The lipid code-dependent phosphoswitch pdk1–d6pk activates pin-mediated auxin efflux in arabidopsis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Directional intercellular transport of the phytohormone auxin mediated by PIN-FORMED (PIN) efflux carriers has essential roles in both coordinating patterning processes and
integrating multiple external cues by rapidly redirecting auxin fluxes. PIN activity is therefore regulated by multiple internal and external cues, for which the underlying molecular
mechanisms are not fully elucidated. Here, we demonstrate that 3′-PHOSPHOINOSITIDE-DEPENDENT PROTEIN KINASE1 (PDK1), which is conserved in plants and mammals, functions as a molecular hub
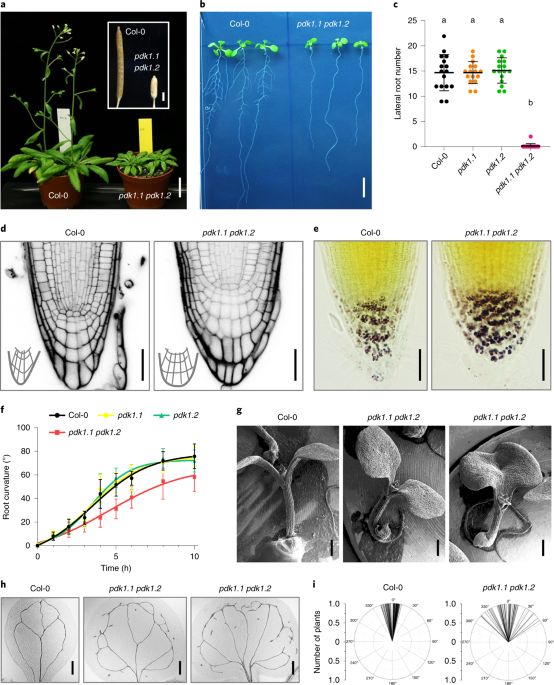
that perceives upstream lipid signalling and modulates downstream substrate activity through phosphorylation. Using genetic analysis, we show that the loss-of-function _Arabidopsis pdk1.1_
_pdk1.2_ mutant exhibits a plethora of abnormalities in organogenesis and growth due to defective polar auxin transport. Further cellular and biochemical analyses reveal that PDK1
phosphorylates D6 protein kinase, a well-known upstream activator of PIN proteins. We uncover a lipid-dependent phosphorylation cascade that connects membrane-composition-based cellular
signalling with plant growth and patterning by regulating morphogenetic auxin fluxes. Access through your institution Buy or subscribe This is a preview of subscription content, access via
your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days
cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS A PHOSPHOINOSITIDE HUB CONNECTS CLE PEPTIDE SIGNALING AND POLAR AUXIN EFFLUX
REGULATION Article Open access 26 January 2023 WAVY GROWTH ARABIDOPSIS E3 UBIQUITIN LIGASES AFFECT APICAL PIN SORTING DECISIONS Article Open access 01 September 2022 TMK-BASED CELL-SURFACE
AUXIN SIGNALLING ACTIVATES CELL-WALL ACIDIFICATION Article Open access 27 October 2021 DATA AVAILABILITY Source data for Figs. 1–6, and Extended Figs. 2–4, 7, 9 and 10 are provided with the
paper. Sequencing data from this Article is provided in the _Arabidopsis_ Genome Initiative databases under the following accession numbers: PIN1 (AT1G73590), PIN2 (AT5G57090), PIN3
(AT1G70940), PIN4 (AT2G01420), PIN7 (AT1G23080), PDK1.1 (AT5G04510), PDK1.2 (AT3G10540), D6PK (AT5G55910), D6PKL1 (AT4G26610), D6PKL2 (AT5G47750), D6PKL3 (AT3G27580), PID (AT2G34650), WAG1
(AT1G53700) and WAG2 (AT3G14370). All data necessary to evaluate the conclusions in the paper or the Supplementary Information are available from the corresponding authors on request. CHANGE
HISTORY * _ 12 JUNE 2020 A Correction to this paper has been published: https://doi.org/10.1038/s41477-020-0719-y _ REFERENCES * Swarup, K. et al. The auxin influx carrier LAX3 promotes
lateral root emergence. _Nat. Cell Biol._ 10, 946–954 (2008). Article CAS PubMed Google Scholar * Petrášek, J. et al. PIN proteins perform a rate-limiting function in cellular auxin
efflux. _Science_ 312, 914–918 (2006). Article PubMed CAS Google Scholar * Adamowski, M. & Friml, J. PIN-dependent auxin transport: action, regulation, and evolution. _Plant Cell_
27, 20–32 (2015). Article CAS PubMed PubMed Central Google Scholar * Geisler, M. et al. Cellular efflux of auxin catalyzed by the _Arabidopsis_ MDR/PGP transporter AtPGP1. _Plant J._
44, 179–194 (2005). Article CAS PubMed Google Scholar * Armengot, L., Marquès-Bueno, M. M. & Jaillais, Y. Regulation of polar auxin transport by protein and lipid kinases. _J. Exp.
Bot._ 67, 4015–4037 (2016). Article CAS PubMed Google Scholar * Friml, J. et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. _Science_ 306,
862–865 (2004). Article CAS PubMed Google Scholar * Dhonukshe, P. et al. Plasma membrane-bound AGC3 kinases phosphorylate PIN auxin carriers at TPRXS(N/S) motifs to direct apical PIN
recycling. _Development_ 137, 3245–3255 (2010). Article CAS PubMed Google Scholar * Grones, P. et al. PID/WAG-mediated phosphorylation of the _Arabidopsis_ PIN3 auxin transporter
mediates polarity switches during gravitropism. _Sci. Rep._ 8, 10279 (2018). Article PubMed PubMed Central CAS Google Scholar * Zourelidou, M. et al. The polarly localized D6 PROTEIN
KINASE is required for efficient auxin transport in _Arabidopsis thaliana_. _Development_ 136, 627–636 (2009). Article CAS PubMed Google Scholar * Zourelidou, M. et al. Auxin efflux by
PIN-FORMED proteins is activated by two different protein kinases, D6 PROTEIN KINASE and PINOID. _eLife_ 3, e02860 (2014). Article PubMed Central CAS Google Scholar * Marhava, P. et al.
A molecular rheostat adjusts auxin flux to promote root protophloem differentiation. _Nature_ 558, 297–300 (2018). Article CAS PubMed Google Scholar * Jia, W. et al. Mitogen-activated
protein kinase cascade MKK7-MPK6 plays important roles in plant development and regulates shoot branching by phosphorylating PIN1 in _Arabidopsis_. _PLoS Biol._ 14, e1002550 (2016). Article
PubMed PubMed Central CAS Google Scholar * Dory, M. et al. Coevolving MAPK and PID phosphosites indicate an ancient environmental control of PIN auxin transporters in land plants.
_FEBS Lett._ 592, 89–102 (2018). Article CAS PubMed Google Scholar * Rigó, G. et al. Inactivation of plasma membrane-localized CDPK-RELATED KINASE5 decelerates PIN2 exocytosis and root
gravitropic response in _Arabidopsis_. _Plant Cell_ 25, 1592–1608 (2013). Article PubMed PubMed Central CAS Google Scholar * Michniewicz, M. et al. Antagonistic regulation of PIN
phosphorylation by PP2A and PINOID directs auxin flux. _Cell_ 130, 1044–1056 (2007). Article CAS PubMed Google Scholar * Dai, M. et al. A PP6-type phosphatase holoenzyme directly
regulates PIN phosphorylation and auxin efflux in _Arabidopsis_. _Plant Cell_ 24, 2497–2514 (2012). Article CAS PubMed PubMed Central Google Scholar * Guo, X. et al. TYPE-ONE PROTEIN
PHOSPHATASE4 regulates pavement cell interdigitation by modulating PIN-FORMED1 polarity and trafficking in _Arabidopsis_. _Plant Physiol._ 167, 1058–1075 (2015). Article CAS PubMed PubMed
Central Google Scholar * Weller, B. et al. Dynamic PIN-FORMED auxin efflux carrier phosphorylation at the plasma membrane controls auxin efflux-dependent growth. _Proc. Natl Acad. Sci.
USA_ 114, E887–E896 (2017). Article CAS PubMed PubMed Central Google Scholar * Laetitia, M. et al. A PtdIns(4)P-driven electrostatic field controls cell membrane identity and signalling
in plants. _Nat. Plants_ 2, 16089 (2016). Article CAS Google Scholar * Wang, P. et al. Phosphatidic acid directly regulates PINOID-dependent phosphorylation and activation of the
PIN-FORMED 2 auxin efflux transporter in response to salt stress. _Plant Cell_ 31, 250–271 (2019). Article CAS PubMed Google Scholar * Barbosa, I. C. R. et al. Phospholipid composition
and a polybasic motif determine D6 PROTEIN KINASE polar association with the plasma membrane and tropic responses. _Development_ 143, 4687–4700 (2016). CAS PubMed Google Scholar * Pearce,
L. R., Komander, D. & Alessi, D. R. The nuts and bolts of AGC protein kinases. _Nat. Rev. Mol. Cell Biol._ 11, 9–22 (2010). Article CAS PubMed Google Scholar * Rintelen, F.,
Stocker, H., Thomas, G. & Hafen, E. PDK1 regulates growth through Akt and S6K in _Drosophila_. _Proc. Natl. Acad. Sci. USA_ 98, 15020–15025 (2001). Article CAS PubMed PubMed Central
Google Scholar * Lawlor, M. A. et al. Essential role of PDK1 in regulating cell size and development in mice. _EMBO J._ 21, 3728–3738 (2002). Article CAS PubMed PubMed Central Google
Scholar * Deak, M., Casamayor, A., Currie, R. A., Peter Downes, C. & Alessi, D. R. Characterisation of a plant 3-phosphoinositide-dependent protein kinase-1 homologue which contains a
pleckstrin homology domain. _FEBS Lett._ 451, 220–226 (1999). Article CAS PubMed Google Scholar * Rentel, M. C. et al. OXI1 kinase is necessary for oxidative burst-mediated signalling in
_Arabidopsis_. _Nature_ 427, 858–861 (2004). Article CAS PubMed Google Scholar * Anthony, R. G., Khan, S., Costa, J., Pais, M. S. & Bögre, L. The _Arabidopsis_ protein kinase PTI1-2
is activated by convergent phosphatidic acid and oxidative stress signaling pathways downstream of PDK1 and OXI1. _J. Biol. Chem._ 281, 37536–37546 (2006). Article CAS PubMed Google
Scholar * Camehl, I. et al. The OXI1 kinase pathway mediates _Piriformospora indica_-induced growth promotion in _Arabidopsis_. _PLoS Pathog._ 7, e1002051 (2011). Article CAS PubMed
PubMed Central Google Scholar * Anthony, R. G. et al. A protein kinase target of a PDK1 signalling pathway is involved in root hair growth in _Arabidopsis_. _EMBO J._ 23, 572–581 (2004).
Article CAS PubMed PubMed Central Google Scholar * Rademacher, E. H. & Offringa, R. Evolutionary adaptations of plant AGC kinases: from light signaling to cell polarity regulation.
_Front. Plant Sci._ 3, 250 (2012). Article PubMed PubMed Central Google Scholar * Zegzouti, H. et al. Structural and functional insights into the regulation of _Arabidopsis_ AGC VIIIa
kinases. _J. Biol. Chem._ 281, 35520–35530 (2006). Article CAS PubMed Google Scholar * Scholz, S. et al. The AGC protein kinase UNICORN controls planar growth by attenuating PDK1 in
_Arabidopsis thaliana_. _PLoS Genet._ 15, e1007927 (2019). Article CAS PubMed PubMed Central Google Scholar * Xiao, Y. & Offringa, R. PDK1 regulates auxin transport and
_Arabidopsis_ vascular development through AGC1 kinase PAX. _Nat. Plants_ https://doi.org/10.1038/s41477-020-0650-2 (2020). * Friml, J. et al. AtPIN4 mediates sink-driven auxin gradients and
root patterning in _Arabidopsis_. _Cell_ 108, 661–673 (2002). Article CAS PubMed Google Scholar * Band, L. R. et al. Root gravitropism is regulated by a transient lateral auxin gradient
controlled by a tipping-point mechanism. _Proc. Natl Acad. Sci. USA_ 109, 4668–4673 (2012). Article CAS PubMed PubMed Central Google Scholar * Sabatini, S. et al. An auxin-dependent
distal organizer of pattern and polarity in the _Arabidopsis_ root. _Cell_ 99, 463–472 (1999). Article CAS PubMed Google Scholar * Friml, J. et al. Efflux-dependent auxin gradients
establish the apical–basal axis of _Arabidopsis_. _Nature_ 426, 147–153 (2003). Article CAS PubMed Google Scholar * Prát, T. et al. WRKY23 is a component of the transcriptional network
mediating auxin feedback on PIN polarity. _PLoS Genet._ 14, e1007177 (2018). Article PubMed PubMed Central CAS Google Scholar * Zadnikova, P. et al. Role of PIN-mediated auxin efflux in
apical hook development of _Arabidopsis thaliana_. _Development_ 137, 607–617 (2010). Article CAS PubMed Google Scholar * Stepanova, A. N. et al. TAA1-mediated auxin biosynthesis is
essential for hormone crosstalk and plant development. _Cell_ 133, 177–191 (2008). Article CAS PubMed Google Scholar * Friml, J., Wiśniewska, J., Benková, E., Mendgen, K. & Palme, K.
Lateral relocation of auxin efflux regulator PIN3 mediates tropism in _Arabidopsis_. _Nature_ 415, 806–809 (2002). Article PubMed Google Scholar * Gray, W. M., Ostin, A., Sandberg, G.,
Romano, C. P. & Estelle, M. High temperature promotes auxin-mediated hypocotyl elongation in _Arabidopsis_. _Proc. Natl Acad. Sci. USA_ 95, 7197–7202 (2002). Article Google Scholar *
Franklin, K. A. et al. PHYTOCHROME-INTERACTING FACTOR 4 (PIF4) regulates auxin biosynthesis at high temperature. _Proc. Natl Acad. Sci. USA_ 108, 20231–20235 (2011). Article CAS PubMed
PubMed Central Google Scholar * Parry, G. & Estelle, M. Auxin receptors: a new role for F-box proteins. _Curr. Opin. Cell Biol._ 18, 152–156 (2006). Article CAS PubMed Google
Scholar * Brumos, J. et al. Local auxin biosynthesis is a key regulator of plant development. _Dev. Cell_ 47, 306–318 (2018). Article CAS PubMed Google Scholar * Luschnig, C., Gaxiola,
R. A., Grisafi, P. & Fink, G. R. EIR1, a root specific protein involved in auxin transport, is required for gravitropism in _Arabidopsis thaliana_. _Genes Dev._ 12, 2175–2187 (1998).
Article CAS PubMed PubMed Central Google Scholar * Swarup, R. et al. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal.
_Nat. Cell Biol._ 7, 1057–1065 (2005). Article CAS PubMed Google Scholar * Zegzouti, H., Anthony, R. G., Jahchan, N., Bogre, L. & Christensen, S. K. Phosphorylation and activation
of PINOID by the phospholipid signaling kinase 3-phosphoinositide-dependent protein kinase 1 (PDK1) in _Arabidopsis_. _Proc. Natl Acad. Sci. USA_ 103, 6404–6409 (2006). Article CAS PubMed
PubMed Central Google Scholar * Zhang, J., Nodzynski, T., Pencik, A., Rolcik, J. & Friml, J. PIN phosphorylation is sufficient to mediate PIN polarity and direct auxin transport.
_Proc. Natl Acad. Sci. USA_ 107, 918–922 (2010). Article CAS PubMed Google Scholar * Van Leeuwen, W., Ökrész, L., Bögre, L. & Munnik, T. Learning the lipid language of plant
signalling. _Trends Plant Sci._ 9, 378–384 (2004). Article PubMed CAS Google Scholar * Geldner, N. et al. The _Arabidopsis_ GNOM ARF-GEF mediates endosomal recycling, auxin transport,
and auxin-dependent plant growth. _Cell_ 112, 219–230 (2003). Article CAS PubMed Google Scholar * Noack, L. C. & Jaillais, Y. Precision targeting by phosphoinositides: how PIs direct
endomembrane trafficking in plants. _Curr. Opin. Plant Biol._ 40, 22–33 (2017). Article CAS PubMed Google Scholar * Platre, M. P. et al. Developmental control of plant Rho GTPase
nano-organization by the lipid phosphatidylserine. _Science_ 364, 57–62 (2019). Article CAS PubMed Google Scholar * Mei, Y., Jia, W., Chu, Y. & Xue, H. _Arabidopsis_
phosphatidylinositol monophosphate 5-kinase 2 is involved in root gravitropism through regulation of polar auxin transport by affecting the cycling of PIN proteins. _Cell Res._ 22, 581–597
(2011). Article PubMed PubMed Central CAS Google Scholar * Stenzel, I. et al. Phosphatidylinositol 4,5-bisphosphate influences PIN polarization by controlling clathrin-mediated membrane
trafficking in _Arabidopsis_. _Plant Cell_ 25, 4894–4911 (2013). PubMed PubMed Central Google Scholar * Tejos, R. et al. Bipolar plasma membrane distribution of phosphoinositides and
their requirement for auxin-mediated cell polarity and patterning in _Arabidopsis_. _Plant Cell_ 26, 2114–2128 (2014). Article CAS PubMed PubMed Central Google Scholar * Gao, H. B.,
Chu, Y. J. & Xue, H. W. Phosphatidic acid (PA) binds PP2AA1 to regulate PP2A activity and PIN1 polar localization. _Mol. Plant_ 6, 1692–1702 (2013). Article CAS PubMed Google Scholar
* Zhang, J. et al. Inositol trisphosphate-induced Ca2+ signaling modulates auxin transport and PIN polarity. _Dev. Cell_ 20, 855–866 (2011). Article CAS PubMed Google Scholar * Xu, J.
et al. A molecular framework for plant regeneration. _Science_ 311, 385–388 (2006). Article CAS PubMed Google Scholar * Benková, E. et al. Local, efflux-dependent auxin gradients as a
common module for plant organ formation. _Cell_ 115, 591–602 (2003). Article PubMed Google Scholar * Xu, J. & Scheres, B. Dissection of _Arabidopsis_ ADP-RIBOSYLATION FACTOR 1
function in epidermal cell polarity. _Plant Cell_ 17, 525–536 (2005). Article CAS PubMed PubMed Central Google Scholar * Clough, S. J. & Bent, A. F. Floral dip: a simplified method
for _Agrobacterium_-mediated transformation of _Arabidopsis thaliana_. _Plant J._ 16, 735–743 (1998). Article CAS PubMed Google Scholar * Liu, W., Xu, Z. H., Luo, D. & Xue, H. W.
Roles of OsCKI1, a rice casein kinase I, in root development and plant hormone sensitivity. _Plant J._ 36, 189–202 (2003). Article CAS PubMed Google Scholar * Rook, F. et al.
Sucrose-specific signalling represses translation of the _Arabidopsis ATB2_ bZIP transcription factor gene. _Plant J._ 15, 253–263 (1998). Article CAS PubMed Google Scholar * Schindelin,
J. et al. Fiji: an open-source platform for biological-image analysis. _Nat. Methods_ 9, 676–682 (2012). Article CAS PubMed Google Scholar * Baster, P. et al. SCFTIR1/AFB-auxin
signalling regulates PIN vacuolar trafficking and auxin fluxes during root gravitropism. _EMBO J._ 32, 260–274 (2013). Article CAS PubMed Google Scholar * Abas, L. et al. Intracellular
trafficking and proteolysis of the _Arabidopsis_ auxin-efflux facilitator PIN2 are involved in root gravitropism. _Nat. Cell Biol._ 8, 249–256 (2006). Article CAS PubMed Google Scholar *
Lewis, D. R. & Muday, G. K. Measurement of auxin transport in _Arabidopsis thaliana_. _Nat. Protoc._ 4, 437–451 (2009). Article CAS PubMed Google Scholar * Tan, S. et al. Salicylic
acid targets protein phosphatase 2A to attenuate growth in plants. _Curr. Biol._ 30, 381–395 (2020). Article CAS PubMed PubMed Central Google Scholar Download references
ACKNOWLEDGEMENTS We thank C. Schwechheimer and B. Scheres for sharing published materials; M. Glanc for providing pET28a-PIN2/3 plasmids; X. Gao for help with SEM imaging, L. Rodriguez for
advice on co-IP; staff at the bioimaging and life science facilities of IST Austria for continuous service and assistance; and the Nottingham _Arabidopsis_ Stock Centre (NASC) and the
_Arabidopsis_ Biological Resource Centre (ABRC) for providing T-DNA insertional mutants. J.P. acknowledges the support from imaging facility of IEB CAS. The research leading to these results
has received funding from Chinese Ten-Thousand Talent Program (to H.-W.X.) and the European Union’s Horizon2020 program (ERC grant agreement no. 742985, to J.F.). S.T. was funded by a
European Molecular Biology Organization (EMBO) long-term postdoctoral fellowship (ALTF 723–2015). X.Z. was supported by a PhD scholarship from China Scholarship Council. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Institute of Science and Technology Austria (IST Austria), Klosterneuburg, Austria Shutang Tan, Xixi Zhang, Gergely Molnár & Jiří Friml * Department of Applied
Genetics and Cell Biology, University of Natural Resources and Life Sciences (BOKU), Vienna, Austria Xixi Zhang & Gergely Molnár * National Key Laboratory of Plant Molecular Genetics,
CAS Centre for Excellence in Molecular Plant Sciences, Shanghai Institute of Plant Physiology and Ecology, Chinese Academy of Sciences, Shanghai, China Wei Kong, Xiao-Li Yang & Hong-Wei
Xue * Institute of Experimental Botany, The Czech Academy of Sciences, Prague, Czech Republic Zuzana Vondráková, Roberta Filepová & Jan Petrášek * Joint Center for Single Cell Biology,
School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai, China Hong-Wei Xue Authors * Shutang Tan View author publications You can also search for this author inPubMed
Google Scholar * Xixi Zhang View author publications You can also search for this author inPubMed Google Scholar * Wei Kong View author publications You can also search for this author
inPubMed Google Scholar * Xiao-Li Yang View author publications You can also search for this author inPubMed Google Scholar * Gergely Molnár View author publications You can also search for
this author inPubMed Google Scholar * Zuzana Vondráková View author publications You can also search for this author inPubMed Google Scholar * Roberta Filepová View author publications You
can also search for this author inPubMed Google Scholar * Jan Petrášek View author publications You can also search for this author inPubMed Google Scholar * Jiří Friml View author
publications You can also search for this author inPubMed Google Scholar * Hong-Wei Xue View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
S.T., J.F. and H.-W.X. designed experiments. S.T., X.Z., W.K. and X.-L.Y. performed experiments. G.M. provided [32P]ATP and helped with kinase assays. J.P., Z.V. and R.F. performed
experiments in BY-2 cells. S.T., J.F. and H.-W.X. analysed and interpreted the data. S.T., J.F. and H.-W.X. wrote the manuscript with input from other co-authors, and all of the authors read
and revised the manuscript. CORRESPONDING AUTHORS Correspondence to Jiří Friml or Hong-Wei Xue. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED DATA
FIG. 1 EXPRESSION PATTERN OF _PDK1.1_ AND _PDK1.2_. GUS staining of _pPDK1.1::GUS_ and _pPDK1.2::GUS_ lines indicated that _PDK1.1_ and _PDK1.2_ were expressed in the vascular tissues in
both roots and shoots at various developmental stages, including young seedlings (A, F; 7 days), root stele (B, G; 7 days), columella cells (C, H, only with expression detected for _PDK1.1_;
7 days), lateral root primordia (D, I; 12 days), and dark-grown seedlings (E, J; 4 days). Representative images of three independent homozygous lines were shown. Scale bars, 1 mm. EXTENDED
DATA FIG. 2 IDENTIFICATION OF _ARABIDOPSIS PDK1.1_ AND _PDK1.2_ T-DNA INSERTIONAL MUTANTS. A, Schematic representation of _PDK1.1_ and _PDK1.2_ genes and positions of T-DNA insertions for
_pdk1.1_ and _pdk1.2_. Introns, exons, and non-coding regions are indicated by lines, black, or blank boxes respectively. Positions of primers are indicated. B, Identification of homozygous
_pdk1.1_ and _pdk1.2_ mutants. Genomic DNA of _pdk1.1_ and _pdk1.2_ mutants was used as templates for PCR amplification. Homozygous lines have a single amplified DNA fragment when using
LBa1/pdk1.1-RP or LBa1/pdk1.2-RP primers. n = 5 biologically independent experiments, with similar results obtained. C, qRT-PCR analysis confirmed the deficient expression of _PDK1.1_ and
_PDK1.2_ genes in _pdk1.1_ and _pdk1.2_ mutants, respectively. Total RNA of 7-day-old WT, _pdk1.1_ and _pdk1.2_ seedlings was extracted, reversely transcribed, and then used for analysis.
_ACTIN7_ was amplified and used as an internal reference to normalize the expression of _PDK1.1_ and _PDK1.2_, and the mean value in Col-0 was set as “1”. The experiments were biologically
repeated for 3 times. Dots represent individual samples, and lines indicate mean ± s.d.. _P_ values were calculated by a Welch’s two-tailed _t_-test. Source Data EXTENDED DATA FIG. 3
DEFICIENCY OF _PDK1.1_ AND _PDK1.2_ IMPAIRED THE HYPOCOTYL GRAVITROPISM UNDER DARK AND PHOTOTROPISM TOWARDS DIRECTIONAL LIGHT. A, Deficiency of _PDK1.1_ and _PDK1.2_ promoted the radial
growth in the root columella cell region. Transverse view of the root columella cells by CLSM. Left, a schematic image to show the positon for the transverse view; middle, Col-0; right,
_pdk1.1 pdk1.2_. Scale bars, 20 µm. n = 3 biologically independent experiments, with similar results obtained. B, Deficiency of _PDK1.1_ and _PDK1.2_ gave rise to more columns, but not
layers of, root columella cells. Quantification is based on CLSM images of PI-stained roots. Layer numbers were counted for both undifferentiated and differentiated columella cells. Dots
represent individual plants, and lines indicate mean ± s.d.. n = 18, 26, 18, and 26, from left to right, respectively. _P_ values were calculated by a Welch’s two-tailed _t_-test. C–E,
Deficiency of _PDK1.1_ and _PDK1.2_ impaired root and shoot gravitropic response in the dark. Etiolated seedlings of Col-0, _pdk1.1_, _pdk1.2_, and _pdk1.1 pdk1.2_ were grown under dark for
90 h and a representative photo was shown (C). Scale bar, 5 mm. n = 5 biologically independent experiments, with similar results obtained (C). (D), Root tip angles are shown as polar bar
charts. n = 61, 62, 69, and 71, respectively. (E), Hypocotyl angles are shown as polar bar charts. n = 51, 50, 59, and 68, respectively. F–G, _pdk1.1 pdk1.2_ showed defects in phototropism.
Seedlings of Col-0, _pdk1.1_, _pdk1.2_, and _pdk1.1 pdk1.2_ were grown under dark for 90 h, exposed to white light for 24 h, and were then subjected to directional white light in a box
covered with aluminium foil from the other sides. A representative photo is shown (f). Scale bar, 5 mm. n = 3 biologically independent experiments, with similar results obtained (F). (G),
hypocotyl angles are shown by polar bar charts. n = 56, 62, 67, and 62, respectively. _P_ values were calculated by a Welch’s two-tailed _t_-test, and also by a further F-test to indicate
differences of variances (D, E, and G). Source Data EXTENDED DATA FIG. 4 DEFICIENCY OF _PDK1.1_ AND _PDK1.2_ IMPAIRED THE NORMAL DEVELOPMENT OF THE APICAL HOOK AND HIGH TEMPERATURE-INDUCED
HYPOCOTYL ELONGATION. A, B, Observation (A, scale bar, 5 mm) and quantification (B) showed that etiolated seedlings (90 h) of _pdk1.1 pdk1.2_ exhibited less tight apical hooks. n = 5
biologically independent experiments, with similar results obtained (A). n = 14, 18, 12 and 17 seedlings for Col-0, _pdk1.1_, _pdk1.2_, and _pdk1.1 pdk1.2_ respectively (B). C, Etiolated
seedlings of _pdk1.1 pdk1.2_ exhibited comparably long hypocotyls to Col-0. Seedlings were grown under dark for 90 h. n = 20, 21, 20 and 17 seedlings for Col-0, _pdk1.1_, _pdk1.2_, and
_pdk1.1 pdk1.2_ respectively. D, Etiolated seedlings of _pdk1.1 pdk1.2_ did not form exaggerated apical hooks in the presence of ACC. Seedlings of Col-0, _pdk1.1_, _pdk1.2_, and _pdk1.1
pdk1.2_ were grown under dark for 90 h in the absence or presence of ACC (10 µM). Scale bars, 500 µm. n = 3 biologically independent experiments, with similar results obtained. E–F, _pdk1.1
pdk1.2_ showed defects in high temperature-induced hypocotyl elongation. Seedlings of Col-0, _pdk1.1_, _pdk1.2_ and _pdk1.1 pdk1.2_ were grown under light at 22 °C (n = 16, 20, 18 and 22
respectively) or 29 °C (n = 20, 21, 17 and 18) for 5 days, and hypocotyl elongation was observed (E, scale bar, 1 cm) and quantified (E). Hypocotyl length was measured by the Image J program
and shown as mean ± s.d. (left) or relative length by setting the hypocotyl length of Col-0 and _pdk1.1 pdk1.2_ at 22 °C as “1”, respectively (right). Dots represent individual plants, and
lines indicate mean ± s.d.. Different letters represent significant difference, _P <_ 0.05, by one-way ANOVA with a Tukey multiple comparison test, and _P_ values are shown for each
genotype compared with Col-0 (B, C, and F). Source Data EXTENDED DATA FIG. 5 LOSS OF FUNCTION OF _PDK1.1_ AND _PDK1.2_ IMPAIRED AUXIN DISTRIBUTION. Observation of the auxin responsive
reporter DR5rev::GFP by CLSM indicated a dramatic decrease of the auxin maxima in _pdk1.1 pdk1.2_ (E–H) compared with Col-0 (A–D). Fused cotyledon exhibiting two sites of auxin maxima in
light-grown 7-day-old seedlings of _pdk1.1 pdk1.2_ (E) compared with one of Col-0 (A); roots of light-grown 10-day-old seedlings (B and F); apical hooks of 10 µM ACC treated 4-day-old
etiolated seedlings (C and G); roots of 10 µM ACC treated 4-day-old etiolated seedlings (D and H). Scale bars, 200 µm. n = 3 biologically independent experiments, with similar results
obtained. EXTENDED DATA FIG. 6 DEFICIENCY OF _PDK1.1_ AND _PDK1.2_ DID NOT AFFECT THE POLARITY OF PIN PROTEINS. A, Deficiency of _PDK1.1_ and _PDK1.2_ did not change the polarity of
PIN1-YFP. Four-day-old seedlings of _pPIN1::PIN1-YFP_ in Col-0 and _pPIN1::PIN1-YF_P in _pdk1.1 pdk1.2_ were imaged by CLSM. Scale bars, 20 µm. n = 2 biologically independent experiments,
with similar results obtained (A-G). B, Deficiency of _PDK1.1_ and _PDK1.2_ did not change the polarity of PIN2-GFP. Four-day-old seedlings of _pPIN2::PIN2-GFP_ in Col-0 and
_pPIN2::PIN2-GFP_ in _pdk1.1 pdk1.2_ were imaged by CLSM. Scale bars, 20 µm. C–F, Deficiency of _PDK1.1_ and _PDK1.2_ did not change the polarity of PIN3-GFP. Four-day-old seedlings of
_pPIN3::PIN3-GFP_ in Col-0 and _pPIN3::PIN3-GFP_ in _pdk1.1 pdk1.2_ were imaged by CLSM. (C) subcellular localisation of PIN3-GFP; (D) an amplified view of PIN3-GFP in the root stele; (E) a
close view of PIN3-GFP in root columella cells; (F) a 3D-projection of PIN3-GFP localisation in root columella cells. The “Green Fire Blue” LUT was used for photo visualization based on
fluorescence intensity by Fiji. Scale bars, 20 µm. G, A transverse view of the root columella cells revealed the overproliferation, with an enlarged region expressing PIN3-GFP. Four-day-old
seedlings of _pPIN3::PIN3-GFP_ in Col-0 and _pPIN3::PIN3-GFP_ in _pdk1.1 pdk1.2_ were stained with PI, and imaged by CLSM, at the position as marked in the left image. Scale bars, 20 µm.
EXTENDED DATA FIG. 7 ANALYSIS OF _PDK1_ TRANSGENIC LINES. A, Expression of _PDK1.1_ or _PDK1.2_ (_p35S::Venus-PDK1.1, p35S::Venus-PDK1.2, p35S::mCherry-PDK1.1_ and _p35S::mCherry-PDK1.2_)
rescued the growth defects of _pdk1.1 pdk1.2_. Adult plants (25-day-old) were observed and representative photos were shown. Scale bar, 2 cm. B, Western blot analysis verified the PDK1.1 or
PDK1.2 expression (_p35S::Venus-PDK1.1_ and _p35S::Venus-PDK1.2_) in _pdk1.1 pdk1.2_, respectively. Seven-day-old T3 homozygous seedlings were used for protein extraction and subjected to
analysis. Upper panel, anti-GFP (1:2000); lower panel, Ponceau staining. C, Western blot verified the PDK1.1 or PDK1.2 expression (_p35S::mCherry-PDK1.1_ and _p35S::mCherry-PDK1.2_) in
_pdk1.1 pdk1.2_, respectively. Seven-day-old T3 homozygous seedlings were used for protein extraction and subjected to analysis. Upper panel, anti-RFP (1:2000); lower panel, Ponceau
staining. D–E, mCherry-fused PDK1.1 (D) and PDK1.2 (E) localised to both cytoplasm and the basal side of PM. Four-day-old seedlings of _p35S::mCherry-PDK1.1_ and _p35S::mCherry-PDK1.2_ were
imaged by CLSM. Open arrowheads indicated the basal polar localisation. Scale bar, 10 µm. F–I, Subcellular localisation of Venus-fused PDK1.1N (F, cytoplasm), Venus-fused PDK1.1C (G, both
cytoplasm and nucleus), Venus-fused PDK1.2N (h, cytoplasm), and Venus-fused PDK1.2C (i, both cytoplasm and nucleus). Four-day-old seedlings expressing corresponding fusion proteins were
imaged by CLSM. Scale bar, 10 µm. n = 4, 2, 3, and 3 biologically independent experiments for (A), (B), (C), and (D) respectively, with similar results obtained. Source Data EXTENDED DATA
FIG. 8 FUNCTIONAL _PPDK1.1::VENUS-PDK1.1_ LOCALISED AT BOTH CYTOPLASM AND THE BASAL SIDE OF PM. A, _pPDK1.1::Venus-PDK1.1_ rescued the growth defects of _pdk1.1 pdk1.2_. 25-day-old adult
plants were observed and representative photos are shown. Scale bar, 2 cm. B, _pPDK1.1::Venus-PDK1.1_ rescued the lateral root defects of _pdk1.1 pdk1.2_. 10-day-old seedlings were observed
and representative photos are shown. Scale bar, 2 cm. C, _pPDK1.1::Venus-PDK1.1_ rescued the defects of _pdk1.1 pdk1.2_ in the hypocotyl gravitropic response. 4-day-old etiolated seedlings
were observed and representative photos are shown. Scale bar, 2 cm. D, Venus-fused PDK1.1 localised to both cytoplasm and PM, especially with a predominant presence at the basal side of PM.
Four-day-old seedlings of _pPDK1.1::Venus-PDK1.1_ were imaged by CLSM. Open arrowheads indicate the basal polar localisation. The “Green Fire Blue” LUT was used for photo visualization based
on fluorescence intensity by Fiji. Scale bar, 20 µm. E, Venus-PDK1.1 localized to cytoplasm (upper image) in interphase tobacco BY-2 cells, and the association with PM was obvious only
during cytokinesis (arrow in lower image). An _XVE>>Venus-PDK1.1_ line was induced for 48 h with 1 μM β-estradiol and were then imaged by spinning disk confocal microscope. Scale bar,
10 μm. n = 3, 3, 2, 3, and 2 biologically independent experiments for (A), (B), (C), (D), and (E) respectively, with similar results obtained. EXTENDED DATA FIG. 9 MCHERRY-D6PKS CO-LOCALISED
WITH VENUS-PDK1.1 AT THE BASAL SIDE OF PM. A, mCherry-fused D6PKs localised to the basal side of PM. Four-day-old seedlings of _p35S::mCherry-D6PK/D6PKLs_ (short as _D0 to D3_) were imaged
by CLSM. The “mpl-inferno” LUT was used for photo visualization based on fluorescence intensity by Fiji. Scale bars, 10 µm. B, Western blot verified the mCherry-D0~D3 protein level
(_35S::mCherry-D0~D3_) in Col-0, respectively. Seven-day-old T3 homozygous seedlings were used for protein extraction and subjected to Western blot analysis. Upper panel, anti-RFP (1:1000),
short exposure (0.5 sec); medium panel, anti-RFP (1:1000), long exposure (5 sec, for low expression of mCherry-D3); lower panel, Ponceau staining. C, Venus-PDK1.1 co-localised with
mCherry-D6PKL2 and mCherry-D6PKL3 at the basal side of PM. Four-day-old seedlings of _p35S::mCherry-_D6PKL2_/ p35S::Venus-PDK1.1_ and _p35S::mCherry-_D6PKL3_/ p35S::Venus-PDK1.1_ were imaged
by CLSM. Scale bar, 10 µm. D, _In vitro_ kinase assay with [32P]-ATP revealed that GST-PDK1.2-conducted phosphorylation of D6PK facilitates its activity towards PIN-HL phosphorylation.
Upper panel, autoradiography; lower panel, CBB staining. E, _In vitro_ kinase assay with [32P]-ATP revealed that GST-PDK1.2-conducted full phosphorylation and activation of D6PK, towards
His-PIN1-HL phosphorylation, required the phosphorylation at S345 for D6PK. Upper panel, autoradiography of 32P; lower panel, CBB staining. n = 3, 2, 2, 3, and 3 biologically independent
experiments for (A), (B), (C), (D), and (E) respectively, with similar results obtained. Source Data EXTENDED DATA FIG. 10 OVEREXPRESSION OF _VENUS-D6PK__S345D_ RESCUED THE DEFECTS OF
LATERAL ROOT FORMATION AND HYPOCOTYL GRAVITROPISM IN _PDK1.1 PDK1.2_. A, B, _35S::Venus-D6PK__S345D_ (L3 as a representative line) rescued the lateral root defects of _pdk1.1 pdk1.2_.
Nine-day-old seedlings were observed. n = 3 biologically independent experiments, with similar results obtained. A representative photo is shown in (A, scale bar, 2 cm), and the lateral root
number was quantified (b). Dots represent individual plants, and lines indicate mean ± s.d.. n = 15, 15, 14 and 14 individual seedlings for Col-0, _pdk1.1 pdk1.2_, _35S::Venus-D6PK__S345D_
in _pdk1.1 pdk1.2_ (L3), and _35S::Venus-D6PK__S345D_ in _pdk1.1 pdk1.2_ (L5), respectively. Different letters represent significant difference, _P <_ 0.05, by one-way ANOVA with a Tukey
multiple comparison test, and _P_ values are shown for each genotype compared with Col-0. C, The subcellular localisations of Venus-D6PK, Venus-D6PKS345A, and Venus-D6PKS345D exhibit
difference in PM targeting, but not show difference in the basal polarity. Four-day-old seedlings of _p35S::Venus-D6PK_, _p35S::Venus-D6PK__S345A_, and _p35S::Venus-D6PK__S345D_, in Col-0
and _pdk1.1 pdk1.2_ respectively, were imaged by CLSM. The “Green Fire Blue” LUT was used for photo visualization based on fluorescence intensity by Fiji. Representative photos of at least
three independent transgenic lines were shown. Scale bars, 10 µm. n = 2 biologically independent experiments, with similar results obtained. Source Data SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION Supplementary Figs. 1–9, Tables 1–7 and references for the Supplementary Information. REPORTING SUMMARY SOURCE DATA SOURCE DATA FIG. 1 Statistical source data.
SOURCE DATA FIG. 2 Statistical source data. SOURCE DATA FIG. 3 Unprocessed western blots and/or gels. SOURCE DATA FIG. 4 Statistical source data. SOURCE DATA FIG. 4 Unprocessed western blots
and/or gels. SOURCE DATA FIG. 5 Statistical source data. SOURCE DATA FIG. 5 Unprocessed western blots and/or gels. SOURCE DATA FIG. 6 Statistical source data. SOURCE DATA FIG. 6 Unprocessed
western blots and/or gels. SOURCE DATA EXTENDED DATA FIG. 2 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 2 Unprocessed western blots and/or gels. SOURCE DATA EXTENDED DATA FIG. 3
Statistical source data. SOURCE DATA EXTENDED DATA FIG. 4 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 7 Unprocessed western blots and/or gels. SOURCE DATA EXTENDED DATA FIG. 9
Statistical source data. SOURCE DATA EXTENDED DATA FIG. 9 Unprocessed western blots and/or gels. SOURCE DATA EXTENDED DATA FIG. 10 Statistical source data. RIGHTS AND PERMISSIONS Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tan, S., Zhang, X., Kong, W. _et al._ The lipid code-dependent phosphoswitch PDK1–D6PK activates PIN-mediated auxin efflux in
_Arabidopsis_. _Nat. Plants_ 6, 556–569 (2020). https://doi.org/10.1038/s41477-020-0648-9 Download citation * Received: 31 August 2019 * Accepted: 25 March 2020 * Published: 11 May 2020 *
Issue Date: 15 May 2020 * DOI: https://doi.org/10.1038/s41477-020-0648-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
