Complementary corrosion protection of cast alsi7mg0. 3 alloy using zr-cr conversion and polyacrylic/siloxane-silica multilayer coatings
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Aluminium cast alloy AlSi7Mg0.3 is a lightweight metal commonly used in automotive, aeronautical and mechanical applications. It has good corrosion resistance but, under harsh
operative conditions, would benefit from additional protection. In this study, a corrosion-protective multilayer coating system for AlSi7Mg0.3 based on hexafluoro-zirconated trivalent
chromium coating (Zr-CrCC) and polyacrylic/siloxane-silica (PEHA-SS) coating was developed. The Zr-CrCC was formed by immersion of the substrate in a commercial conversion bath (SurTec®
650). PEHA-SS synthesis was based on organic precursors (2-ethylhexyl acrylate and [3-(methacryloyloxy)propyl]trimethoxysilane) and an inorganic precursor, tetraethyl orthosilicate. After
deposition on AlSi7Mg0.3, each coating was first characterised individually, followed by the analysis of the multilayer using scanning electron microscopy and energy-dispersive X-ray
spectroscopy. The adhesion of the coatings was evaluated with a cross-hatch cut test. The corrosion studies in sodium chloride solution using electrochemical impedance spectroscopy and salt
spray testing showed that the multilayer system is superior to individual Zr-CrCC and PEHA-SS coatings. After 4 months in 0.1 M NaCl, the multilayer-coated samples exhibited the impedance at
10 mHz in the range of GΩ cm2, while scribed samples withstood the corrosion attack in a salt spray chamber for one week. Thus, albeit only about 100 nm thick, the Zr-CrCC deposited between
the substrate and a 9-micrometre thick barrier sol-gel PEHA-SS coating acts as an active corrosion protection interlayer and contributes to the overall protectiveness of the multilayer
system. SIMILAR CONTENT BEING VIEWED BY OTHERS STUDY OF THE ANTICORROSIVE BEHAVIOR OF SAMARIUM AS A CORROSION INHIBITOR IN MULTILAYER SYSTEMS FOR ALUMINUM ALLOY Article Open access 23
February 2023 MULTILAYER PEO COATINGS WITH ENCAPSULATED CERIUM FOR ACTIVE CORROSION PROTECTION OF ALUMINIUM Article Open access 07 March 2025 CORROSION SUPPRESSION AND STRENGTHENING OF THE
AL-10ZN ALLOY BY ADDING SILICA NANORODS Article Open access 08 July 2024 INTRODUCTION The relevant casting characteristics of alloys 3xx.x series are the main reasons that almost 90% of all
cast aluminium alloys produced belong to this series1. These alloys contain silicon, copper and/or magnesium as alloying elements1. Alloys containing silicon have improved castability, wear
resistance, and reduced melting point. Silicon and magnesium form the Mg2Si intermetallics (IMs), contributing to better strength2, but alloys with a greater proportion of copper and
magnesium have lower corrosion resistance2,3,4. The most common cast alloy AlSi7Mg0.3 (also classified as EN AC-42100)2 is used in industry for various parts of engines, automobile wheels,
aircraft parts, housings, compressors and pumps. Over the last few decades, many research studies in corrosion protection have focused on developing effective coatings to replace
environmentally hazardous chromate conversion coatings containing chromium(VI)5,6,7,8. In the last twenty years, various environmentally acceptable and efficient corrosion protective
conversion coating systems based on manganese9,10, titanium11,12, cerium13,14,15,16, trivalent chromium process (TCP)17,18,19,20, and zirconium12,21,22,23,24,25,26,27,28,29,30 have been
investigated. The zirconium-based coatings (ZrCCs) are already commercialised12. When analysing the literature data and commercial products, it should be distinguished that some ZrCCs
contain only Zr-bearing components, while others also contain trivalent chromium salt. ZrCC conversion baths most often contain hexafluoro zirconic acid (H2ZrF6)12,21,22,25,27,28,29, but in
some studies, potassium fluorozirconate (K2ZrF6)26, and H2ZrF6 and hexafluorotitanic acid (H2TiF6) were used29,31. Some products also contain a small amount of cupric ions27,28 for
stimulating the precipitation of the coating, phosphate26 or borate ions21. TCP baths contain, usually in addition to the Zr-bearing component, a trivalent chromium salt ( ≤ 5 wt.%) to form
a Zr-Cr-rich oxide coating on the metal substrate17,18,19,20. The terminology in the literature is not very strict, so the term ZrCC has often been used when the coating contained Zr and
Cr-bearing components, and the term TCP has often been used albeit the coating contained not only Cr(III) but also Zr-bearing component. The terms ZrCCs and TCP are thus used generically,
often not specifying the actual coating composition, which can sometimes be misleading. The formation of the conversion coating can be generally divided into the following steps: (i)
degreasing using an alkaline cleaner to remove organic contaminants and part of a naturally grown oxide layer on the aluminium surface, (ii) desmutting of the smut layer formed during
alkaline cleaning to remove some of the insoluble intermetallic particles, and at the same time, to passivate aluminium surface in the presence of HNO3, and (iii) coating formation in the
conversion bath24,25,26,32,33. The deposition of conversion coating is a pH-dependent process. Metal substrate dipped into a conversion bath containing Zr-bearing species becomes covered by
Zr-hydrated oxide precipitated on the metal surface once the pH reaches the value required for precipitation (usually around pH ≈ 4). This process is the most pronounced at the cathodic
sites where, due to oxygen reduction reaction and concomitant OH− formation, the pH rises to the values suitable for the precipitation of Zr(IV) hydroxide (ZrO2 × 2H2O), which is then stable
up to highly alkaline pH values12,21,25,30,34,35. In the case of conversion baths containing both zirconium and trivalent chromium, the precipitation of Cr(III) hydroxide proceeds at
similar pH values35. The ZrCCs deposited on the aluminium alloys enhance the corrosion properties24,25 and adhesion of the upper organic layer10,36,37,38. Namely, the role of the conversion
coating is not solely corrosion protection but also adhesion to the upper organic layer, as in industrial applications, the coating system consists of the primer, organic and/or top layers.
For that reason, several studies have focused on the effect of adhesion of ZrCCs as an interlayer between metal and organic coatings. The variety of combinations investigated is very broad.
As substrates, mainly steels, galvanised steels and wrought Al alloys were used. The general conclusion is that the ZrCC interlayer coating, albeit being only 50−150 nm thick, improves the
adhesion strength of the several tens of micrometres thick upper organic coating to the underlying metal substrate; this finding was ascribed to conversion coating providing stronger
bonding, increasing the surface roughness and reducing the cathodic disbondment of the organic coating by inhibiting the formation of large water aggregates. The adhesion is improved under
both dry and wet conditions. These issues were reported for mild steel36,39,40, cold-rolled steel41, carbon steel42 and galvanised steel31 using ZrCC coatings prepared from H2ZrF6 and
H2TiF631, Jiuhe Zr solution41 and MAVOM 1742 coating42. As organic layers, epoxy coatings39,40,41,42, epoxy-containing ZnAl polyphosphate pigments36 and polyester-based resins31 were used.
As additives to ZrCC, Zn sulphate40 or various organic components were used31. The former acted as anti-corrosion pigments, whereas the latter initially improved the interfacial stability of
conversion coating-treated substrates; however, in the long term, organic additives were shown to be detrimental to polyester coil coat adhesion. The combination of conversion and organic
coatings was investigated on various wrought Al alloys: AA105043, AA106044, AA601645, AA606046,47, AA606348,49 and AA7A5238. As Zr-based interlayers, conversion coatings prepared from
H2ZrF643, H2ZrF6 and H2TiF645, H2ZrF6, H2TiF6, sodium vanadate and phosphoric acid44,48, potassium hexafluoro zirconate, hexafluorotitanate and amino trimethylene phosphonic acid (ATMP)38
and commercial Zr/Ti Alodine 5200® (Henkel)49 were used. In addition, cerium-based conversion layers combined with organic layers were also studied46,47. As organic layers, polyester
coating47, water-based polyurethane resin44, epoxy26,48, polyvinylidene fluoride (PVDF) and acrylic resin49, acrylic resin45 and epoxy/polyamide43 were used. In one study, a thin ZrO2
sol-gel layer was applied above the Ce-based conversion coating46. The presence of ZrCC as an interlayer between the substrate and organic coatings also improves the corrosion properties. If
we focus more on the Al-based substrates, it was reported that when prepared with the Ti/Zr/V conversion interlayer, polyurethane coating exhibited excellent corrosion resistance and
eliminated the post-film washing step, thus simplifying the process44. Ti/Zr interlayer between the AA7A52 substrate and epoxy coating demonstrated very good results in the salt spray
chamber, ascribed to the conversion layer’s barrier properties38. Another study reported that the conversion coating (Alodine 5200®) achieved poor results in the salt spray chamber because
of its small thickness of only 20 nm, which is insufficient to produce a barrier effect49. However, when combined with a PVDF/acrylic resin, the corrosion resistance of the system as a whole
improved49. Similar results were published also for ZrCC/epoxy-polyamide-coated AA105043. It was reported in previous publications that ZrCCs prepared from H2ZrF6 on wrought aluminium alloy
AA3005 have the ability of so-called self-sealing, i.e. improvement of protective properties of the coatings with prolonged immersion in sodium chloride solution24,25. The self-sealing was
explained by the transformation of ZrF4/ZrOxFy to ZrO2·1.2H2O(s), i.e. progressive loss of fluoride from the coating and densification of the Zr-oxide layer24,25. Similar properties were
noted for the commercial hexafluoro-zirconate trivalent chromium coating SurTec 650® in sodium chloride solution and simulated acid rain24. Compared to wrought aluminium alloys, relatively
little is known about the electrochemical performance of conversion coatings on cast alloys14,50. Given these considerations and the overview of the literature studies given in the above
text, it can be stated that corrosion and adhesion research study based on ZrCCs on cast aluminium alloys would bring novel results, not only for the individual coating but also in
combination with organic overlayer, i.e. in a multilayer system. As Zr-based conversion coating, the commercial hexafluoro-zirconate trivalent chromium coating SurTec 650® was used (denoted
as Zr-CrCC). Instead of commonly used organic coatings, usually several tens of micrometres thick, we explored a much thinner hybrid sol-gel coating. In our previous study, a
polyacrylic/siloxane-silica coating (abbreviated PEHA-SS) was developed using tetraethyl orthosilicate (TEOS) as an inorganic precursor, and organically modified silane precursor
[3-(methacryloyloxy)propyl]trimethoxysilane (MAPTMS) and an organic monomer 2-ethylhexyl acrylate (2-EHA)51. The nine micrometres thick PEHA-SS coating achieved durable corrosion (barrier)
protection for the cast AlSi7Mg0.3 alloy in 0.1 M NaCl during the first four months of immersion or under accelerated corrosion conditions in a Machu chamber containing NaCl, acetic acid,
and hydrogen peroxide at 37 °C51. The coating synthesis and characterisation details are given in our previous publication51. This study aimed to research the individual coatings (Zr-CrCC
and PEHA-SS) and a combination of both on cast aluminium alloy AlSi7Mg0.3 (Supplementary Figure 1). This particular combination has not been investigated in the literature, mainly focusing
on combining various ZrCCs and organic coatings on steels or wrought Al alloys. The study’s rationale and experimental approach is thus to combine two methodologies on cast AlSi7Mg0.3 alloy
and obtain a protective system with improved interfacial properties gained through SurTec 650® Zr-CrCC and efficient barrier properties gained through environmentally suitable sol-gel
PEHA-SS coating. The coatings’ characterisation was assessed using scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDS), adhesion evaluation and
corrosion testing in 0.1 M NaCl and salt spray chamber. RESULTS AND DISCUSSION PREPARATION OF ZR-CR CONVERSION COATING The deposition of the Zr-Cr conversion coating consists of three steps:
i) degreasing by immersion of the samples in an alkaline cleaning solution, ii) desmutting in an acidic solution and iii) surface passivation using the zirconium conversion process. After
each step, the samples were rinsed with deionised water and dried. A detailed procedure of individual steps of Zr-CrCC deposition is given in the Methods section. Figure 1a shows the SEM
image of the ground alloy surface recorded using a secondary electrons (SE) detector. Cast AlSi7Mg0.3 alloy has high hardness (Brinell hardness ~ 55 HBW)3; therefore, the mechanical grinding
with SiC paper left longitudinal marks on the surface. The EDS analysis is given in Table 1, revealing that x1 belongs to the α-Al matrix consisting mainly of Al with a small amount of Si
(1.9 at.%). Site x2 refers to the intermetallic particle (IMP) containing Mg (0.9 at.%) and approximately ten times more Si than at the matrix (14.5 at.%) (Table 1). In contrast to the SE
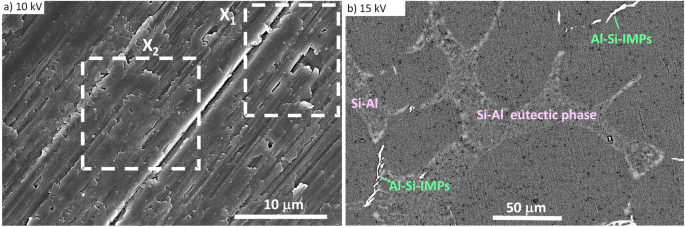
SEM image on the ground surface given in Fig. 1a, the image in Fig. 1b was recorded on the polished surface using a circular scattered electrons (CBS) detector. The heterogeneity of the
surface is more pronounced than in Fig. 1a. A typical microstructure of AlSi7Mg0.3 alloy consists of an α-Al matrix, eutectic phase and intermetallic particles. The patterned area is a
eutectic phase containing more than 60 at.% of Si and the rest of Al and C4. Bright intermetallic particles refer to Al-Si-based IMPs. In AlSi7Mg0.3 alloy, there are several types of IMPs:
Al-Si-Mg, Ali-Si-Mg(Fe), Al-Si-Fe-Mn and Al-Si-Fe-Mn(Mg)4,25. The presence of Mg-rich intermetallics can reduce the corrosion resistance of the alloy compared to aluminium
matrix3,16,25,52,53. Figure 2a–c present the SEM images of the ground AliSi7Mg0.3 sample coated with Zr-CrCC; images were recorded in CBS mode using different magnifications. The coverage of
the Zr-CrCC was not homogeneous (Fig. 2a). Three regions are recognised, differing in the composition and thickness of the coating (detailed in Fig. 2b). The first characteristic regions
are several micrometres broad areas of irregular shape (exampled by x3 and x6). When analysed using 10 kV acceleration voltage and higher magnification (Fig. 2a), it seems that no coating
was formed since only the longitudinal grinding marks of the underlying substrate were visible. EDS analysis (Table 1) of areas x3 and x6 (coated sample, Fig. 2) and x1 (non-coated sample,
Fig. 1a) confirmed the similar compositions comprised mainly of Al and a small amount of Si and O. Higher magnification (Fig. 2b) revealed the presence of a thin layer in these areas
(exampled by x6), which could not be observed when imaged at smaller magnification (Fig. 2a). However, EDS analysis at 10 kV (area x6) still did not detect any element originating from the
coating. In contrast, when using the smaller voltage of 5 kV, Zr and F were detected also in these areas (area x9, Fig. 2c, Table 1). This discrepancy in the identification of coating
elements is due to different analysis depths at 5 kV and 10 kV. Namely, the analysis depths are 300 and 1000 nm, respectively. The analysis depth was assessed using a Monte Carlo-based
simulation; details are given in Supplementary Figure 2. The second observation is that only Zr and F, not Cr, were detected at 5 kV. Cr cannot be unambiguously identified under these
conditions because, in contrast to Zr, its most intense peak is above 5 kV. Kα and Lα peaks for Zr are at 15.7 keV and 2.0 keV, respectively. Kα and Lα peaks for Cr are at 5.4 keV and 0.57
keV, respectively, which means that chromium’s most intense peak cannot be identified when using an acceleration voltage of 5 kV (Cr Lα peak at 0.57 keV is partially overlapping with the Kα
peak of O at 0.525 keV). Therefore, Cr can be detected reliably only when the EDS analysis is performed at 10 kV, while Zr can be detected at 5 kV. Notably, the concentration of Al
originating from the underlying substrate is high (Table 1) due to a nanometric-size Zr-CrCC thickness and a micrometre-deep EDS analysis depth. The second characteristic region refers to
the rest of the surface visibly covered by the coating (areas x4 and x7, Fig. 2a, b). EDS analysis of these sites detected Zr, Cr, F, and higher O concentrations (Table 1). The concentration
of Zr doubled that of Cr, and the concentrations of Cr and F in the coating were similar. The concentration of O was more prominent than on the non-coated sample, and the concentration of
Al was reduced to about 80 at.%. The coating formation suppressed the detection of Al from the substrate. Another important issue concerned the concentration of Si, which is at an area of x4
considerable (5 at.%). The origin of Si is from the IMPs of the underlying substrate (Fig. 1); this result indicates that the coating formed above the Al-Si containing IMPs (areas x4 and
x7) was much thicker than when formed above the α-matrix (areas x3 and x6). The third characteristic area refers to agglomerated grain-like products formed on the coating (exampled by areas
x5 and x8, Fig. 2a, b). The grains are several hundred nanometres up to micrometre-sized. The coating thickness further increased in this area since the grains were much brighter in the CBS
image than the underlying coating (i.e. areas x4 and x7). Indeed, EDS analysis detected higher amounts of Zr, Cr and F (Table 1). In addition, Mg was detected, along with a high Si
concentration of 23 and 27 at.%, i.e. more than five times larger than in the homogeneous coating (areas x4 and x7). Considering the high Si contents detected, the parts of the coating
growing above the area rich in Si (and Mg) are thicker and more abundant than those growing above areas with less Si or α-Al matrix. Notably, the concentration of Al was decreasing in the
order x3, x6 > x4, x7 > x5, x8. The thickness of the Zr-CrCC coating increased in the reverse order. The coating thickness determined at the interface between the first and the second
layer is around 100 nm (Fig. 2d). Above the matrix (x3), the coating is probably a few tens of nanometres thick, reaching above 100 nm in agglomerated parts (x5). Due to the small thickness
of Zr-CrCC on the alloy surface, the adhesion cannot be evaluated with a cross-cut test according to the ASTM D3359–23 standard (see below). During coating formation and growth, some
micro-cracks appeared (arrows in Fig. 2a). This observation is quite common for conversion coatings; the shrinkage in volume induced by tensile stresses was particularly pronounced at
thicker coatings, leading to cracking25,37. Another reason for micro-cracking may be dehydration during drying in the SEM chamber. The inhomogeneity of the coating coverage indicates that
its formation proceeded laterally across the surface, being thicker at some sites at the alloy surface. As described above, the inhomogeneity of the coating thickness and composition are
related to the inhomogeneity of the underlying substrate (Figs. 1, 2); it is the thinnest above the α-matrix (x3), increases above the Al-Si-IMPs (x4) and is the largest above
Al-Si-Mg-containing IMPs (x5). These regions differ in their electrochemical activity as well. In heterogeneous alloys like Al alloys, IMPs represent sites of increased anodic or cathodic
activity; notably, due to the ongoing selective dissolution of individual elements from the IMP, the activity can change from anodic to cathodic during immersion in chloride solution54. In
Al-Si-Mg alloys, IMPs containing Al, Si and Mg are subject to selective dissolution of the less noble metal (Mg or Al). This process depends on the pH; Al and Mg are selectively dissolved in
acidic (Al, Mg) and near-neutral solutions (Mg), leaving behind Si-rich remnants, which then serve as a cathode54,55. The surrounding α-Al matrix acts as an anodic site. Cathodic reaction
at the local cathodic sites, in this case Si-containing IMPs, is represented by reaction (1): $$2{\rm{H}}_{2}{\rm{O}}+{{\rm{O}}}_{2}+4{e}^{-}\to 4{{\rm{OH}}}^{-}$$ (1) Considering the
formation of areas of different thicknesses and morphologies (Fig. 2), it is reasonable to assume that IMPs containing more silicon act as a stronger cathode, readily leading to increased pH
(reaction 1) and faster precipitation of Zr- and Cr-(hydr)oxides. Namely, locally increased pH on the micro-cathodic sites results in the hydrolysis of the soluble fluorometalate precursor
species in the conversion bath34. According to thermodynamic data, the pH values at which the conditions for the precipitation of hydrated metal oxide coating (e.g., ZrO2 × 2H2O) are
established are still in the acidic range, i.e., between 2 and 4−4.5, depending on the concentration of Zr-bearing species34. Using the SurTec® ST650 conversion bath with pH = 3.9, the pH
conditions at which the deposition of the coating at the metal surface are readily achieved. In general, the deposition of Zr(IV) hydroxide from the hexafluorozirconic acid bath proceeds
according to reaction (2)34. $${{\rm{ZrF}}}_{6}^{2-}+4{{\rm{OH}}}^{-}\to {{\rm{ZrO}}}_{2}\times 2{{\rm{H}}}_{2}{\rm{O}}+6{{\rm{F}}}^{-}$$ (2) Once deposited, Zr(IV) hydrated oxide is the
most Zr-stable species at higher pHs34. In addition to the Zr-bearing component, the SurTec® ST650 also contains trivalent Cr salt, resulting in incorporating both Zr and Cr in the
conversion coating, as shown by EDS analysis (Table 1). The deposition of Cr(III) hydroxide is feasible at the pH of the conversion bath. The formation of trivalent Cr hydr(oxide) along
Zr(IV) hydr(oxide) was identified previously by X-ray photoelectron spectroscopy (XPS)17,18,19,20,24,56,57. At pH around 4, the deposition of Ce3+ ions proceeds according to the reaction
(3): $${{\rm{Cr}}}^{3+}+3{{\rm{OH}}}^{-}\to {\rm{Cr}}{({\rm{OH}})}_{3}$$ (3) As shown by EDS analysis, the Zr-CrCC also contains fluoride (Table 1), as observed in previous studies24,25. The
coating can be, therefore, described by a Zr-Cr-(hydro)oxide or Zr-Cr-(hydro)oxyfluoride. The corrosion behaviour of bare and Zr-CrCC-coated alloy was investigated by measuring
electrochemical impedance after one hour of immersion. The selected representative Bode plots shown in Fig. 3 confirmed the differences in the corrosion resistance between ground non-coated
and Zr-CrCC-coated samples in a corrosive medium of 0.1 M NaCl. The Bode plot of impedance magnitude (Fig. 3a) showed the typical behaviour of a ground non-coated AlSi7Mg0.3 sample. For
comparative purposes, the general measure of corrosion resistance can be represented by the value of |_Z_10mHz| in the low-frequency (0.01 Hz) region (Fig. 3a). After 1 h of immersion, the
|_Z_10 mHz| was ~22 kΩ cm2, reflecting relatively low corrosion resistance. The phase angle plot showed the maximum phase angle of −79° at 23 Hz (Fig. 3b). This phenomenon is attributed to
the forming of an aluminium oxide/hydroxide layer on the surface with some pores and defects allowing diffusion paths for corrosive species. These also can be reflected as a minimum phase
angle at low frequencies (0.1 Hz), forming a tail related to the local corrosion process4,58. However, after one week of immersion, the corrosion resistance slightly improved, which can be
ascribed to the densification of the aluminium oxide/hydroxide layer; the |_Z_10 mHz| increased to ~36 kΩ cm2. The tail at low frequencies disappeared, indicating that the degree of the
local corrosion attack did not increase with extended immersion time. The Bode plots of Zr-CrCC coated alloy are also shown in Fig. 3. At high and middle frequencies, the impedance and phase
angle plots did not differ considerably from the non-coated sample, but a more significant difference occurred at low frequencies in the phase angle plot. After 1 h of immersion, the |_Z_10
mHz| for the Zr-CrCC-coated sample was 36 kΩ cm2 (Fig. 3a). The coated sample also showed a broader phase angle plot in the middle frequencies with a maximum phase angle of −81° at 22 Hz
(Fig. 3b). More importantly, no minimum at low frequencies appeared, reflecting the protective nature of the Zr-CrCC. When extending the immersion time to one week, the corrosion protection
by the Zr-CrCC was still efficient, as evidenced by the |_Z_10 mHz| of 72 kΩ cm2 and broadening of the frequency region where the coating shows a capacitive character. Based on performed
electrochemical measurements, it can be stated that the Zr-CrCC, despite being around 100 nanometres thick, enhanced the corrosion protection of the AlSi7Mg0.3 cast alloy in the tested
corrosive medium after short (1 week) immersion. POLYACRYLIC/SILOXANE-SILICA COATING The sols at various preparation steps and the final coating were detailed in our separate paper51. The
polyacrylic siloxane-silica sol (abbreviated as PEHA-SS) was prepared from two separate sols (Fig. 4). Briefly, Sol 1 consists of organic precursor MAPTMS copolymerise with 2-EHA in the
presence of butyl acetate (BA) and benzoyl peroxide (BPO) to start radical copolymerisation between MAPTMS and 2-EHA51. Inorganic precursor TEOS mixed with ethanol is the main component of
Sol 2. The hydrolysis of TEOS was initiated by acidified water. The final hybrid sol-gel solution was obtained by combining Sol 1 and Sol 2. A detailed procedure is given in the Methods
section. The polyacrylic/siloxane-silica coating was evaluated using SEM/EDS (Fig. 5a). The surface of the coating was very smooth, without cracks or visible pores, which confirmed that the
coating evenly covered the alloy surface. SEM analysis of the scribed sample showing the coating interior showed that the coating contained small, randomly arranged silicon-based domains of
a few tens of nanometres in size seen as bright spots (Fig. 5b). Differences in the composition of the coating matrix and silica-rich domains were checked by spot EDS analysis. At point x10,
i.e., at the bulk of the coating, the composition of the coating in weight percentage refers to Si (16.9 at.%) and O (83.1 at.%) (Fig. 5a). These are (in addition to carbon, which EDS
cannot quantitatively evaluate due to the deposition of carbon layer prior the analysis), the main elements in an organosilane coating consisting of 2-EHA, MAPTMS and TEOS. Since only these
two elements are in the spectrum, it can be concluded that the thickness of the coating is in the range of a few micrometres where the beam did not reach the aluminium alloy substrate. The
estimated thickness of the coating along the coating cross-section is around ~9 µm, which is at least 90 × times thicker than Zr-CrCC (Fig. 2d). At the x11 site located on the nano-domain,
the composition differed from point x10 (Fig. 5). Greater amounts of Si and O were detected at site x11 than at site x10, i.e. Si (22.9 at.%) and O (77.1 at.%) referring to the silica
(Si−O−Si) rich domain based on TEOS, as has already been noticed for similar polyacrylate siloxane-silica coatings51,59,60. The presence of these domains confirmed highly condensed coating,
which presents the efficient barrier corrosion protection between corrosion medium and aluminium surface51,59,60. Figure 6 a, b show SE-SEM images along the artificially made scribe of the
AlSi7Mg0.3 surface coated with a PEHA-SS. The coating was removed with a sharp razor. The coating did not crack, but the peeling occurred in certain areas due to scribing, confirmed by
SEM/EDS analysis, as the spectrum x12 had a slightly higher proportion of Si and O (coating residues). The composition of the alloy at x13 was identical to the ground non-coated surface
(Fig. 1a). The adhesion of the PEHA-SS coating was evaluated with an X-cross incision adhesion test (Fig. 6c, d, Supplementary Figure 3). PEHA-SS coating did not visibly peel or form flakes
during the test, which confirmed that the coating adhered firmly to the surface. After deposition, the PEHA-SS coating formed a strong covalent bond Si−O−Al between Si−OH and Al(OH)3, which
provides good adhesion to the alloy surface61. The adhesion of both coatings on the surface was assessed by designation 5B, the best assessment according to standard D3359–2262. After
adhesion testing, the sample surface was characterised with a confocal microscope at the site where the intersection between the incisions occurred (Fig. 6c). There were only small signs of
peeling on the microscopic scale for the alloy coated with the PEHA-SS coating (Fig. 6d). Figure 7a shows the results of electrochemical impedance spectroscopy (EIS) measurements for alloy
coated with PEHA-SS as a function of frequency after 1 h, 1 week, and 4 months. In the Bode diagram, characteristics of the curves at low and medium-high frequencies record the events at the
phase boundary between the coating and the substrate59. In contrast, measurements at high frequencies describe the behaviour of the interface between the coating and the solution after the
sample is exposed to an aggressive corrosive medium. The |Z10mHz| values for PEHA-SS are significantly higher (6 GΩ cm2, for more than five orders of magnitude) than for the ground
non-coated and Zr-CrCC-coated samples (Fig. 3), confirming efficient barrier protection of the alloy surface. These values are comparable with other polyacrylate siloxane-silica
coatings59,60,63. The phase angle values were above −80° over a wide frequency range (over 4–5 decades in the mid- and high-frequency range), showing behaviour close to that known for a
quasi-ideal capacitor (Fig. 7b). Values are related to the capability of the coating to completely blocks the entrance of a corrosive medium confirming that the preparation of hybrid PEHA-SS
coating was adequate to achieve appropriate barrier protection51. The coating was also characterised as a function of immersion time to evaluate its durability. A significant drop in
impedance at the lowest frequencies of about 1.5 decades was observed after 1 week of immersion (Fig. 7a). The changes were also noticed in the phase angle plot because the values above −80°
appeared in a much narrower frequency range (Fig. 7b). At longer times (4 months), the decrease was slighter. Despite the decreasing trend, the impedance value at these frequencies remained
high (42 MΩ cm2). Thus, the lifespan of the coating is limited, but the impedance values remained above 1 MΩ cm2, indicating durable (barrier) corrosion protection during testing time. The
limited durability is probably related mainly to the swelling effect of the organic phase in contact with a corrosive medium (aqueous chloride solution). MULTILAYER ZR-CR BASED AND
POLYACRYLIC/SILOXANE-SILICA COATING The multilayer Zr-CrCC+PEHA-SS coating was prepared by combining Zr-CrCC and PEHA-SS coatings (Supplementary Figure 1). The scribe made on this multilayer
system (Fig. 8) exhibits somewhat different behaviour than observed for only PEHA-SS-coated alloy (Fig. 6). The multilayer coating did not crack (Fig. 8a). Some cracks were noted but
probably caused by dehydration in low vacuum, and electron beam during SEM imaging (Fig. 8b). Again, the peeling of the coating occurred at certain spots along the scribe, but Zr-CrCC
remained on the surface, observed as bright spots along the scribe (area marked with the blue arrow in Fig. 8b). The squared areas were additionally analysed with EDS. The spectrum x14 was
recorded underneath the PEHA-SS coating with the interface with Zr-CrCC. Here, a high content of Si from the PEHA-SS was detected (57.7 at.%) along with Zr (9.1 at.%) and F (0.8 at.%) from
the Zr-CrCC. Notably, Cr was not detected at 5 kV for reasons explained in the above text. The Zr-CrCC remained firmly adhered to the surface and enhanced the adhesion of the PEHA-SS coating
to the substrate. The composition of the scribe (x15) refers to the underlying substrate surface (Fig. 1a). The adhesion of the Zr-CrCC+PEHA-SS coating on the alloy surface was evaluated
with an X-cross incision adhesion test (Fig. 8c, d, Supplementary Fig. 4). The coating multilayer system did not peel or form flakes, which confirms that the coatings adhered firmly to the
surface. The Zr-CrCC yielded greater surface area than ground alloy due to its more rough surface (see Fig. 1a). The Zr-CrCC contains OH− groups64, which can form a strong covalent bond with
the Al alloy surface (Zr−O−Al, Cr−O−Al) and at the same time with polyacrylic/siloxane-silica coating (Zr−O−Si, Cr−O−Si). Hence, increased adhesion results from more surface interactions
and bonding between the pretreated alloy surface and coating due to higher surface area and the chance of bond formation. Therefore, the Zr-CrCC on the AlSi7Mg0.3 surface is a good promotor
of adhesion of the sol-gel coating36,39. The adhesion of the multilayer coating system on the surface was assessed by designation 5B (Fig. 8c, d). Confocal microscope images showed no
cracking or peeling on the microscopic scale. Some differences in the adhesion between the PEHA-SS and Zr-CrCC+PEHA-SS were observed (Fig. 6c, d, Fig. 8c, d and Supplementary Figs. 3, 4).
The coating peeling along the scribe occurred only for the PEHA-SS-coated sample (Fig. 6c, d). In addition, the scribe edges were sharper for the alloy coated with ZrCC+PEHA-SS coating (Fig.
8). This can be related to the enhanced adhesion of the PEHA-SS coating to the Zr-CrCC pretreated aluminium surface. The corrosion properties of multilayer Zr-CrCC+PEHA-SS coating were
evaluated using EIS measurements as a function of immersion time. The results are presented as Bode plots (a) impedance magnitude and (b) phase angle (Fig. 9). After 1 h of immersion, the
Zr-CrCC+PEHA-SS coating exhibited an impedance magnitude value of |Z10 mHz | = 11.3 GΩ cm2 (Fig. 9a, c), one order of magnitude higher than PEHA-SS coating (Fig. 7a). Figure 9b shows the
negative phase angle as a function of frequency. A Zr-CrCC+PEHA-SS coating has a constant phase angle of almost −90° at an even broader frequency range than PEHA-SS, reflecting the
insulating properties of the multilayer coating. EIS measurements were performed for an extended period to evaluate the corrosion protection of the polyacrylic/siloxane-silica coating and
the Zr-CrCC+PEHA-SS coating (1 hour, 1 week and 4 months). At prolonged immersion, a decrease in impedance values was again noticed at lower frequencies, but the drop at low frequencies was
much lower than for PEHA-SS (Figs. 7 and 9). Impedance values remained at almost 0.9 GΩ cm2 (Fig. 9c). The durability of the coating could also be confirmed by the phase angle curves, which
remained unchanged (Fig. 9b). This is related to the presence of the Zr-CrCC inner layer, which contributes an additional degree of protection and promotes the adhesion of the upper PEHA-SS
coating on the surface. Their behaviour is similar to other multilayer systems on other metals consisting of conversion coating and polymer coating36,42,46,48,49,65,66,67, despite the
multilayer Zr-CrCC+PEHA-SS coating being much thinner compared to systems including polymer coatings. This result represents a good starting point for further improvements in coating
deposition. The corrosion protection of metals used for various industrial applications typically requires a salt-spray corrosion test following the standard ASTM B117. Figure 9 shows the
test results after selected periods (1 day, 2 days, 4 days, and 1 week) for the uncoated sample (Fig. 10a), Zr-CrCC-coated sample (Fig. 10b), PEHA-SS-coated sample (Fig. 10c), and
Zr-CrCC+PEHA-SS-coated sample (Fig. 10d). After just one day, the first corrosion products appeared on the non-coated AlSi7Mg0.3 sample (Fig. 10a). The surface colour changed from light grey
to dark grey, probably related to oxidation and the formation of hydrolysed aluminium oxide layer containing some chloride (see below). White spots of corrosion products (presumably
Al(OH)2Cl) were observed at localised sites. The amount of these corrosion products increased rapidly with the exposure time, and at the same time, the number of white spots increased. After
one week, the surface was completely covered with a thick layer of corrosion products with many white dots distributed over the entire surface. The Zr-CrCC-coated sample showed better
corrosion resistance than the uncoated (Fig. 10b). In individual spots, corrosion products appeared on the surface after 2 days, while most of the surface remained protected. More corrosion
products were observed after 4 days, but corrosion progressed slowly and remained localised. The PEHA-SS-coated sample expressed more durable barrier protection during the testing period.
The corrosion occurred mainly in the scribed area, where dark corrosion products were observed after 1 day (Fig. 10c). Two common compounds of a dark layer of corrosion products on the
AlSi7Mg0.3 surface (magnesium-containing alloy) are magnesium hydroxide (Mg(OH)2) and magnesium oxide (MgO). Both of these compounds can contribute to the darkening of surfaces over time
during exposure to moisture in the air. Additionally, magnesium can form other corrosion products depending on the specific environmental conditions it is exposed to, such as (MgCO3) or
magnesium chloride (MgCl2) when exposed to carbon dioxide or chloride ions, respectively. The amount of corrosion products then increased further after a more extended testing period. The
multilayer protection (Zr-CrCC+PEHA-SS) achieved the most efficient corrosion protection. Corrosion products were only visible along with the scribe. At the same time, they were not detected
on the coated area of the sample (Fig. 10d). The corrosion along the scribe was significantly reduced compared to the alloy coated with only a PEHA-SS (Fig. 10c). The Zr-CrCC thus acts as a
source of complementary protection12,36 and, together with barrier protection ensured by the polyacrylic/siloxane-silica coating, results in long-lasting protection of the underlying
substrate. After salt spray testing for two days, the parts of the scribed areas (Supplementary Figure 5) were evaluated by SEM and point and mapping EDS analyses to assess the complementary
protection of the Zr-CrCC coating. The scribed area of the PEHA-SS-coated AlSi7Mg0.3 sample showed areas exposing the underlying substrate and areas covered by corrosion products (Fig. 11
and EDS mapping in Supplementary Fig. 6). The layer of corrosion products was relatively thick and rather voluminous, containing Al, Si, O and some Cl (S is probably the impurity from the
solution) (site x17). The area not covered by corrosion products (site x18) contained more Al and Si originating from the substrate. Once exposed to salt spraying, the substrate within the
scribe underwent selective anodic dissolution of more active metals (e.g. Mg and Al), resulting in a more voluminous corrosion product, which may redeposit at the surface. The PEHA-SS
coating along the scribe (site x16) showed a composition similar to that given in Fig. 8b, site x12. The slight difference is due to the presence of Al and Na related to the non-soluble
salts, such as aluminium oxides/hydroxides formed at the surface when exposed to a corrosive medium (NaCl). Local corrosion attack was not observed in the scribed areas on the sample coated
with Zr-CrCC+PEHA-SS coating (Fig. 12 and Supplementary Fig. 7). In contrast to the PEHA-SS-coated sample (Fig. 11), no voluminous corrosion products were observed. The scribed area (site
x21) showed only 6 at.% of oxygen compared to up to 55 at.% for the PEHA-SS, sites x17 in Fig. 11. Furthermore, some Mg was detected, indicating that the selective dissolution of Mg was
inhibited, which was not the case for the PEHA-SS-coated sample (Fig. 11). EDS maps in Supplementary Figures 6 and 7 show the difference in contents of Al and O within the scribe. The
scribed area was additionally analysed at higher magnification (x22 and x23). No elements related to corrosion (Na, Cl) were detected. A notable finding is that in addition to substrate
elements, those originating from Zr-CrCC were detected: Zr, Cr and F (area x22). This means that the scribed area was recovered after exposure to salt spray by spreading the Zr-CrCC within
the scribe. In addition to Zr, Cr was identified. The spreading of the Zr-CrCC by the self-release of Zr and Cr resulted in the prevention or mitigation of corrosion of the underlying
substrate, as proven by the salt spray test (Fig. 10d). At the area along the scribe x20, Zr, F and Cr related to Zr-CrCC coating were also detected, whereas the upper layer consisted of
PEHA-SS (spot x19). The presence of Cr and Zr within the scribe is evidenced by the EDS mapping (Supplementary Fig. 7). CONTRIBUTION OF INDIVIDUAL COATINGS TO THE PROTECTION MECHANISM OF
MULTILAYER COATING The experimental observations confirmed that the multilayer Zr-CrCC+PEHA-SS deposited on AlSi7Mg0.3 was less susceptible to corrosion attack, evidenced by the absence of
corrosion products in the scribed area. Evidence also showed that the Zr-CrCC contributed to active corrosion protection since the elements originating from the conversion coating (Zr, Cr)
were identified within the scribe. This process supposedly involves the release of Zr and Cr species and their transfer and formation of related (hydro)oxides at the sites within the scribe.
Although the exact mechanism cannot be postulated based solely on the results presented in this study, some premises can be proposed, supported by the literature data on similar systems.
The protection mechanisms of the scribed PEHA-SS and Zr-CrCC+PEHA-SS coatings are schematically proposed in Fig. 13. During Zr-CrCC coating formation in an acidic conversion bath (pH ≈ 3.9),
less noble metals such as Mg and Al are dissolved, additionally supported by the action of fluoride ions to attack the thin native oxide layer, thus facilitating access of ZrF62 − species
to the metal substrate (reaction 2). In the absence of F-containing species in the solution, the favourable reaction is the formation of Zr hydroxide through the chemical reaction of
hydrolysis34: $$m{{\rm{Zr}}}^{{4}_{+}}+q{{\rm{H}}}_{2}{\rm{O}}\rightleftharpoons {{\rm{Zr}}}_{m}{({\rm{OH}})}_{{\rm{q}}}^{{({\rm{4m}}-{\rm{q}})}_{+}}+q{{\rm{H}}}^{+}$$ (4) The role of
fluoride in the conversion baths is dual: (i) thinning of the oxide layer formed spontaneously on the metal surface and (ii) shift the pH at which Zr hydroxide precipitates to higher pH
values (between 3 and 6 depending on the concentration of Zr species) due to the formation of stable ZrFq4-q complexes34; the increase of precipitation pH has an important practical meaning
since it is beneficial for aluminium substrate tending to corrode at acidic pH12,30,34. The concomitant cathodic reaction (1) of oxygen reduction provides the conditions for the deposition
of Zr and Cr (hydr)oxides formed through reactions (2) and (3) once the pH reaches values around 434. The Zr-CrCC layer, around 100 nanometres thick (Fig. 2), acts as a first line of
defence, preventing further oxidation of the alloy in a corrosive medium for the first days of testing (Fig. 10b). Similar Zr/Cr-containing coatings were investigated in the literature. Li
et al. reported that the Alodine® T5900 (Henkel) comprised a biphasic structure of hydrated zirconia (ZrO2·2H2O) mixed with Cr(III) (hydr)oxides (Cr(OH)3, CrOOH and Cr2O3) precipitated above
the fluoroaluminate interlayer on AA202468,69. The distribution of Cr was not uniform throughout the coating but was elevated around the pits. The coating provided both anodic and cathodic
protection by physically blocking Al-rich sites and Cu-rich IMPs68. Guo and Frenkel found that the Cr content in the Alodine® T5900 (Henkel) coating was only one-fourth of the Zr content.
The coating thickness was 40−120 nm, considerably thicker than the conversion coating without Cr species70. Qi et al. reported17,18 that SurTec® ST650 (SurTec) contained ZrO2, ZrF4, Cr(OH)3,
Cr2(SO4)3, CrF3, CrO3, and CrO42 − , similar to our previous study24. Nine micrometres thick PEHA-SS sol-gel forms a smooth, uniform coating that acts as a barrier to the dissolution of the
underlying substrate (Fig. 7). However, the locally damaged, scribed PEHA-SS surface represents the starting point for the exposure of underlying AlSi7Mg0.3 substrate to the corrosive
environment (Fig. 10c), schematically presented in Fig. 13c. As a result, the aluminium and magnesium ions are dissolved, forming corrosion products consisting mainly of hydrated aluminium
oxide containing chloride (Al(OH)2Cl). Due to the absence of any active protection, the area freely corrodes in the corrosion medium (Fig. 13c). A multilayer coating system (Fig. 13b)
consisting of a thin Zr-CrCC and micrometre-thick hybrid sol-gel (PEHA-SS) coating provides (i) less coating peeling and (ii) durable active corrosion protection once damaged (Fig. 13d, f).
These experimental findings can be substantiated as follows. (i) Due to the presence of Zr-CrCC, better adhesion of PEHA-SS to the alloy surface is achieved, resulting in less peeling along
the scribe (Fig. 8). PEHA-SS coating offers a dense polymerised Si−O−Si structure (Fig. 13e) due to the combined benefits of organic and inorganic components. With a Zr-CrCC as an
interlayer, strong covalent bonds Al−O−Zr, Al−O−Cr, Cr−O−Si, and Zr−O−Si are formed34,36, and the coating firmly adhering to the alloy surface, preventing the coating from peeling (Figs. 8,
10d, 12). The beneficial effect on the conversion coating as an interlayer between the substrate and outer organic-based coating are in line with the results published for similar
systems36,38,43,48. (ii) Zr-CrCC redeposition at the scribed surface acts as an active inhibition and repassivates the damaged area along the scribe (Fig. 12). In fact, the ability of active
inhibition of conversion coatings containing trivalent chromium was already reported70 and connected to the possibility of transient formation of Cr(VI) species during more extended
immersion in the electrolyte, as shown by Raman spectroscopy68,69. The latter process would be enabled by the locally produced H2O2 as a product of oxygen reduction at the cathodic sites
according to: $$2{{\rm{H}}}_{2}{\rm{O}}+{{\rm{O}}}_{2}+2{{\rm{e}}}^{-}\to {{\rm{H}}}_{2}{{\rm{O}}}_{2}+2{{\rm{OH}}}^{-}$$ (5) Hydrogen peroxide would then act as an oxidation agent for
Cr(III) species69 through reaction (6); chromate species formed are then reduced, resulting in the formation of passivating Cr(III) hydroxide through reaction (7):
$$2{\rm{Cr}}{({\rm{OH}})}_{3}+3{{\rm{H}}}_{2}{{\rm{O}}}_{2}+4{{\rm{OH}}}^{-}\to 2{{\rm{CrO}}}_{4}^{2-}+8{{\rm{H}}}_{2}{\rm{O}}$$ (6)
$${{\rm{Al}}+{\rm{CrO}}}_{4}^{2-}+4{{\rm{OH}}}^{-}+4{{\rm{H}}}_{2}{\rm{O}}\to {[{\rm{Al}}{({\rm{OH}})}_{6}]}^{3-}+{[{\rm{Cr}}{({\rm{OH}})}_{6}]}^{3-}$$ (7) It was proposed that Cr(VI)
species are mobile and thus be involved in repairing damaged sites69, similar to the mechanism of chromate conversion coatings but with a much smaller concentration of Cr(VI) species. Using
the artificial scratch cell70, Guo and Frankel proved that Cr species, in contrast to Zr, were detected in the exposed electrolyte and could thus be released and transferred to distant
sites. As shown by XPS, the amount of Cr(VI) species is small, i.e. less than 3% of the total chromium17 and 0.1 to 1% of the coating weight18. Whether the transient formation of Cr(VI)
species is responsible for the active protection by Zr-CrCC observed in this study cannot be stated since speciation is impossible using only EDS. In our previous study on SurTec® ST650
coating, XPS could not identify chromate species unambiguously due to the overlapping with CrF3 peaks24. If present, their concentration would be minimal. However, the results presented in
this study prove that the active corrosion protection mechanism is operative. Another possible mechanism, not necessarily involving the formation of chromate species, can be suggested.
Namely, the Zr-CrCC can be regarded as condensed sol going through olation (reaction 8) and oxolation (reaction 9), resulting in polymerisation accompanied by the release of water
molecules34: $${\rm{M}}-{\rm{OH}}+{\rm{M}}-{{\rm{OH}}}_{2}\to {\rm{M}}-{\rm{OH}}-{\rm{M}}+{{\rm{H}}}_{2}{\rm{O}}$$ (8) $${\rm{M}}-{\rm{OH}}+{\rm{HO}}-{\rm{M}}\to
{\rm{M}}-{\rm{O}}-{\rm{M}}+{{\rm{H}}}_{2}{\rm{O}}$$ (9) where M is Zr or Cr (Fig. 13f). Both Zr and Cr belong to classes of elements which form polycations involved in the condensation of
metal complexes in solution, in which the cations are linked by hydroxo (HO−) or oxo (O2−) bridges. The condensation is initiated by hydroxylation71.
$${[{\rm{M}}{({{\rm{OH}}}_{2})}_{6}]}^{3+}+{{\rm{H}}}_{2}{\rm{O}}\to {[{\rm{M}}({\rm{OH}}){({{\rm{OH}}}_{2})}_{5}]}^{2+}+{{\rm{H}}}_{3}{{\rm{O}}}_{{\rm{solv}}}^{+}$$ (10) In the case of Zr,
a general hydrolysis model for mononuclear and polynuclear Zr-species can be presented as reaction (11)71:
$${{\rm{Zr}}({\rm{H}}}_{2}{{\rm{O}})}_{{\rm{N}}}^{4+}+q{{\rm{H}}}_{2}{\rm{O}}\rightleftarrows
{{\rm{Zr}}({\rm{OH}})}_{{\rm{q}}}{{({\rm{H}}}_{2}{\rm{O}})}_{{\rm{N}}-{\rm{q}}}^{(4-{\rm{q}})+}+{q}{{\rm{H}}}^{+}$$ (11) Tetrameric species, Zr4(OH)88+(aq), or more precisely
[Zr4(OH)8(OH2)16]8+, were shown to be the main building block of ZrCC34,72. In the case of Cr, dimer [Cr2(OH)2(OH2)8]4+, trimer [Cr3(OH)4(OH2)9]5+ and tetramer [Cr4(OH)6(OH2)10]6+ may form
among which is the trimer stable and most chemically inert of all Cr polycations71. The distribution, however, depends on the Cr species concentration and pH71. Entities of polycations are
linked by [H3O2]− double bridges ligands. Since the Cr polycations are inert towards ligand substitution, the structure, by ageing, is transformed into characteristic amorphous chromium gels
and oxyhydroxide. It has already been proposed that the formation of chromate conversion coatings is consistent with sol-gel chemistry principles73, so similar consideration of
hydrolisation and condensation may also be viable for trivalent chromium coatings without necessarily forming chromate species17,74. The released water molecules, reactions (8) and (9),
facilitate the diffusion of Zr and also Cr species within the coating, filling the gaps and defects in the damaged regions caused by the dissolution of aluminium exposed to the chloride
medium (Fig. 13f). Zr and Cr species react with atmospheric oxygen and moisture to form a new layer of Zr/Cr hydroxide, effectively sealing the damaged region. Further polymerisation of the
Zr and Cr species results in forming new bonds and creating a network of Zr− O − Zr34,61 and Cr − O − Cr57,74,75, which gradually regenerates the Zr/Cr-based protective layer, effectively
healing the damaged region23,29 (Fig. 13f). This regeneration can occur over a certain period, and the coating can regain its protective properties. The mechanism of active corrosion
prevention along the scribe can be thus explained as a combination of chemical reactions and physical processes (Fig. 13f). These findings are consistent with previous studies of
self-healing coatings23,69,70,73,76 and were confirmed with EDS analysis along the scribe (e.g., during salt spray testing) presented in Figs. 8, 10, 12 and Supplementary Fig. 7. Overall,
the active properties of Zr-CrCC contribute to the long-term, four-months long corrosion protection of multilayer Zr-CrCC+PEHA-SS by continuously repairing and maintaining the integrity of
the coating, thus prolonging the service life of the coated aluminium alloy. It is noteworthy, however, that the active corrosion properties of Zr-CrCC are generally effective for minor
damages, such as a scribe or other small defects. Severe or extensive damage may exceed the self-healing capabilities of the coating. The specific rate and effectiveness of self-healing can
vary depending on factors such as the coating formulation, environmental conditions, and the extent of damage. In summary, this study showed that the two modes of corrosion protection of
AlSi7Mg0.3 using hexafluoro-zirconated trivalent chromium coating Zr-CrCC (as primary active corrosion protection) and polyacrylic/siloxane-silica coating PEHA-SS (as barrier protection)
achieved a complementary effect when deposited as a multilayer system. Albeit differing in thickness, morphology and composition, individual layers achieved synergy when used as a
multilayer. The novelty of the study lies in developing the multilayer corrosion protection comprising a thin Zr-CrCC and several micrometres thick sol-gel coatings, in contrast to commonly
used organic coatings several tens of micrometres thick. Further, the majority of previous studies have been performed on wrought Al alloys; the possibility to effectively protect cast Al
alloys is of importance to their increasing use in industry due to their economic advantages in terms of shorter processing cycles and assembly costs. These promising results offer an
insight into the interfacial process of the multilayer and represent a good basis for further studies focusing on combining several types of coatings to achieve the optimal multilayer system
mutually supplying each other advantageous properties. It was postulated that the sol-gel nature of zirconium and chromium oxyhydroxides is related to the self-healing ability of Zr-Cr
conversion coatings. METHODS METAL SUBSTRATE The study was performed on the aluminium alloy AlSi7Mg0.3 (EN AC-42100) distributed by Talum d. d., Slovenia. The alloy composition is given in
weight percentage in Table 2. The bulk plate was cut into cuboids sized 6 cm × 4 cm × 1 cm. The sample surface was ground with a LaboPol-6 grinding machine (Struers) with SiC emery papers of
grades 320, 500, 800, 1000, 1200, and 2400 distributed by Struers. Grinding was carried out in tap water to remove grinding residues and prevent local overheating of the material. The
process was carried out until the oxide layer and other impurities were removed from the surface to obtain an evenly ground surface. After grinding, the samples were rinsed with deionised
water, followed by a two-minute cleaning by immersion in ethanol (99%, Carlo Erba) in an ultrasonic bath. COATING DEPOSITION Ground metal samples were subject to the three-step process:
degreasing with alkaline cleaner, acid desmutting and passivation (Table 3). Commercial reagents supplied by SurTec® (SurTec International GmbH) were used; their concentrations and related
parameters (immersion times and bath temperatures), as recommended by the producer, are summarised in Table 3. Following standard procedure, the degreasing, desmutting and formation of
conversion coating (passivation) were performed in a 250-mL polyethylene cup. ST061® is multi-metal degreasing for light metals, SurTec® ST089 is a recyclable soak detergent SurTec® 089
contains non-ionic surfactant alcohols such as amines, coco alkyl, and ethoxylated fatty alcohol. SurTec® ST496 is a standard desmutter for aerospace, electronics, and automotive
applications, applicable for desmutting Si-, Zn-, and Cu- containing aluminium alloys. SurTec® ST650, a hexafluoro-zirconate conversion bath with trivalent chromium Cr(III), was used to
prepare the final coating according to the conditions in Table 2. The commercial conversion coating SurTec® ST650 in the text was denoted as Zr-CrCC. For SEM analysis of the microstructure
(Fig. 1b), non-coated AlSi7Mg0.7 samples were water-ground successively using SiC-paper up to 4000 grit and then diamond-polished using polishing cloth (MD-Nap, Struers), non-water polishing
using 1 μm diamond paste (DP-Paste P, Struers) and alcohol-based lubricant (DP-Lubricant Blue, Struers). The polyacrylic/siloxane-silica (hybrid sol-gel) sols were synthesised from51:
acrylate monomer 2-ethylhexyl acrylate (2-EHA; > 99%, Sigma-Aldrich), initiator benzoyl peroxide (BPO, > 99%, Aldrich), solvent butyl acetate (BA; > 99%, Sigma-Aldrich), the
organically modified precursor [3-(methacryloyloxy)propyl]trimethoxysilane (also known as 3-(trimethoxysilyl)propyl methacrylate or silane A174) (MAPTMS, > 98%, Sigma-Aldrich), and the
inorganic precursor tetraethyl orthosilicate (TEOS, 99.9%, Aldrich), HNO3 ( > 70%, Sigma-Aldrich), deionised water prepared with a Milli-Q direct instrument, with an electrical
resistivity of water of 18.2 MΩ cm at 25 °C (Millipore) and anhydrous ethanol (99%, Carlo Erba). The synthesis steps of the sol-gel are shown in Fig. 4. Sol 1 was prepared from 0.128 g of
BPO, 14 mL of BA, 1.888 mL of MAPTMS and 10.5 mL of 2-EHA while stirring. The reaction mixture (Sol 1) was heated at reflux (at ∼ 130 °C) for one hour. In the meantime, the inorganic sol was
prepared (Sol 2) from 4.2 mL of TEOS in a 25 mL reactor and 9.3 mL of ethanol. The flask was placed on a magnetic stirrer, and 0.7 mL of H2O/HNO3 solution (pH 1.0) was added dropwise with
constant stirring. The mixture was then stirred for 15 minutes at room temperature. After 1 h of refluxing Sol 1, the reaction mixture was cooled to ambient temperature. Then, the prepared
Sol 2 was added dropwise at constant stirring in Sol 1. After combining, the mixture was stirred for another hour at room temperature. The prepared polyacrylic/siloxane-silica coating was
applied to the pre-prepared surface of the alloys by dipping method with a dip-coater RDC 15 (Bungard). The one-step deposition of the coating was performed with a dip- and pull-rate speed
of 14 cm/min. The sample was immersed in the sol for 3 s. The deposited coatings on alloys were thermally cured in the oven, where the temperature was slowly increased (heating rate 5
°C/min) to a final temperature of 180 °C. The curing lasted 1 h. SURFACE AND COATING CHARACTERISATION The morphology of the ground alloy AlSi7Mg0.3 samples and samples coated with Zr-CrCC,
PEHA-SS and Zr-CrCC+PEHA-SS was analysed with a field emission electron microscope (FESEM) FEI Helios Nanolab 650 Dual beam associated with energy dispersive X-ray spectrometer (EDS) Oxford
Instruments X-max SDD (50 mm2), using Aztec software. Before analysis, the sol-gel coatings were scribed with a diamond tip; the thickness of the coating was determined at the scribe. The
samples were coated with a thin carbon layer with BAL-TEC SCD 005. Samples’ surface imaging was performed with secondary electrons (SE) and a circular backscatter detector (CBS) at
acceleration voltages of 0.5 kV, 2 kV, 5 kV, 10 kV or 15 kV. The chemical composition of the selected areas on the surface was analysed using EDS at 5 and 10 kV in point and mapping modes.
The cross-hatch tester kit (brand Cgoldenwall) was used to test the adhesion of AlSi7Mg0.3 samples coated with PEHA-SS and Zr-CrCC+PEHA-SS. Coatings were cross-hatched with the specified
tool (diamond razors) to produce a net-patterned surface (Supplementary Fig. 4a). Finally, the patterned surface was covered with # 810 ScotchMagicTM tape, which was pressed firmly against
the surface and slowly peeled off. Based on the fragments removed from the coating, the degree of adhesion was assessed according to ASTM standard D3359–23 (ISO-2409)62 on a scale from 0B to
5B. After the adhesion test, the selected crossed area was additionally characterised with the confocal microscope at 10× and 20× magnification (Axio, CSM 700, Zeiss, Göttingen, Germany and
Axio CSM 700 3D software). Electrochemical measurements were performed in a three-electrode system in a 250 mL corrosion cell at room temperature. The samples were fixed to the cell with a
holder, and the exposed sample surface (1 cm2) served as the working electrode. The reference electrode was a saturated silver/silver chloride electrode (Ag/AgCl) with _E_ = 0.197 V vs.
standard hydrogen electrode, and a graphite rod with a diameter of 5 mm acted as a counter electrode. Measurements were performed with a potentiostat/galvanostat Autolab 204 M (Metrohm
Autolab) with Nova 2.1 software to control the measurement and analyse the obtained data. A corrosive medium solution (0.1 M NaCl) was prepared using sodium chloride ( > 99.5%, Fisher)
and deionised water (Milli-Q Direct). Electrochemical impedance spectroscopy was performed in the frequency range between 10 mHz and 100 kHz. For ground and ZrCC-coated samples, the EIS
measurements were conducted after 1 h of immersion in 0.1 M NaCl. For the samples coated with PEHA-SS and Zr-CrCC+PEHA-SS coatings, measurements were also performed after 1 week and 4 months
of immersion in the tested medium. The measurements were performed at least in triplicate, and the representative one was presented in graphs. The corrosion testing in a salt-spray chamber
was conducted according to the international standard ASTM B11777. The test was performed in a controlled atmosphere in a chamber with a volume of 0.17 m3 (ASCOTT). NaCl solution (_γ_ = 58.5
g/L) was pumped into the chamber through the filter columns. The temperature in the chamber was 35 °C ± 2 °C. Before testing, the edges of the samples were protected with adhesive tape to
minimise the corrosion of unprotected parts. The X-cross was made with a sharp diamond cutter on the sample coated with the PEHA-SS coating. The samples were placed into a plastic holder at
an angle of 45°. The sample surface was photographed at the selected time up to 168 h (7 days). After 2 days of exposure, the samples were also analysed along with the scribe using SEM/EDS.
DATA AVAILABILITY The data that support the findings of this study are available from the corresponding author upon reasonable request. REFERENCES * Berlanga-Labari, C., Biezma-Moraleda, M.
V. & Rivero, P. J. Corrosion of Cast Aluminum Alloys: A Review. _Metals_ 10, 1384 (2020). Article CAS Google Scholar * Davis, J. R. _Corrosion of Aluminum and Aluminum Alloys_. (ASM
International, Novelty, Ohio, The United States of America, 1999). * Kuchariková, L., Liptáková, T., Tillová, E., Kajánek, D. & Schmidová, E. Role of Chemical Composition in Corrosion of
Aluminum Alloys. _Metals_ 8, 581 (2018). Article Google Scholar * Milošev, I. et al. Mechanism of Corrosion of Cast Aluminum-Silicon Alloys in Seawater. Part 1: Characterization and Field
Testing of Bare Alloys in the Adriatic Sea. _Corrosion_ 79, 193–212 (2022). Article Google Scholar * Zhao, J. et al. Effects of chromate and chromate conversion coatings on corrosion of
aluminum alloy 2024-T3. _Surf. Coat. Technol._ 140, 51–57 (2001). Article CAS Google Scholar * Kendig, M. W. & Buchheit, R. G. Corrosion Inhibition of Aluminum and Aluminum Alloys by
Soluble Chromates, Chromate Coatings, and Chromate-Free Coatings. _Corrosion_ 59, 379–400 (2003). Article CAS Google Scholar * Gharbi, O., Thomas, S., Smith, C. & Birbilis, N.
Chromate replacement: what does the future hold? _Npj Mater. Degrad._ 12, 1–8 (2018). Google Scholar * Becker, M. Chromate-free chemical conversion coatings for aluminum alloys. _Corros.
Rev._ 37, 321–342 (2019). Article CAS Google Scholar * Santa Coloma, P. et al. Chromium-free conversion coatings based on inorganic salts (Zr/Ti/Mn/Mo) for aluminum alloys used in
aircraft applications. _Appl. Surf. Sci._ 345, 24–35 (2015). Article CAS Google Scholar * Chauhan, L. R. et al. Development of eco-friendly chemical conversion coating for aluminium
substrate. _J. Indian Chem. Soc._ 99, 100392 (2022). Article CAS Google Scholar * Cristoforetti, A., Fedel, M., Deflorian, F. & Rossi, S. Influence of deposition parameters on the
behavior of nitro-cobalt-based and Ti-hexafluoride-based pretreatments. _J. Coat. Technol. Res._ 19, 859–873 (2022). Article CAS Google Scholar * Milošev, I. & Frankel, G. S.
Review—Conversion Coatings Based on Zirconium and/or Titanium. _J. Electrochem. Soc._ 165, C127–C144 (2018). Article Google Scholar * Dabalà, M., Armelao, L., Buchberger, A. &
Calliari, I. Cerium-based conversion layers on aluminum alloys. _Appl. Surf. Sci._ 172, 312–322 (2001). Article Google Scholar * Sainis, S. & Zanella, C. A Study of the Localized Ceria
Coating Deposition on Fe-Rich Intermetallics in an AlSiFe Cast Alloy. _Materials_ 14, 3058 (2021). Article CAS PubMed PubMed Central Google Scholar * Harvey, T. G. Cerium-based
conversion coatings on aluminium alloys: a process review. _Corros. Eng. Sci. Technol._ 48, 248–269 (2013). Article CAS Google Scholar * Rodič, P., Milošev, I. & Frankel, G. S.
Corrosion of Synthetic Intermetallic Compounds and AA7075-T6 in Dilute Harrison’s Solution and Inhibition by Cerium(III) Salts. _J. Electrochem. Soc._ 170, 031503 (2023). Article Google
Scholar * Qi, J.-T. et al. Trivalent chromium conversion coating formation on aluminium. _Surf. Coat. Technol._ 280, 317–329 (2015). Article CAS Google Scholar * Qi, J. et al. Influence
of pre- and post-treatments on formation of a trivalent chromium conversion coating on AA2024 alloy. _Thin Solid Films_ 616, 270–278 (2016). Article CAS Google Scholar * Qi, J.,
Światowska, J., Skeldon, P. & Marcus, P. Chromium valence change in trivalent chromium conversion coatings on aluminium deposited under applied potentials. _Corros. Sci._ 167, 108482
(2020). Article CAS Google Scholar * Li, L., Doran, K. P. & Swain, G. M. Electrochemical Characterization of Trivalent Chromium Process (TCP) Coatings on Aluminum Alloys 6061 and
7075. _J. Electrochem. Soc._ 160, C396 (2013). Article CAS Google Scholar * George, F. O., Skeldon, P. & Thompson, G. E. Formation of zirconium-based conversion coatings on aluminium
and Al–Cu alloys. _Corros. Sci._ 65, 231–237 (2012). Article CAS Google Scholar * Golru, S. S., Attar, M. M. & Ramezanzadeh, B. Morphological analysis and corrosion performance of
zirconium based conversion coating on the aluminum alloy 1050. _J. Ind. Eng. Chem._ 24, 233–244 (2015). Article CAS Google Scholar * Yoganandan, G., Pradeep Premkumar, K. & Balaraju,
J. N. Evaluation of corrosion resistance and self-healing behavior of zirconium–cerium conversion coating developed on AA2024 alloy. _Surf. Coat. Technol._ 270, 249–258 (2015). Article CAS
Google Scholar * Mujdrica Kim, M., Kapun, B., Tiringer, U., Šekularac, G. & Milošev, I. Protection of Aluminum Alloy 3003 in Sodium Chloride and Simulated Acid Rain Solutions by
Commercial Conversion Coatings Containing Zr and Cr. _Coatings_ 9, 563 (2019). Article Google Scholar * Šekularac, G., Kovač, J. & Milošev, I. Prolonged protection, by zirconium
conversion coatings, of AlSi7Mg0.3 aluminium alloy in chloride solution. _Corros. Sci._ 169, 108615 (2020). Article Google Scholar * Zhou, P. et al. Critical role of pretreatment on the
corrosion resistance of Zr conversion coating on 6061 aluminum alloy: The combined effect of surface topography and potential difference between different phases. _Surf. Coat. Technol._ 377,
124904 (2019). Article CAS Google Scholar * Liu, X. et al. Environmentally Friendly Zr-Based Conversion Nanocoatings for Corrosion Inhibition of Metal Surfaces Evaluated by Multimodal
X-ray Analysis. _ACS Appl. Nano Mater._ 2, 1920–1929 (2019). Article CAS Google Scholar * Nabizadeh, M. et al. Unraveling the formation mechanism of hybrid Zr conversion coating on
advanced high strength stainless steels. _Surf. Coat. Technol._ 441, 128567 (2022). Article CAS Google Scholar * Zhan, W. et al. Preparation and Characterization of Synchronous Chemical
Conversion Coating on 6061 Aluminum Alloy/7075 Aluminum Alloy/Galvanized Steel Substrates. _Metals_ 12, 2011 (2022). Article CAS Google Scholar * Samaei, A. & Chaudhuri, S. Role of
zirconium conversion coating in corrosion performance of aluminum alloys: An integrated first-principles and multiphysics modeling approach. _Electrochim. Acta._ 433, 141195 (2022). Article
CAS Google Scholar * Fockaert, L. I. et al. Effect of organic additives in fluoacid-based Ti and Zr-treatments for galvanized steel on the stability of a polymer coated interface. _Prog.
Org. Coat._ 146, 105738 (2020). Article CAS Google Scholar * Cerezo, J. et al. The effect of surface pre-conditioning treatments on the local composition of Zr-based conversion coatings
formed on aluminium alloys. _Appl. Surf. Sci._ 366, 339–347 (2016). Article CAS Google Scholar * Shen, G.-T., Chen, S.-Y., Huang, C.-Y. & Lin, C.-S. Microstructural evolution and
corrosion behavior of constituent particles of AA2024-T3 Al alloy during zirconium conversion coating. _Appl. Surf. Sci._ 635, 157657 (2023). Article CAS Google Scholar * Kraš, A. &
Milošev, I. The Aqueous Chemistry of Zirconium as a Basis for Better Understanding the Formation of Zirconium Conversion Coatings: Updated Thermodynamic Data. _J. Electrochem. Soc._ 170,
021508 (2023). Article Google Scholar * Pourbaix, M. _Atlas of Electrochemical Equilibria in Aqueous Solutions_. (National Association of Corrosion Engineers, 1974). * Asemani, H. R.,
Ahmadi, P., Sarabi, A. A. & Eivaz Mohammadloo, H. Effect of zirconium conversion coating: Adhesion and anti-corrosion properties of epoxy organic coating containing zinc aluminum
polyphosphate (ZAPP) pigment on carbon mild steel. _Prog. Org. Coat._ 94, 18–27 (2016). Article CAS Google Scholar * Moreira, V. B., Meneguzzi, A., Jiménez-Piqué, E., Alemán, C. &
Armelin, E. Aluminum Protection by Using Green Zirconium Oxide Layer and Organic Coating: An Efficient and Adherent Dual System. _Sustainability_ 13, 9688 (2021). Article CAS Google
Scholar * Liu, Q. et al. Investigation on adhesion strength and corrosion resistance of Ti-Zr aminotrimethylene phosphonic acid composite conversion coating on 7A52 aluminum alloy. _Appl.
Surf. Sci._ 458, 350–359 (2018). Article CAS Google Scholar * Ghanbari, A. & Attar, M. M. Surface free energy characterization and adhesion performance of mild steel treated based on
zirconium conversion coating: A comparative study. _Surf. Coat. Technol._ 246, 26–33 (2014). Article CAS Google Scholar * Golabadi, M., Aliofkhazraei, M. & Toorani, M. Corrosion
behavior of zirconium-pretreated/epoxy-coated mild steel: New approach for determination of cathodic disbondment resistance by electrochemical impedance spectroscopy. _J. Alloy. Compd._ 873,
159800 (2021). Article CAS Google Scholar * Wu, A.-H. et al. Corrosion resistance properties of colored zirconium conversion coating and powder coating on cold-rolled steel. _Rare Met._
42, 1005–1010 (2023). Article CAS Google Scholar * Sababi, M., Terryn, H. & Mol, J. M. C. The influence of a Zr-based conversion treatment on interfacial bonding strength and
stability of epoxy coated carbon steel. _Prog. Org. Coat._ 105, 29–36 (2017). Article CAS Google Scholar * Sharifi Golru, S., Attar, M. M. & Ramezanzadeh, B. Effects of surface
treatment of aluminium alloy 1050 on the adhesion and anticorrosion properties of the epoxy coating. _Appl. Surf. Sci._ 345, 360–368 (2015). Article CAS Google Scholar * Han, X. et al.
Corrosion resistance and microstructural characterization of Ti-Zr-V/waterborne resin composite conversion coating on 1060 aluminum alloy. _Colloids Surf. Physicochem. Eng. Asp._ 678, 132503
(2023). Article CAS Google Scholar * Andreatta, F. et al. SKPFM and SEM study of the deposition mechanism of Zr/Ti based pre-treatment on AA6016 aluminum alloy. _Surf. Coat. Technol._
201, 7668–7685 (2007). Article CAS Google Scholar * De Nicolò, A. et al. Cerium conversion coating and sol–gel multilayer system for corrosion protection of AA6060. _Surf. Coat. Technol._
287, 33–43 (2016). Article Google Scholar * Živković, Lj. S., Jegdić, B. V., Popić, J. P., Bajat, J. B. & Mišković-Stanković, V. B. The influence of Ce-based coatings as pretreatments
on corrosion stability of top powder polyester coating on AA6060. _Prog. Org. Coat._ 76, 1387–1395 (2013). Article Google Scholar * Zhu, W., Li, W., Mu, S., Fu, N. & Liao, Z.
Comparative study on Ti/Zr/V and chromate conversion treated aluminum alloys: Anti-corrosion performance and epoxy coating adhesion properties. _Appl. Surf. Sci._ 405, 157–168 (2017).
Article Google Scholar * Banjo, N., Sasaki, T. T. & Hono, K. Microstructural origin of adhesion and corrosion properties of Ti-based conversion coatings on A6063 alloy. _Appl. Surf.
Sci._ 604, 154411 (2022). Article CAS Google Scholar * Sainis, S., Roșoiu, S., Ghassemali, E. & Zanella, C. The role of microstructure and cathodic intermetallics in localised
deposition mechanism of conversion compounds on Al (Si, Fe, Cu) alloy. _Surf. Coat. Technol._ 402, 126502 (2020). Article CAS Google Scholar * Rodič, P., Kapun, B. & Milošev, I.
Durable Polyacrylic/Siloxane-Silica Coating for the Protection of Cast AlSi7Mg0.3 Alloy against Corrosion in Chloride Solution. _Polymers_ 15, 3993 (2023). Article PubMed PubMed Central
Google Scholar * Arrabal, R. et al. Pitting corrosion of rheocast A356 aluminium alloy in 3.5wt.% NaCl solution. _Corros. Sci._ 73, 342–355 (2013). Article CAS Google Scholar * Milošev,
I., Kapun, B. & Rodič, P. The Relation Between the Microstructure of Aluminum Alloy 7075-T6 and the Type of Cerium Salt in the Formation of the Cerium Conversion Layer. _J. Electrochem.
Soc._ 169, 091501 (2022). Article Google Scholar * Buchheit, R. G., Grant, R. P., Hlava, P. F., Mckenzie, B. & Zender, G. L. Local Dissolution Phenomena Associated with S Phase
(Al2CuMg) Particles in Aluminum Alloy 2024‐T3. _J. Electrochem. Soc._ 144, 2621–2628 (1997). Article CAS Google Scholar * Gharbi, O. & Birbilis, N. Clarifying the Dissolution
Mechanisms and Electrochemistry of Mg2Si as a Function of Solution pH. _J. Electrochem. Soc._ 165, C497 (2018). Article CAS Google Scholar * Qi, J. et al. Formation of a Trivalent
Chromium Conversion Coating on AA2024-T351 Alloy. _J. Electrochem. Soc._ 163, C25 (2015). Article Google Scholar * Qi, J. et al. Chromate Formed in a Trivalent Chromium Conversion Coating
on Aluminum. _J. Electrochem. Soc._ 164, C442 (2017). Article CAS Google Scholar * Denissen, P. J. & Garcia, S. J. Reducing subjectivity in EIS interpretation of corrosion and
corrosion inhibition processes by in-situ optical analysis. _Electrochim. Acta_ 293, 514–524 (2019). Article CAS Google Scholar * Milošev, I. et al. Siloxane polyacrylic sol-gel coatings
with alkyl and perfluoroalkyl chains: Synthesis, composition, thermal properties and long-term corrosion protection. _Appl. Surf. Sci._ 574, 151578 (2022). * Hamulić, D. et al. The Effect of
the Methyl and Ethyl Group of the Acrylate Precursor in Hybrid Silane Coatings Used for Corrosion Protection of Aluminium Alloy 7075-T6. _Coatings_ 10, 172 (2020). Article Google Scholar
* Rodič, P., Iskra, J. & Milošev, I. A hybrid organic–inorganic sol–gel coating for protecting aluminium alloy 7075-T6 against corrosion in Harrison’s solution. _J. Sol.-Gel Sci.
Technol._ 70, 90–103 (2014). Article Google Scholar * Standard Test Methods for Rating Adhesion by Tape Test. https://www.astm.org/d3359-23.html. * Harb, S. V. et al. Organic-Inorganic
Hybrid Coatings for Corrosion Protection of Metallic Surfaces. in _New Technologies in Protective Coatings_ (eds. Giudice, C. & Canosa, G.) (InTech, 2017). * Fockaert, L. I. et al.
Effect of zirconium-based conversion treatments of zinc, aluminium and magnesium on the chemisorption of ester-functionalized molecules. _Appl. Surf. Sci._ 508, 145199 (2020). Article CAS
Google Scholar * Liu, T. et al. Machine learning assisted discovery of high-efficiency self-healing epoxy coating for corrosion protection. _Npj Mater. Degrad._ 8, 1–11 (2024). Article
Google Scholar * Schottner, G. Hybrid Sol−Gel-Derived Polymers: Applications of Multifunctional Materials. _Chem. Mater._ 13, 3422–3435 (2001). Article CAS Google Scholar * Pereira, G.
S. et al. Cerium conversion coating and sol-gel coating for corrosion protection of the WE43 Mg alloy. _Corros. Sci._ 206, 110527 (2022). Article CAS Google Scholar * Li, L., Swain, G.
P., Howell, A., Woodbury, D. & Swain, G. M. The Formation, Structure, Electrochemical Properties and Stability of Trivalent Chrome Process (TCP) Coatings on AA2024. _J. Electrochem.
Soc._ 158, C274 (2011). Article CAS Google Scholar * Li, L., Kim, D. Y. & Swain, G. M. Transient Formation of Chromate in Trivalent Chromium Process (TCP) Coatings on AA2024 as Probed
by Raman Spectroscopy. _J. Electrochem. Soc._ 159, C326 (2012). Article CAS Google Scholar * Guo, Y. & Frankel, G. S. Characterization of trivalent chromium process coating on
AA2024-T3. _Surf. Coat. Technol._ 206, 3895–3902 (2012). Article CAS Google Scholar * Jolivet, J.-P. _Metal Oxide Nanostructures Chemistry: Synthesis from Aqueous Solutions_. (Oxford
University Press, 2019). https://doi.org/10.1093/oso/9780190928117.001.0001. * Kraš, A., Milošev, I., Seyeux, A. & Marcus, P. Investigating the presence of tetrameric forms in the
zirconium conversion coatings on cold-rolled steel: proof of concept. _npj Mater. Degrad._ (2024). * Osborne, J. H. Observations on chromate conversion coatings from a sol–gel perspective.
_Prog. Org. Coat._ 41, 280–286 (2001). Article CAS Google Scholar * Pearlstein, F. & Agarawala, V. S. Trivalent chromium conversion coatings for aluminum. _Plat. Surf. Finish_. JULY,
50–55 (1994). * Xia, L. & McCreery, R. L. Chemistry of a Chromate Conversion Coating on Aluminum Alloy AA2024‐T3 Probed by Vibrational Spectroscopy. _J. Electrochem. Soc._ 145, 3083
(1998). Article CAS Google Scholar * Zhong, X., Wu, X., Jia, Y. & Liu, Y. Self-repairing vanadium–zirconium composite conversion coating for aluminum alloys. _Appl. Surf. Sci._ 280,
489–493 (2013). Article CAS Google Scholar * Standard Practice for Operating Salt Spray (Fog) Apparatus. https://www.astm.org/b0117-19.html. Download references ACKNOWLEDGEMENTS The
authors would like to acknowledge student Veronika Bračič for her assistance in preparing the Zr-CrCC and sol-gel coatings and samples for testing. The funding was provided by the Slovenian
Research and Innovation Agency (core programme funding No. P1–0134 and P2–0393). The authors acknowledge the Centre of Excellence in Nanoscience and Nanotechnology-Nanocenter, Ljubljana,
Slovenia, to access the scientific equipment including FIB-SEM/EDXS. The company Talum d.d. is acknowledged for supplying the substrate plate (M-Era.Net grant COR_ID). AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Jožef Stefan Institute, Department of Physical and Organic Chemistry, Jamova c. 39, SI-1000, Ljubljana, Slovenia Peter Rodič, Barbara Kapun & Ingrid Milošev
Authors * Peter Rodič View author publications You can also search for this author inPubMed Google Scholar * Barbara Kapun View author publications You can also search for this author
inPubMed Google Scholar * Ingrid Milošev View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS P.R. and I.M. conceptualised the work, P.R.
conducted experimental work (synthesis of the sols, coatings preparation and deposition, electrochemical measurements, and data analysis), B.K. performed FIB-SEM/EDS analysis. P.R. and I.M.
prepared and wrote the paper. All authors have read and agreed to the published version of the manuscript. CORRESPONDING AUTHOR Correspondence to Ingrid Milošev. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps
and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL MATERIAL PDF RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Rodič, P., Kapun, B. & Milošev, I. Complementary corrosion protection of cast
AlSi7Mg0.3 alloy using Zr-Cr conversion and polyacrylic/siloxane-silica multilayer coatings. _npj Mater Degrad_ 8, 58 (2024). https://doi.org/10.1038/s41529-024-00467-5 Download citation *
Received: 21 October 2023 * Accepted: 19 April 2024 * Published: 28 May 2024 * DOI: https://doi.org/10.1038/s41529-024-00467-5 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
