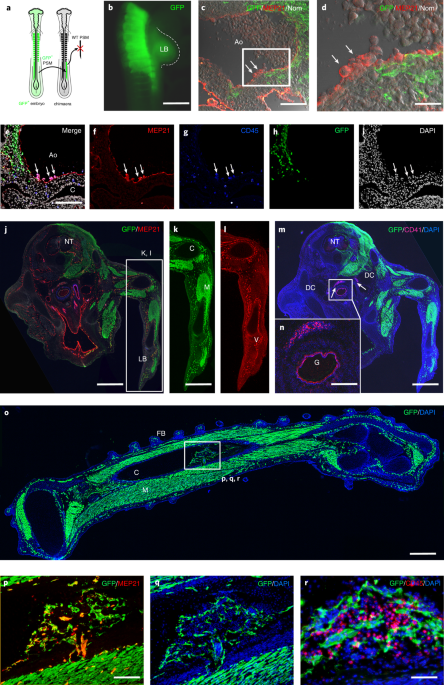
In vivo generation of haematopoietic stem/progenitor cells from bone marrow-derived haemogenic endothelium
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT It is well established that haematopoietic stem and progenitor cells (HSPCs) are generated from a transient subset of specialized endothelial cells termed haemogenic, present in the
yolk sac, placenta and aorta, through an endothelial-to-haematopoietic transition (EHT). HSPC generation via EHT is thought to be restricted to the early stages of development. By using
experimental embryology and genetic approaches in birds and mice, respectively, we document here the discovery of a bone marrow haemogenic endothelium in the late fetus/young adult. These
cells are capable of de novo producing a cohort of HSPCs in situ that harbour a very specific molecular signature close to that of aortic endothelial cells undergoing EHT or their immediate
progenies, i.e., recently emerged HSPCs. Taken together, our results reveal that HSPCs can be generated de novo past embryonic stages. Understanding the molecular events controlling this
production will be critical for devising innovative therapies. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS
OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn
more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to
full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our
FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS MAPPING HUMAN HAEMATOPOIETIC STEM CELLS FROM HAEMOGENIC ENDOTHELIUM TO BIRTH Article 13 April 2022 IDENTIFICATION OF A
RETINOIC ACID-DEPENDENT HAEMOGENIC ENDOTHELIAL PROGENITOR FROM HUMAN PLURIPOTENT STEM CELLS Article 28 April 2022 HAEMATOPOIETIC STEM AND PROGENITOR CELL HETEROGENEITY IS INHERITED FROM THE
EMBRYONIC ENDOTHELIUM Article Open access 17 July 2023 DATA AVAILABILITY Microarray data that support the findings of this study have been deposited in the Gene Expression Omnibus (GEO)
under accession codes GSE122857, GSE133804 and GSE133812. All other data supporting the findings of this study are available from the corresponding author on reasonable request. REFERENCES *
Sugimura, R. et al. Haematopoietic stem and progenitor cells from human pluripotent stem cells. _Nature_ 545, 432–438 (2017). Article CAS PubMed PubMed Central Google Scholar * Lis, R.
et al. Conversion of adult endothelium to immunocompetent haematopoietic stem cells. _Nature_ 545, 439–445 (2017). Article CAS PubMed PubMed Central Google Scholar * Jaffredo, T.,
Gautier, R., Eichmann, A. & Dieterlen-Lievre, F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. _Development_ 125, 4575–4583 (1998). Article CAS
PubMed Google Scholar * Zovein, A. C. et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. _Cell Stem Cell_ 3, 625–636 (2008). Article CAS PubMed PubMed
Central Google Scholar * Chen, M. J., Yokomizo, T., Zeigler, B. M., Dzierzak, E. & Speck, N. A. Runx1 is required for the endothelial to haematopoietic cell transition but not
thereafter. _Nature_ 457, 887–891 (2009). Article CAS PubMed PubMed Central Google Scholar * Kissa, K. & Herbomel, P. Blood stem cells emerge from aortic endothelium by a novel type
of cell transition. _Nature_ 464, 112–115 (2010). Article CAS PubMed Google Scholar * Swiers, G., Rode, C., Azzoni, E. & de Bruijn, M. F. A short history of hemogenic endothelium.
_Blood Cells Mol. Dis._ 51, 206–212 (2013). Article CAS PubMed PubMed Central Google Scholar * Boisset, J. C. et al. In vivo imaging of haematopoietic cells emerging from the mouse
aortic endothelium. _Nature_ 464, 116–120 (2010). Article CAS PubMed Google Scholar * Mikkola, H. K. & Orkin, S. H. The journey of developing hematopoietic stem cells. _Development_
133, 3733–3744 (2006). Article CAS PubMed Google Scholar * Dzierzak, E. & Speck, N. A. Of lineage and legacy: the development of mammalian hematopoietic stem cells. _Nat. Immunol._
9, 129–136 (2008). Article CAS PubMed PubMed Central Google Scholar * Yokomizo, T. & Dzierzak, E. Three-dimensional cartography of hematopoietic clusters in the vasculature of whole
mouse embryos. _Development_ 137, 3651–3661 (2010). Article CAS PubMed PubMed Central Google Scholar * Boisset, J. C. & Robin, C. On the origin of hematopoietic stem cells:
progress and controversy. _Stem Cell Res._ 8, 1–13 (2012). Article CAS PubMed Google Scholar * Drevon, C. & Jaffredo, T. Cell interactions and cell signaling during hematopoietic
development. _Exp. Cell Res._ 329, 200–206 (2014). Article CAS PubMed Google Scholar * Dejana, E., Hirschi, K. K. & Simons, M. The molecular basis of endothelial cell plasticity.
_Nat. Commun._ 8, 14361 (2017). Article CAS PubMed PubMed Central Google Scholar * Thambyrajah, R. et al. New insights into the regulation by RUNX1 and GFI1(s) proteins of the
endothelial to hematopoietic transition generating primordial hematopoietic cells. _Cell Cycle_ 15, 2108–2114 (2016). Article CAS PubMed PubMed Central Google Scholar * Blaser, B. W.
& Zon, L. I. Making HSCs in vitro: don’t forget the hemogenic endothelium. _Blood_ 132, 1372–1378 (2018). Article CAS PubMed PubMed Central Google Scholar * Ottersbach, K.
Endothelial-to-haematopoietic transition: an update on the process of making blood. _Biochem. Soc. Trans._ 47, 591–601 (2019). Article CAS PubMed PubMed Central Google Scholar *
Dzierzak, E. & Bigas, A. Blood development: hematopoietic stem cell dependence and independence. _Cell Stem Cell_ 22, 639–651 (2018). Article CAS PubMed Google Scholar * Pardanaud,
L. et al. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. _Development_ 122, 1363–1371 (1996). Article CAS PubMed Google Scholar * Pouget, C., Gautier,
R., Teillet, M. A. & Jaffredo, T. Somite-derived cells replace ventral aortic hemangioblasts and provide aortic smooth muscle cells of the trunk. _Development_ 133, 1013–1022 (2006).
Article CAS PubMed Google Scholar * Yvernogeau, L., Auda-Boucher, G. & Fontaine-Perus, J. Limb bud colonization by somite-derived angioblasts is a crucial step for myoblast
emigration. _Development_ 139, 277–287 (2012). Article CAS PubMed Google Scholar * Ambler, C. A., Nowicki, J. L., Burke, A. C. & Bautch, V. L. Assembly of trunk and limb blood
vessels involves extensive migration and vasculogenesis of somite-derived angioblasts. _Dev. Biol._ 234, 352–364 (2001). Article CAS PubMed Google Scholar * Christ, B., Huang, R. &
Scaal, M. Amniote somite derivatives. _Dev. Dyn._ 236, 2382–2396 (2007). Article CAS PubMed Google Scholar * McGrew, M. J. et al. Efficient production of germline transgenic chickens
using lentiviral vectors. _EMBO Rep._ 5, 728–733 (2004). Article CAS PubMed PubMed Central Google Scholar * McNagny, K. M. et al. Thrombomucin, a novel cell surface protein that defines
thrombocytes and multipotent hematopoietic progenitors. _J. Cell Biol._ 138, 1395–1407 (1997). Article CAS PubMed PubMed Central Google Scholar * Thornton, M. A. & Poncz, M.
Characterization of the murine platelet αIIb gene and encoded cDNA. _Blood_ 94, 3947–3950 (1999). Article CAS PubMed Google Scholar * Yvernogeau, L. et al. An in vitro model of hemogenic
endothelium commitment and hematopoietic production. _Development_ 143, 1302–1312 (2016). CAS PubMed Google Scholar * Lassila, O., Eskola, J., Toivanen, P. & Dieterlen-Lievre, F.
Lymphoid stem cells in the intraembryonic mesenchyme of the chicken. _Scand. J. Immunol._ 11, 445–448 (1980). Article CAS PubMed Google Scholar * Jaffredo, T., Gautier, R., Brajeul, V.
& Dieterlen-Lièvre, F. Tracing the progeny of the aortic hemangioblast in the avian embryo. _Dev. Biol._ 224, 204–214 (2000). Article CAS PubMed Google Scholar * Taoudi, S. et al.
Progressive divergence of definitive haematopoietic stem cells from the endothelial compartment does not depend on contact with the foetal liver. _Development_ 132, 4179–4191 (2005). Article
CAS PubMed Google Scholar * Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. _Genesis_ 45, 593–605 (2007). Article CAS
PubMed Google Scholar * Sorensen, I., Adams, R. H. & Gossler, A. DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. _Blood_ 113, 5680–5688 (2009).
Article PubMed CAS Google Scholar * Oguro, H., Ding, L. & Morrison, S. J. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent
progenitors. _Cell Stem Cell_ 13, 102–116 (2013). Article CAS PubMed PubMed Central Google Scholar * Kiel, M. J., Yilmaz, O. H., Iwashita, T., Terhorst, C. & Morrison, S. J. SLAM
family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. _Cell_ 121, 1109–1121 (2005). Article CAS PubMed Google Scholar *
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. _BMC Bioinformatics_ 9, 559 (2008). Article PubMed PubMed Central CAS Google Scholar *
Richard, C. et al. Endothelio-mesenchymal interaction controls _runx1_ expression and modulates the _notch_ pathway to initiate aortic hematopoiesis. _Dev. Cell_ 24, 600–611 (2013). Article
CAS PubMed PubMed Central Google Scholar * Pardanaud, L. & Dieterlen-Lievre, F. Manipulation of the angiopoietic/hemangiopoietic commitment in the avian embryo. _Development_ 126,
617–627 (1999). Article CAS PubMed Google Scholar * Noden, D. M. Embryonic origins and assembly of blood vessels. _Am. Rev. Respir. Dis._ 140, 1097–1103 (1989). Article CAS PubMed
Google Scholar * Pudliszewski, M. & Pardanaud, L. Vasculogenesis and angiogenesis in the mouse embryo studied using quail/mouse chimeras. _Int. J. Dev. Biol._ 49, 355–361 (2005).
Article CAS PubMed Google Scholar * Nguyen, P. D. et al. Haematopoietic stem cell induction by somite-derived endothelial cells controlled by _meox1_. _Nature_ 512, 314–318 (2014).
Article CAS PubMed Google Scholar * Qiu, J. et al. Embryonic hematopoiesis in vertebrate somites gives rise to definitive hematopoietic stem cells. _J. Mol. Cell Biol._ 8, 288–301
(2016). Article CAS PubMed PubMed Central Google Scholar * Palis, J. Hematopoietic stem cell-independent hematopoiesis: emergence of erythroid, megakaryocyte and myeloid potential in
the mammalian embryo. _FEBS Lett._ 590, 3965–3974 (2016). Article CAS PubMed Google Scholar * Chen, M. J. et al. Erythroid/myeloid progenitors and hematopoietic stem cells originate from
distinct populations of endothelial cells. _Cell Stem Cell_ 9, 541–552 (2011). Article CAS PubMed PubMed Central Google Scholar * Christensen, J. L., Wright, D. E., Wagers, A. J. &
Weissman, I. L. Circulation and chemotaxis of fetal hematopoietic stem cells. _PLoS Biol._ 2, E75 (2004). Article PubMed PubMed Central Google Scholar * Beaudin, A. E. et al. A
transient developmental hematopoietic stem cell gives rise to innate-like B and T cells. _Cell Stem Cell_ 19, 768–783 (2016). Article CAS PubMed PubMed Central Google Scholar * Plein,
A., Fantin, A., Denti, L., Pollard, J. W. & Ruhrberg, C. Erythro-myeloid progenitors contribute endothelial cells to blood vessels. _Nature_ 562, 223–228 (2018). Article CAS PubMed
PubMed Central Google Scholar * Chevallier, A., Kieny, M. & Mauger, A. Limb–somite relationship: origin of the limb musculature. _J. Embryol. Exp. Morphol._ 41, 245–258 (1977). CAS
PubMed Google Scholar * Christ, B., Jacob, H. J. & Jacob, M. Experimental analysis of the origin of the wing musculature in avian embryos. _Anat. Embryol._ _(__Berl__)_ 150, 171–186
(1977). Article CAS Google Scholar * Hamburger, V. & Hamilton, H. L. A series of normal stages in the development of the chick embryo. _J. Morphol._ 88, 49–92 (1951). Article CAS
PubMed Google Scholar * Boisset, J. C., Andrieu-Soler, C., van Cappellen, W. A., Clapes, T. & Robin, C. Ex vivo time-lapse confocal imaging of the mouse embryo aorta. _Nat. Protoc._ 6,
1792–1805 (2011). Article CAS PubMed Google Scholar * Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. _BMC Dev. Biol._ 1,
4 (2001). Article CAS PubMed PubMed Central Google Scholar * Engleka, K. A. et al. Insertion of Cre into the _Pax3_ locus creates a new allele of _splotch_ and identifies unexpected
_Pax3_ derivatives. _Dev. Biol._ 280, 396–406 (2005). Article CAS PubMed Google Scholar * Huang, D. W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large
gene lists using DAVID bioinformatics resources. _Nat. Protoc._ 4, 44–57 (2009). Article CAS Google Scholar * Huang, D. W. et al. Extracting biological meaning from large gene lists with
DAVID. _Curr. Protoc. Bioinformatics_ 13, 13.11 (2009). Google Scholar Download references ACKNOWLEDGEMENTS We apologize to those investigators whose important work we were unable to cite
or describe due to space constraints. We thank D. Traver for critical reading of the manuscript and S. Gournet for help in image preparation. We are indebted to R. Adams for his sharing of
the Tg(Cdh5Cre/ERT2) transgenic mouse line and to S. Germain for providing us with the founders. We thank the Cell Imaging and Flow Cytometry facility of the IBPS (Paris, France) for access
and technical support in confocal image acquisition and the Genom’IC platform at Cochin Institute, Paris for their invaluable help with transcriptomic samples treatment. This work was
supported by CNRS, UPMC, Fondation Les Treilles (L.Y.), an EMBO short-term fellowship (L.Y.), MERI and FRM PhD fellowships (H.K.) and grants from FRM (DEQ20100318258) and an ANR/CIRM joint
grant (ANR/CIRM 0001–02) in T.J.’s laboratory. The production of the GFP+ chicken embryos was supported by grants from BBSRC and the Wellcome Trust. Part of the work and L.Y. were supported
by a European Research Council grant (ERC, project no. 220-H75001EU/HSCOrigin-309361) and a TOP subsidy from NWO/ZonMw (912.15.017) in C.R.’s laboratory. AUTHOR INFORMATION Author notes *
Laurent Yvernogeau Present address: Hubrecht Institute, Utrecht, Netherlands * Michèle Souyri Present address: UMRS 1131, Institut Universitaire d’Hématologie, Hôpital Saint Louis, Paris,
France * These authors contributed equally: Rodolphe Gautier, Laurence Petit. AUTHORS AND AFFILIATIONS * IBPS, Laboratoire de Biologie du Développement CNRS UMR7622, Inserm U1156, Sorbonne
Université, Paris, France Laurent Yvernogeau, Rodolphe Gautier, Laurence Petit, Hanane Khoury, Pierre Charbord, Michèle Souyri & Thierry Jaffredo * Institut Mondor de Recherches
Biomédicales, Créteil, France Frédéric Relaix * Institut Jacques Monod, Paris, France Vanessa Ribes * The Roslin Institute and R(D)SVS, University of Edinburgh, Edinburgh, UK Helen Sang *
Hubrecht Institute, Utrecht, Netherlands Catherine Robin * Regenerative Medicine Center, University Medical Center Utrecht, Utrecht, Netherlands Catherine Robin Authors * Laurent Yvernogeau
View author publications You can also search for this author inPubMed Google Scholar * Rodolphe Gautier View author publications You can also search for this author inPubMed Google Scholar *
Laurence Petit View author publications You can also search for this author inPubMed Google Scholar * Hanane Khoury View author publications You can also search for this author inPubMed
Google Scholar * Frédéric Relaix View author publications You can also search for this author inPubMed Google Scholar * Vanessa Ribes View author publications You can also search for this
author inPubMed Google Scholar * Helen Sang View author publications You can also search for this author inPubMed Google Scholar * Pierre Charbord View author publications You can also
search for this author inPubMed Google Scholar * Michèle Souyri View author publications You can also search for this author inPubMed Google Scholar * Catherine Robin View author
publications You can also search for this author inPubMed Google Scholar * Thierry Jaffredo View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
L.Y. and T.J. conceived the project. L.Y. and T.J. designed the experiments on chicken embryos, performed chicken somite grafts and interpreted data. L.Y. performed chicken grafts, carried
out immunohistological analysis and performed FACS analysis. R.G. performed chicken graft analysis. L.P. performed all mouse work, including transplantation studies, and analysed the data.
H.S. provided GFP transgenic chickens. V.R. and F.R. provided the Pax3CRE X Rosa mTmG mice and critical associated materials. H.K. analysed the YFP+ transcriptome versus the YFP−
transcriptome. P.C. analysed transcriptomes, performed network analyses and interpreted data. M.S. designed Pax3CRE X ROSAmTmG and the initial VECAD-Cre ERT X Rosa 26 approaches, analysed
the data and provided the additional mouse embryonic and BM adult transcriptomes. C.R. and L.Y. designed and supervised the experiments on adult chickens and the live imaging on sections.
T.J. wrote the manuscript. L.Y. and C.R. provided significant input for data analyses, findings, interpretations and manuscript writing. CORRESPONDING AUTHOR Correspondence to Thierry
Jaffredo. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 PSM REMOVAL EXPERIMENT. A, E2 (15-somite stage, HH11/12) wild-type chicken embryo
submitted to a PSM ablation. White arrows located the regions of transverse histological analysis at two representative anterior and posterior levels displayed in 1 and 2. MEP21 (red) and
DAPI (blue) staining showed that only the PSM was removed without disturbing the surrounding tissues (10 chicken embryos). B, Whole mounts of two PSMs isolated from six E2 (15-somite stage,
HH11/12) wild-type embryos and stained with DAPI (blue), MEP21 (red) and CD45 (green) antibodies, showing that PSMs do not contain any ECs or HCs after dissection. Two independent examples
are shown (#1, #2) (6 chicken embryos). C, Mid trunk, thick transverse section of an E3 chicken embryo stained with MEP21 (red) and CD45 (green) antibodies shown as positive control of the
immunostainings. Of note, PSMs (B) and thick slice (C) were stained at the same time, using the same antibody solutions. D, Representative FACS analysis on single PSMs isolated from 8
independent 13-15 somite stage embryos showing no contamination by haematopoietic (CD45) or endothelial (MEP21) cells. Controls are two whole embryos without PSMs showing the presence of
CD45+ haematopoietic and MEP21+ endothelial cells. Ao, Aorta; Ec, Ectoderm; En, Endoderm; Nc, Notochord; NT, Neural Tube; PSM, Presomitic mesoderm; So, Somatopleura; Sp, Splanchnopleura.
Scale bars, 200µm in A, 25μm in A1, A2; 50μm in B and C. EXTENDED DATA FIG. 2 GFP+CD45+ HAEMATOPOIETIC CELLS IN CIRCULATING BLOOD OF THE GRAFTED OR CONTROL EMBRYOS WITH AGE. A-F, CD45
expression in the sample (left) and GFP plot in the CD45+ population (right). A-D, chimeric embryos. A, E4 embryo. B, E8 embryo. C, E12 embryo. D, E16 embryo. Of note, only E16 embryos show
GFP+ cells within the CD45+ fraction. E, E12 GFP transgenic embryo. F, E12 WT embryo. 23 chicken embryos analysed. See Extended Data Table 1 for details. Data representative of 4 independent
experiments. EXTENDED DATA FIG. 3 GFP+ SOMITE-DERIVED CELLS CONTRIBUTE TO E16/E17 BONE MARROW CD45+ HAEMATOPOIETIC PRODUCTION. A-C, Representative fluorescent cross section of the limb bone
showing the co-distribution of GFP+ sinusoid cells and CD45+ cells. B, Enlargement of the bone marrow showing GFP+CD45+ cells lining the sinusoids. C, Enlargement of the frame in B, showing
the double positive cells. D-L, Series of confocal cross sections separated by 1µm, showing a GFP+CD45+ cell (white arrow). D, G, J, CD45 staining. E, H, K, GFP signal. F, I, L, Merged
CD45, GFP and DAPI. Right and bottom banners correspond to YZ and XZ projections of the confocal image, respectively. Experiments were repeated 16 times with similar results (see Extended
Data Table 1 for details). Confocal analysis was performed 5 times with similar results. Scale bars in A, 200µm; in B, 40µm; in C, 15µm; in D-L, 20µm. EXTENDED DATA FIG. 4 FLOW CYTOMETRY
ANALYSIS REVEALS THE PRESENCE OF ENDOTHELIAL AND HAEMATOPOIETIC CELLS EXPRESSING GFP IN THE E16-GRAFTED CHICKEN BM. Distribution of CD45, MEP21, CD41, c-KIT, and CD3 populations in grafted
(A, B) or wild-type (C, D) BM mononucleated cells. A, n = 12 independently grafted animals were used for CD45, CD41, c-KIT and CD3 expression and n = 8 for MEP21. B, Representative flow
cytometry analysis of an E16/E17 grafted BM showing that GFP+ cells derived from the PSM graft contributed to all lineages. Numbers in green indicate the percentage of GFP+ cells in each
population (that is in CD45+, MEP21+, CD41+, c-KIT+ and CD3+ populations). C, n = 3 independent WT animals were used for CD45, CD41, c-KIT expression analysis, n = 2 animals were used for
MEP21 and CD3 expression analysis. D, Representative flow cytometry analysis of an E16/E17 WT BM. Data in B representative of 4 independent experiments, 3 in D. Error bars are mean ± SEM.
Source data EXTENDED DATA FIG. 5 PSM-DERIVED CELLS PROVIDE HAEMATOPOIETIC PRECURSORS ABLE TO COLONIZE THE SECONDARY HAEMATOPOIETIC ORGANS OF E16/E17 GRAFTED CHICKENS. A, F, I, Global views
of the thymus (A), spleen (F) and bursa of Fabricius (I) of an E16 chicken colonized by GFP+ PSM-derived grafted cells. A, Most of the thymus lobes were colonized by GFP+ cells (3 chickens).
B, Immunostaining (with DAPI) of a thymus transverse section. C-D, Higher magnification of the frame in (B). GFP (C) and merge of DAPI and GFP (D). E, Flow cytometry analysis of an E16/E17
thymus (2 chickens) revealed the contribution of GFP+ PSM-derived cells to the whole thymus and more precisely to the T lineage (GFP+CD4+). G, Higher magnification of the frame in (F),
showing dispersed GFP+ cells in the spleen (2 chickens). H, Flow cytometry analysis of the spleen, showing the contribution of GFP+ PSM-derived cells to the whole spleen and more precisely
to the CD45+ haematopoietic lineage (2 chickens). J, Higher magnification of the frame in (I) showing dispersed GFP+ cells in the bursa (2 chickens). K, Flow cytometry analysis showing that
the bursa of Fabricius, located far away from the PSM graft, was also colonized by PSM-derived haematopoietic progenitors that derived from the graft (2 chickens). Drawings are shown on the
left side of the organ pictures to locate the thymus, spleen and bursa of Fabricius in an E16/E17 chicken. Scale bars in A, B, F, I: 100µm. EXTENDED DATA FIG. 6 FLOW CYTOMETRY ANALYSIS
REVEALS THE PRESENCE OF ENDOTHELIAL AND HAEMATOPOIETIC CELLS EXPRESSING GFP IN ADULT BM GRAFTED CHICKENS. Distribution of CD45, MEP21, CD41, c-KIT, CD3 and KUL1 populations in grafted (A, B)
and wild-type (C, D) adult BM mononucleated cells. A, Grafted chickens. CD45 (n = 7), MEP21 (n = 4), CD41 (n = 7), c-KIT (n = 7), CD3 (n = 7) and KUL1 (n = 6) independent chickens were used
for the analysis. B, Representative multilineage flow cytometry analysis of a grafted adult BM showing that GFP+ cells derived from the PSM graft contributed to all lineages. Numbers in
green indicate the percentages of GFP+ cells in each population (that is in CD45+, MEP21+, CD41+, c-KIT+, CD3+ and KUL1+ populations). C, Wild-type animals. CD45 (n = 5), CD41 (n = 4), c-KIT
(n = 5), CD3 (n = 4) and KUL1 (n = 4); independent animals were used for the analysis. n = 2 animals were used for MEP21 expression analysis. D, Representative multilineage flow cytometry
analysis of a wild-type adult BM showing the absence of GFP staining. Error bars represent mean ± SEM. Data in B and D representative of 4 independent experiments. Source data EXTENDED DATA
FIG. 7 PAX3+ SOMITE-DERIVED CELLS DO NOT CONTRIBUTE TO THE AORTA HAEMATOPOIEISIS BUT CONTRIBUTE TO THE ENDOTHELIAL AND HAEMATOPOIETIC LINEAGES IN THE LATE FETAL BM. A, Transverse section
through the aorta of an E10.5 Pax3 GFP+ mouse embryo at the mid-trunk level. CD31 (red, left), Pax3-GFP (green, middle) double staining counterstained with DAPI (blue). Right panel
represents the merge (5 mice). B, Cross section of a Pax3KICRE; ROSA GFP embryo at E8.5 showing that this model tags the somite and recapitulates the Pax3 GFP expression at the same stage (5
mice). C, Flow cytometry analysis of the endothelial and haematopoietic lineages in the BM of Pax3-Cre/mTmG fetuses at E19-E21. n = 3 fetuses from 3 independent experiments. D, Flow
cytometry analysis showing GFP+ cell (that is somite-derived cell) contribution to the different endothelial and haematopoietic populations. Mouse somite-derived cells mostly contributed to
fetal/new-born BM CD3+, Sca1+c-Kit+ and CD144+CD45+ cell populations. n = 3 foetuses from 3 independent experiments. E, Representative GFP expression in mononucleated BM cells. F,
Representative flow cytometry analysis of the mononucleated BM cells revealed GFP+ cells mainly in the CD144+CD45+ population. G, Flow cytometry analysis showing the presence of GFP+ cells
in the Sca1+c-KIT+ population (that is HSPC population). H, Negative controls are non-recombined fetuses. Data represent mean ± SEM. Data in E-H representative of 2 independent experiments.
Scale bars 350µm in A; 200µm in B. Source data EXTENDED DATA FIG. 8 YFP EXPRESSION ON NEGATIVE CONTROLS AND YFP TRACING FOLLOWING INDUCTION IN VE-CADHERIN+ CELLS AT DIFFERENT TIME POINTS
AFTER BIRTH. A, Flow cytometry analysis for the presence of YFP+ cells in the CD45+ fraction of the BM immediately after the last tamoxifen injection. Representative new-born mice injected
with tamoxifen at post-natal days 1, 2, and 3 and analysed for the expression of CD45 and YFP at day 4. No CD45+ YFP+ cells were found indicating the absence of contamination by
haematopoietic progenitors expressing VE-Cadherin (11 animals in 3 independent experiments). The gating strategy is shown for mouse #1. B, C, BM from a representative non-induced mouse at 27
post-natal days. B, Representative flow cytometry analysis showing the absence of YFP expression in mononucleated BM cells and in the CD144−CD45+, CD144+CD45+ and CD144+CD45− cell fractions
(4 animals in 2 independent experiments). C, Representative flow cytometry analysis of the mononucleated BM cells showing the absence of YFP expression in the Sca1+c-KIT+ population (that
is HSPC population) and in the different HSC, HPC and MPP populations (2 independent experiments). D, Scheme showing the activation of YFP in VE-Cadherin+ (CDH5) cells by tamoxifen injection
at different time points after birth (coloured arrows). E, Analysis of the percentages of total YFP+ cells, and YFP+ cells in CD144+CD45− and LSK populations at 21 days post tamoxifen
injection. Of note, the x axis values represent the age of the mice from the time of tamoxifen injection. n = 3 mice for d1, n = 6 for d10, n = 7 for d21, n = 7 for d36, n = 5 for d68. Error
bars are mean ± SEM. Data in A-C representative of 2 independent experiments. Source data EXTENDED DATA FIG. 9 HAEMOGENIC POTENTIAL OF BM ECS AND TRANSCRIPTOME CHARACTERISATION OF THE YFP+
AND YFP− LSK CELLS. A, representative flow cytometry profile showing the gates used to isolate the CD144+CD45− and the CD144−CD45+ populations. Image representative of 7 mice. B-E,
representative pictures of the CD144−CD45+ (B) and CD144+CD45− (C, D) cells from 8-day old mouse bone marrow in culture in the endothelial/haematopoietic medium. A high number of floating
cells are present in the CD144−CD45+ cells fraction (B). Flat adherent cells (in C) and round, floating, haematopoietic-like cells (arrows in C and D) are present in the CD144+CD45− cell
fraction after 4 days of culture. Image representative of 3 experiments. Bar = 10µm. E, F, representative FACS analysis of the CD45+ populations generated after 4 days of culture from the
CD144−CD45+ (E) and the CD144+CD45− (F) cell populations. Image representative of 3 experiments. G, PCA with the entire set of mRNAs (30,922 genes) as variables and the basic set of YFP+
(green) and YFP− (red) LSK cells as observations. The two types of transcriptomes were strongly separated (3 biological replicates per population). H, Major GO categories given by DAVID for
the gene sets up-regulated in YFP+ (green) and YFP− (red) LSK cells. I, Hierarchical clustering obtained with the 23 samples (3 biological replicates per population except for HC BM and LSK
CD150+ BM where quadruplicates were used) as observations and the gene set of 2056 DEGs (986 up, 1070 down) as variables was generated from the PCA displayed in Fig. 6a, bottom panel. Branch
organisation reflects the association between the different samples displayed on the PCA. Scale Bars: 50µm in B, C, D. EXTENDED DATA FIG. 10 LONG-TERM REPOPULATION ANALYSIS IN THE
PERIPHERAL BLOOD OF RECIPIENTS TRANSPLANTED EITHER WITH YFP− OR YFP+ LSK CELLS. A, B, Flow cytometry analysis of circulating blood from 6 (A) and 4 (B) C57Bl/6-CD45.1 recipient mice at 11
weeks injected with 2,000 YFP− (A) or YFP+ (B) LSK cells (C57Bl/6-CD45.2), respectively. Mice recipients transplanted with YFP− LSK cells displayed a more robust reconstitution than the ones
transplanted with YFP+ LSK cells. Of note, two mice in the LSK YFP+ series died before two weeks post-injection. C, Back-gating within the GFP+ population of the mononucleated bone marrow
cells from mouse #d at 14 weeks of reconstitution. Dot plot representation of flow cytometry multilineage analysis showing Gr1 (granulocytes), CD11b (macrophages/monocytes), B220 and CD19 (B
cells), CD4 (T cells), CD8 (T cells), Ter119 and CD71 (red cells), CD41 and CD61 (megakaryocytes) staining. The YFP population was more prominent in B220, CD19, CD71, Gr1 and CD8 fractions.
Data in A-C representative of 2 independent experiments. SUPPLEMENTARY INFORMATION REPORTING SUMMARY SUPPLEMENTARY VIDEO 1 Whole-mount staining of E16 chicken bone marrow after GFP+ PSM
graft. The bone marrow was stained with anti-MEP21 (endothelial marker, red), anti-CD45 (haematopoietic marker, blue) and anti-GFP (green, which revealed grafted PSM-derived cells)
antibodies. The marrow was heavily colonized by GFP+ PSM-derived cells that contributed to the whole bone marrow vascularization (_n_ = 3). SUPPLEMENTARY VIDEO 2 A GFP+ endothelial cell
co-expressing CD45 as revealed by confocal analysis of an E16-grafted BM. Series of confocal sections separated by 1 µm, showing a GFP+CD45+ cell (white arrow). Panels: upper left, DAPI;
upper right, CD45; lower left, GFP; lower right, merged. Right and bottom banners correspond to _y–z_ and _x–z_ projections of the confocal image, respectively (_n_ = 5 in three independent
embryos). SUPPLEMENTARY VIDEO 3 GFP+ endothelial cells co-expressing CD41 are integrated in the vascular endothelium of E16-grafted BM. Series of confocal sections of 1 µm, showing a
GFP+CD41+ cell integrated in the GFP+ vascular endothelium (_n_ = 8 in three independent embryos). SUPPLEMENTARY VIDEO 4 Emergence of CD41+ haematopoietic precursors from GFP+ endothelial
cells in the bone marrow. A, Time-lapse live imaging of a transversal E16 bone marrow slice showing the emergence of a GFP+ cell from the endothelium of a blood vessel. B, After time-lapse
imaging (A) the section was stained again with anti-CD41-PE antibodies. The newly emerged GFP+ cell expressed the haematopoietic marker CD41. Of note, this cell was CD41- at the beginning of
imaging. Time is in hours and minutes (_n_ = 2 independent experimental animals with five different sections per slide). Scale bar, 15 µm. SUPPLEMENTARY VIDEO 5 Whole-mount staining of an
E16 chicken thymus after GFP+ PSM graft. The thymus was stained with anti-MEP21 (endothelial marker, red), anti-CD45 (haematopoietic marker, blue) and anti-GFP (green, which revealed grafted
PSM-derived cells) antibodies. The thymus was colonized by GFP+ PSM-derived cells, some of the lobes being more colonized than others. GFP+ cells could colonize secondary haematopoietic
organs that are located far from the grafting site. (_n_ = 2 independent experimental animals). EXTENDED DATA TABLE 1 Number of grafted chickens, time points of analyses and type of analyses
performed. This table summarizes the number of chicken samples according to the age at analysis; the organs or tissues analysed, that is, the thymus, bone marrow, spleen; the kind of
analysis applied, that is, FACS, immunostaining, confocal imaging or videos; and the number of cases analysed for each time point. EXTENDED DATA TABLE 2 List of anti-mouse and anti-chicken
antibodies. List of anti-mouse (first) and anti-chicken (second) antibodies used in the study, including the conjugated fluorochrome when applicable, clone of origin, cell specificity,
supplier and catalogue number. SOURCE DATA SOURCE DATA FIG. 2 Statistical source data SOURCE DATA FIG. 4 Statistical source data SOURCE DATA FIG. 5 Statistical source data SOURCE DATA FIG. 6
Statistical source data SOURCE DATA EXTENDED DATA FIG. 4 Statistical source data SOURCE DATA EXTENDED DATA FIG. 6 Statistical source data SOURCE DATA EXTENDED DATA FIG. 7 Statistical source
data SOURCE DATA EXTENDED DATA FIG. 8 Statistical source data RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yvernogeau, L., Gautier, R., Petit, L. _et
al._ In vivo generation of haematopoietic stem/progenitor cells from bone marrow-derived haemogenic endothelium. _Nat Cell Biol_ 21, 1334–1345 (2019).
https://doi.org/10.1038/s41556-019-0410-6 Download citation * Received: 06 November 2018 * Accepted: 23 September 2019 * Published: 04 November 2019 * Issue Date: November 2019 * DOI:
https://doi.org/10.1038/s41556-019-0410-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
