, sample vibration, and fast instabilities of the image-forming system. With further improved coherence of
the electron source, such as a cold field-emission gun, a larger scan step sizes with a reduced overlap ratio could be achieved54. Loss of speckle contrast in diffraction, which has similar
effects as partial spatial coherence of the probe, can be introduced due to a finite detector pixel size and limit the largest applicable probe size20,55. Therefore, with further improvement
of the source coherence and the area of the detectors, increased scan step sizes can be utilized, which further enlarges the FOV given the same number of scanning positions. Furthermore,
requirements of the illumination stability in ptychography can be relaxed by use of multiple scans with shared object information56 or other probe relaxation extension49,57. These approaches
can largely overcome the long-term stability limitations of the current scanning systems, which makes even micron length scale with sub-angstrom resolution imaging feasible. Ptychography
requires a forward model for the interaction of the beam with the sample. One limitation of the current mixed-state ptychographic imaging is that it can only be applied in relatively thin
samples because it uses a generalized strong phase approximation that neglects the effects of beam propagation. The generalized phase grating approximation for the interaction of the
incident probe with a projected object function can be written as, \(\psi _{{\mathrm{exit}}}({\mathbf{r}}_i,{\mathbf{r}}) = \psi _{{\mathrm{in}}}\left( {{\mathbf{r}} - {\mathbf{r}}_i}
\right)O\left( {\mathbf{r}} \right)\), where _ψ_exit (R_i_, R) is the electron wavefunction passing through the sample, _ψ_in (R − R_i_) is the incident electron wavefunction centered at
position \({\mathbf{r}}_i\), with \({\mathbf{r}}_i\) and \({\mathbf{r}}\) being 2D coordinates. The complex object function, _O_ (R) is a generalized strong phase object, \(O({\mathbf{r}}) =
A\left( {\mathbf{r}} \right){\mathrm{exp}}\left( {i\sigma V\left( {\mathbf{r}} \right)} \right)\), where _A_ (R) is the amplitude, _σ_ is the interaction constant depending on the electron
energy and _V_ (R) is the projected electrostatic potential of the sample. The amplitude term is included to allow for a weak absorption effect, e.g., scattering outside the detector and
should be close to unity if the sample is thin58. Failures of the model for practical samples might be suspected if the amplitude either deviates by more than 10% from unity or resembles the
phase term such that phase-amplitude mixing has likely occurred59. If the probe shape changes significantly during propagation within the sample, then the probe–sample interaction cannot be
well described in a single plane and a full multi-slice calculation may need to be considered. Both probe free-space propagation and scattering by the sample can change the probe shape. The
thickness limit _T_ due to the propagation effect can be expressed as60, \(T = 1.3\lambda /\theta _{{\mathrm{max}}}^2\), where _θ_max is the maximum scattering angle of the diffraction
pattern and \(\lambda\) is the wavelength of electrons. For a typical scattering angle targeting a resolution better than 0.5 Å, \(\theta _{{\mathrm{max}}} = 20\) mrad at 300 keV, the
thickness limit is ~6.4 nm, which is within the achievable thickness for many samples. For heavy elements, a single atom can induce a large phase shift and a strong amplitude damping to the
electron wavefunction, and the probe shape can be changed significantly by only a few atoms. Therefore, a much more rigorous thickness limit must be adapted for samples containing high
atomic number elements61. Recent attempts to solve the multiple scattering problem in thick samples include multi-slice ptychography60,62,63 and scattering matrix phase retrieval64. Although
the robustness and convergence must be further improved to achieve practical applications in thick samples63,65, mixed-state ptychography could be extended to include multiple scattering49.
Data processing speed is another limiting factor for applications of ptychography. However, with graphics processing unit (GPU) acceleration, the reconstruction of the large FOV image shown
in Fig. 2a only takes less than one hour on a typical GPU card. The processing time largely scales linearly with number of diffraction patterns, therefore, fewer patterns with the defocused
probe setup can significantly accelerate the reconstruction. The flexibility and robustness of mixed-state electron ptychography enable many potential applications. Large FOV high
resolution imaging is critical for uncovering both the overall morphology and the local atomic arrangement in complex nanostructures66. High contrast and high precision coupled with low-dose
imaging can be used to measure the atomic scale dynamics of light elements, such as Li, O, or S in lithium battery materials. Fast single-pass acquisition with scan drift correction opens
the door to in situ phase-contrast STEM imaging of dynamical processes during heating, cooling, or even chemical reaction. Use of a large-illuminated-area probe enables a larger scan step
size and fewer diffraction patterns for a given FOV, which reduces the computational effort during data reconstruction and analysis and accelerates data processing of ptychographic
reconstruction. Rapid data processing is critical for live imaging and 3D structural reconstruction, such as ptychographic tomography67,68. Our demonstration of low-dose imaging at
atomic-resolution is within the allowable dose for many beam-sensitive materials69. Further dose reduction could be potentially realized by improvements of the reconstruction algorithm and
experimental setup such as the averaging of multiple images from structurally identical particles that is commonly used in single-particle Cryo-EM. The high dose efficiency of mixed-state
ptychography may also be helpful for reconstructing biological molecules using cryo-electron microscopy, potentially reducing the number of particles needed in an averaging class to achieve
a desired resolution41. With the anticipated next generation pixel-array detectors that will be even larger and faster, the probe size can be further increased and thus a larger scan step
size can be chosen, which will further enlarge the FOV and increase the acquisition speed. Furthermore, mixed-state electron ptychography, besides the retrieval of the probe mixture, can
also be adapted to retrieve mixed quantum states within a sample21,32, which could expand the reach of quantum-state tomography by using a matter wave. METHODS EXPERIMENTAL METHOD The
scanning diffraction experiments were carried out using an electron microscope pixel-array detector (EMPAD)30 installed on a probe aberration corrected Thermo Scientific™ Titan Themis
electron microscope. The EMPAD has 128 × 128 pixels, a readout speed of 0.86 ms per frame, and 1,000,000:1 electron linear response. All the datasets were acquired using a probe at 80 keV
beam energy, 21.4 mrad probe-forming semi-angle, and ~55 nm defocus value. The exposure time was 1 ms per frame. The beam current varied from 0.09 to 14.3 pA via defocusing a monochromator.
The coherence of the electron probe increases slightly when the beam current reduces but the change is not significant (<3%) as the beam current used (0.1–14 pA) is always much lower than
the coherent current of the source (~50 pA). The large 30 × 30 nm2 FOV image in Fig. 2 is reconstructed from a dataset with a scan step size of 2.36 Å containing 128 × 128 diffraction
patterns, which were selected in an interval of two from a larger dataset with 256 × 256 diffraction patterns. A reconstruction of a four-times down-sampled dataset with a 4.72 Å scan step
size is shown in Supplementary Fig. 4. The imaged sample contains MoSe2 islands on a large area of monolayer WS2. The relative orientation of MoSe2 and WS2 is identical, as we have verified
using the Fourier transform of the ADF image shown in Supplementary Fig. 2. MIXED-STATE PTYCHOGRAPHY We adapted the generalized maximum-likelihood ptychography method49,70 initially
developed for X-ray ptychography. This method solves the phase retrieval problem by preconditioned gradient descent-based optimization. Optimization of amplitude likelihood function49,70
provides more robust convergence than direction optimization of Poisson likelihood. Multiple optimization directions, such as probe and object updates, probe position displacements, or
wavefront variation can be carried out jointly in a consistent way. In combination with a neural-networks-inspired mini-batch optimization scheme49, our approach enables a compromise between
convergence speed and noise robustness, and thus it significantly improves the usability of ptychography for low-dose imaging. The mixed-state description is implemented using the modal
decomposition approach21. Minor time variations of the illumination probe due to the instability of the electron optics or small sample height variation are accounted for by using an
illumination wavefront correction method49, which is a computationally faster and more memory-efficient approximation of the orthogonal probe relaxation (OPR) approach57. The OPR method
describes small variations of the illumination probe by a linear decomposition into a set of several orthogonal mutually coherent modes. The workflow of the algorithm is schematically shown
in Supplementary Fig. 1 and more details are described as a ptychography toolkit in ref. 71. We have observed that the reconstruction quality of the mixed-state ptychography algorithm is not
sensitive to the initial guess of the illumination probe and the number of the mixed modes for datasets with a sufficient probe overlap, although a good initial guess may accelerate the
convergence. On the other hand, the single probe mode ptychography requires a good initial probe guess to provide stable convergence for our experimental datasets. This seems
counterintuitive but it is not surprising. Because mixed-state ptychography can account for the nonnegligible partial coherence of the probe and provide a more accurate reciprocal model,
whereas single-mode ptychography is not sufficient for modeling the probe incoherence and its convergence can become instable if the initial probe deviates from the real probe significantly.
FOURIER RING CORRELATION For Fourier ring correlation (FRC)33,72, we used two phase images reconstructed from two separate datasets from the same scan region, which serves as two
independent measurements. Practically, two datasets were selected from one single dataset in every two diffractions at each dimension but with different starting points. After ptychographic
reconstruction, a global linear phase term due to the inherent ambiguities of ptychography73 is removed by fitting as a 2D linear function. Two phase images are aligned using the sub-pixel
precision cross-correlation algorithm74. Before FRC analysis, the edges of the phase images were Apodized to avoid the artifacts introduced from the boundary discontinuities33. The
resolution is estimated by using the 1-bit threshold33. DATA AVAILABILITY A small (200 Mb) data subset is available from PARADIM, a National Science Foundation Materials Innovation Platform
[https://doi.org/10.34863/G4WA-0J57]75. Full datasets are available from the corresponding author ([email protected]) on request. CODE AVAILABILITY The codes developed at Cornell
University is published on GitHub, muller-group-cornell [https://github.com/muller-group-cornell]. The ptychography reconstruction toolkit, PtychoShelves developed at Paul Scherrer Institut,
Switzerland, is available on the website [https://www.psi.ch/en/sls/csaxs]. REFERENCES * Huang, P. Y. et al. Grains and grain boundaries in single-layer graphene atomic patchwork quilts.
_Nature_ 469, 389–392 (2011). Article ADS CAS PubMed Google Scholar * Fujita, T. et al. Atomic origins of the high catalytic activity of nanoporous gold. _Nat. Mater._ 11, 775–780
(2012). Article ADS CAS PubMed Google Scholar * Furukawa, H., Cordova, K. E., O'Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal-organic frameworks. _Science_
341, 1230444 (2013). Article PubMed CAS Google Scholar * Green, M. A., Ho-Baillie, A. & Snaith, H. J. The emergence of perovskite solar cells. _Nat. Photonics_ 8, 506–514 (2014).
Article ADS CAS Google Scholar * Jiang, Y. et al. Electron ptychography of 2D materials to deep sub-ångström resolution. _Nature_ 559, 343–349 (2018). Article ADS CAS PubMed Google
Scholar * Grigorieff, N. & Harrison, S. C. Near-atomic resolution reconstructions of icosahedral viruses from electron cryo-microscopy. _Curr. Opin. Struct. Biol._ 21, 265–273 (2011).
Article CAS PubMed PubMed Central Google Scholar * Saxberg, B. E. H. & Saxton, W. O. Quantum noise in 2D projections and 3D reconstructions. _Ultramicroscopy_ 6, 85–89 (1981).
Article Google Scholar * Hovden, R. & Muller, D. A. Efficient elastic imaging of single atoms on ultrathin supports in a scanning transmission electron microscope. _Ultramicroscopy_
123, 59–65 (2012). Article CAS PubMed Google Scholar * Kimoto, K. et al. Local crystal structure analysis with several picometer precision using scanning transmission electron
microscopy. _Ultramicroscopy_ 110, 778–782 (2010). Article CAS PubMed Google Scholar * Sang, X. & LeBeau, J. M. Revolving scanning transmission electron microscopy: Correcting sample
drift distortion without prior knowledge. _Ultramicroscopy_ 138, 28–35 (2014). Article CAS PubMed Google Scholar * Yankovich, A. B. et al. Picometre-precision analysis of scanning
transmission electron microscopy images of platinum nanocatalysts. _Nat. Commun._ 5, 4155 (2014). Article ADS CAS PubMed Google Scholar * Rodenburg, J. M. & Bates, R. H. T. The
theory of super-resolution electron microscopy via Wigner-distribution deconvolution. _Philos. Trans. R. Soc. A_ 339, 521–553 (1992). ADS Google Scholar * Yang, H. et al. Simultaneous
atomic-resolution electron ptychography and Z-contrast imaging of light and heavy elements in complex nanostructures. _Nat. Commun._ 7, 12532 (2016). Article ADS CAS PubMed PubMed
Central Google Scholar * Thibault, P. et al. High-resolution scanning X-ray diffraction microscopy. _Science_ 321, 379–382 (2008). Article ADS CAS PubMed Google Scholar * Maiden, A.
M. & Rodenburg, J. M. An improved ptychographical phase retrieval algorithm for diffractive imaging. _Ultramicroscopy_ 109, 1256–1262 (2009). Article CAS PubMed Google Scholar *
Pennycook, T. J., Martinez, G. T., Nellist, P. D. & Meyer, J. C. High dose efficiency atomic resolution imaging via electron ptychography. _Ultramicroscopy_ 196, 131–135 (2019). Article
CAS PubMed Google Scholar * Humphry, M. J., Kraus, B., Hurst, A. C., Maiden, A. M. & Rodenburg, J. M. Ptychographic electron microscopy using high-angle dark-field scattering for
sub-nanometre resolution imaging. _Nat. Commun._ 3, 730 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Putkunz, C. T. et al. Atom-scale ptychographic electron
diffractive imaging of boron nitride cones. _Phys. Rev. Lett._ 108, 073901 (2012). Article ADS PubMed CAS Google Scholar * Dwyer, C., Erni, R. & Etheridge, J. Measurement of
effective source distribution and its importance for quantitative interpretation of STEM images. _Ultramicroscopy_ 110, 952–957 (2010). Article CAS Google Scholar * Nellist, P. D. &
Rodenburg, J. M. Beyond the conventional information limit: the relevant coherence function. _Ultramicroscopy_ 54, 61–74 (1994). Article Google Scholar * Thibault, P. & Menzel, A.
Reconstructing state mixtures from diffraction measurements. _Nature_ 494, 68–71 (2013). Article ADS CAS PubMed Google Scholar * Miao, J., Ishikawa, T., Robinson, I. K. & Murnane,
M. M. Beyond crystallography: Diffractive imaging using coherent X-ray light sources. _Science_ 348, 530–535 (2015). Article ADS MathSciNet CAS PubMed MATH Google Scholar * Pfeiffer,
F. X-ray ptychography. _Nat. Photonics_ 12, 9–17 (2017). Article ADS CAS Google Scholar * Whitehead, L. W. et al. Diffractive imaging using partially coherent x rays. _Phys. Rev. Lett._
103, 243902 (2009). Article ADS CAS PubMed Google Scholar * Enders, B. et al. Ptychography with broad-bandwidth radiation. _Appl. Phys. Lett._ 104, 171104 (2014). Article ADS CAS
Google Scholar * Hue, F., Rodenburg, J. M., Maiden, A. M., Sweeney, F. & Midgley, P. A. Wave-front phase retrieval in transmission electron microscopy via ptychography. _Phys. Rev. B_
82, 121415 (2010). Article ADS CAS Google Scholar * Song, B. et al. Hollow electron ptychographic diffractive imaging. _Phys. Rev. Lett._ 121, 146101 (2018). Article ADS CAS PubMed
Google Scholar * Cao, S., Kok, P., Li, P., Maiden, A. M. & Rodenburg, J. M. Modal decomposition of a propagating matter wave via electron ptychography. _Phys. Rev. A_ 94, 063621 (2016).
Article ADS Google Scholar * Cao, S., Maiden, A. M. & Rodenburg, J. M. Image feature delocalization in defocused probe electron ptychography. _Ultramicroscopy_ 187, 71–83 (2018).
Article CAS PubMed Google Scholar * Tate, M. W. et al. High dynamic range pixel array detector for scanning transmission electron microscopy. _Microsc. Microanal._ 22, 237–249 (2016).
Article ADS CAS PubMed Google Scholar * Dowell, W. C. T. Selected-area diffraction in the shadow electron microscope. _Z. Naturforsch._ 31A, 1435 (1976). Article ADS CAS Google
Scholar * Batey, D. J., Claus, D. & Rodenburg, J. M. Information multiplexing in ptychography. _Ultramicroscopy_ 138, 13–21 (2014). Article CAS PubMed Google Scholar * van Heel, M.
& Schatz, M. Fourier shell correlation threshold criteria. _J. Struct. Biol._ 151, 250–262 (2005). Article PubMed CAS Google Scholar * Lin, Y.-C., Dumcenco, D. O., Huang, Y.-S. &
Suenaga, K. Atomic mechanism of the semiconducting-to-metallic phase transition in single-layered MoS2. _Nat. Nano_ 9, 391–396 (2014). Article CAS Google Scholar * Azizi, A. et al.
Dislocation motion and grain boundary migration in two-dimensional tungsten disulphide. _Nat. Commun._ 5, 4867 (2014). Article ADS CAS PubMed Google Scholar * Bunk, O. et al. Influence
of the overlap parameter on the convergence of the ptychographical iterative engine. _Ultramicroscopy_ 108, 481–487 (2008). Article CAS PubMed Google Scholar * Shannon, C. E. A
mathematical theory of communication. _Bell Syst. Tech. J._ 27, 379–423 (1948). Article MathSciNet MATH Google Scholar * Ophus, C., Ercius, P., Sarahan, M., Czarnik, C. & Ciston, J.
Recording and using 4D-STEM datasets in materials science. _Microsc. Microanal._ 20, 62–63 (2014). Article ADS Google Scholar * Mir, J. A. et al. Characterisation of the Medipix3 detector
for 60 and 80 keV electrons. _Ultramicroscopy_ 182, 44–53 (2017). Article CAS PubMed Google Scholar * Song, J. et al. Atomic resolution defocused electron ptychography at low dose with
a fast, direct electron detector. _Sci. Rep._ 9, 3919 (2019). Article ADS PubMed PubMed Central CAS Google Scholar * Pelz, P. M., Qiu, W. X., Bucker, R., Kassier, G. & Miller, R.
J. D. Low-dose cryo electron ptychography via non-convex Bayesian optimization. _Sci. Rep._ 7, 9883 (2017). Article ADS PubMed PubMed Central Google Scholar * Uhlemann, S., Müller, H.,
Hartel, P., Zach, J. & Haider, M. Thermal magnetic field noise limits resolution in transmission electron microscopy. _Phys. Rev. Lett._ 111, 046101 (2013). Article ADS PubMed CAS
Google Scholar * Henderson, R. The potential and limitations of neutrons, electrons and X-rays for atomic resolution microscopy of unstained biological molecules. _Q. Rev. Biophys._ 28,
171–193 (1995). Article CAS PubMed Google Scholar * Maiden, A. M., Humphry, M. J., Sarahan, M. C., Kraus, B. & Rodenburg, J. M. An annealing algorithm to correct positioning errors
in ptychography. _Ultramicroscopy_ 120, 64–72 (2012). Article CAS PubMed Google Scholar * Beckers, M. et al. Drift correction in ptychographic diffractive imaging. _Ultramicroscopy_ 126,
44–47 (2013). Article CAS PubMed Google Scholar * Zhang, F. et al. Translation position determination in ptychographic coherent diffraction imaging. _Opt. Express_ 21, 13592–13606
(2013). Article ADS PubMed Google Scholar * Cao, M. C. et al. Theory and practice of electron diffraction from single atoms and extended objects using an EMPAD. _Microscopy_ 67,
i150–i161 (2018). Article CAS PubMed Google Scholar * Treacy, M. M. J. Z dependence of electron scattering by single atoms into annular dark-field detectors. _Microsc. Microanal._ 17,
847–858 (2011). Article ADS CAS PubMed Google Scholar * Odstrcil, M., Menzel, A. & Guizar-Sicairos, M. Iterative least-squares solver for generalized maximum-likelihood
ptychography. _Opt. Express_ 26, 3108–3123 (2018). Article ADS PubMed Google Scholar * Liu, X. et al. Top-down fabrication of sub-nanometre semiconducting nanoribbons derived from
molybdenum disulfide sheets. _Nat. Commun._ 4, 1776 (2013). Article ADS PubMed PubMed Central CAS Google Scholar * Komsa, H.-P., Kurasch, S., Lehtinen, O., Kaiser, U. &
Krasheninnikov, A. V. From point to extended defects in two-dimensional MoS2: evolution of atomic structure under electron irradiation. _Phys. Rev. B_ 88, 035301 (2013). Article ADS CAS
Google Scholar * Tian, X. et al. Correlating the three-dimensional atomic defects and electronic properties of two-dimensional transition metal dichalcogenides. _Nat. Mater_.
https://doi.org/10.1038/s41563-020-0636-5 (2020). * Clark, J. N., Huang, X., Harder, R. J. & Robinson, I. K. Dynamic imaging using ptychography. _Phys. Rev. Lett._ 112, 113901 (2014).
Article ADS PubMed CAS Google Scholar * Stachnik, K. et al. Influence of finite spatial coherence on ptychographic reconstruction. _Appl. Phys. Lett._ 107, 011105 (2015). Article ADS
CAS Google Scholar * Edo, T. B. et al. Sampling in x-ray ptychography. _Phys. Rev. A_ 87, 053850 (2013). Article ADS CAS Google Scholar * Guizar-Sicairos, M. et al. High-throughput
ptychography using Eiger: scanning X-ray nano-imaging of extended regions. _Opt. Express_ 22, 14859–14870 (2014). Article ADS PubMed Google Scholar * Odstrcil, M. et al. Ptychographic
coherent diffractive imaging with orthogonal probe relaxation. _Opt. Express_ 24, 8360–8369 (2016). Article ADS CAS PubMed Google Scholar * Humphreys, C. J. The scattering of fast
electrons by crystals. _Rep. Prog. Phys._ 42, 1825 (1979). Article ADS CAS Google Scholar * Liu, C., Walther, T. & Rodenburg, J. M. Influence of thick crystal effects on
ptychographic image reconstruction with moveable illumination. _Ultramicroscopy_ 109, 1263–1275 (2009). Article CAS PubMed Google Scholar * Tsai, E. H., Usov, I., Diaz, A., Menzel, A.
& Guizar-Sicairos, M. X-ray ptychography with extended depth of field. _Opt. Express_ 24, 29089–29108 (2016). Article ADS PubMed Google Scholar * Close, R., Chen, Z., Shibata, N.
& Findlay, S. D. Towards quantitative, atomic-resolution reconstruction of the electrostatic potential via differential phase contrast using electrons. _Ultramicroscopy_ 159, 124–137
(2015). Article CAS PubMed Google Scholar * Maiden, A. M., Humphry, M. J. & Rodenburg, J. M. Ptychographic transmission microscopy in three dimensions using a multi-slice approach.
_J. Opt. Soc. Am. A_ 29, 1606–1614 (2012). Article ADS CAS Google Scholar * Jiang, Y. et al. Breaking the Rayleigh limit in thick samples with multi-slice ptychography. _Microsc.
Microanal._ 24, 192–193 (2018). Article Google Scholar * Brown, H. G. et al. Structure retrieval at atomic resolution in the presence of multiple scattering of the electron probe. _Phys.
Rev. Lett._ 121, 266102 (2018). Article ADS CAS PubMed Google Scholar * Jiang, Y. Investigation of Advanced Image Reconstruction Algorithms for Electron Microscopy. Ph.D. thesis,
Cornell University (2018). * Ly, T. H. et al. Hyperdislocations in van der Waals layered materials. _Nano Lett._ 16, 7807–7813 (2016). Article ADS CAS PubMed Google Scholar * Dierolf,
M. et al. Ptychographic X-ray computed tomography at the nanoscale. _Nature_ 467, 436–439 (2010). Article ADS CAS PubMed Google Scholar * Li, P. & Maiden, A. Multi-slice
ptychographic tomography. _Sci. Rep._ 8, 2049 (2018). Article ADS PubMed PubMed Central CAS Google Scholar * Karuppasamy, M., Karimi Nejadasl, F., Vulovic, M., Koster, A. J. &
Ravelli, R. B. Radiation damage in single-particle cryo-electron microscopy: effects of dose and dose rate. _J. Synchrotron Radiat._ 18, 398–412 (2011). Article CAS PubMed PubMed Central
Google Scholar * Thibault, P. & Guizar-Sicairos, M. Maximum-likelihood refinement for coherent diffractive imaging. _N. J. Phys._ 14, 063004 (2012). Article Google Scholar *
Wakonig, K. et al. PtychoShelves, a versatile high-level framework for high-performance analysis of ptychographic data. _J. Appl. Crystallogr._ 53, 574–586 (2020). Article CAS PubMed
PubMed Central Google Scholar * Vila-Comamala, J. et al. Characterization of high-resolution diffractive X-ray optics by ptychographic coherent diffractive imaging. _Opt. Express_ 19,
21333–21344 (2011). Article ADS PubMed Google Scholar * Guizar-Sicairos, M. et al. Phase tomography from x-ray coherent diffractive imaging projections. _Opt. Express_ 19, 21345–21357
(2011). Article ADS PubMed Google Scholar * Guizar-Sicairos, M., Thurman, S. T. & Fienup, J. R. Efficient subpixel image registration algorithms. _Opt. Lett._ 33, 156–158 (2008).
Article ADS PubMed Google Scholar * Chen, Z. et al. Data set: Mixed-state electron ptychography enables sub-angstrom resolution imaging with picometer precision at low dose. _PARADIM, an
NSF Materials Innovation Platform_, https://doi.org/10.34863/G4WA-0J57 (2020). Download references ACKNOWLEDGEMENTS Z.C. and D.A.M. are supported by the PARADIM Materials Innovation
Platform program in-house program by NSF Grant DMR-1539918. Y.H. is supported by the NSF MRSEC program (DMR-1429155). This work made use of the Cornell Center for Materials Research facility
supported by NSF grant DMR-1719875. We thank Tsai Esther Hsiao Rho and Manuel Guizar-Sicairos for useful discussions. AUTHOR INFORMATION Author notes * Michal Odstrcil Present address: Carl
Zeiss SMT, Carl-Zeiss-Straße 22, 73447, Oberkochen, Germany * Yimo Han Present address: Department of Molecular Biology, Princeton University, Princeton, NJ, 08544, USA * Lain-Jong Li
Present address: Department of Electronic Engineering and Green Technology Research Center, Chang-Gung University, Taoyuan 333, Taiwan AUTHORS AND AFFILIATIONS * School of Applied and
Engineering Physics, Cornell University, Ithaca, NY, 14853, USA Zhen Chen, Yimo Han & David A. Muller * Paul Scherrer Institut, 5232, Villigen PSI, Switzerland Michal Odstrcil * Advanced
Photon Source, Argonne National Laboratory, Lemont, IL, 60439, USA Yi Jiang * Physical Science and Engineering, King Abdullah University of Science and Technology, Thuwal, 23955-6900, Saudi
Arabia Ming-Hui Chiu & Lain-Jong Li * Kavli Institute at Cornell for Nanoscale Science, Ithaca, NY, 14853, USA David A. Muller Authors * Zhen Chen View author publications You can also
search for this author inPubMed Google Scholar * Michal Odstrcil View author publications You can also search for this author inPubMed Google Scholar * Yi Jiang View author publications You
can also search for this author inPubMed Google Scholar * Yimo Han View author publications You can also search for this author inPubMed Google Scholar * Ming-Hui Chiu View author
publications You can also search for this author inPubMed Google Scholar * Lain-Jong Li View author publications You can also search for this author inPubMed Google Scholar * David A. Muller
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Experiments and data analysis were performed by Z.C. under the supervision of D.A.M. The
main ptychographic algorithms were implemented by M.O. for X-ray ptychography with the adaption to electron ptychography by Z.C. and Y.J. Sample preparation was done by Y.H. from thin films
synthesized by M.C. and L.J. Z.C. wrote the manuscript with revisions from M.O. and D.A.M. All authors discussed the results and implications throughout the investigation, and all authors
have given approval to the final version of the manuscript. CORRESPONDING AUTHOR Correspondence to David A. Muller. ETHICS DECLARATIONS COMPETING INTERESTS Cornell University has licensed
the EMPAD hardware to Thermo Scientific. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks Christian Dwyer, Jianwei Miao and the other, anonymous, reviewer(s) for
their contribution to the peer review of this work. Peer review reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published
maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chen, Z., Odstrcil, M., Jiang, Y. _et al._ Mixed-state electron ptychography
enables sub-angstrom resolution imaging with picometer precision at low dose. _Nat Commun_ 11, 2994 (2020). https://doi.org/10.1038/s41467-020-16688-6 Download citation * Received: 14
September 2019 * Accepted: 13 May 2020 * Published: 12 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16688-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
