Generation of tolerogenic antigen-presenting cells in vivo via the delivery of mrna encoding pdl1 within lipid nanoparticles
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

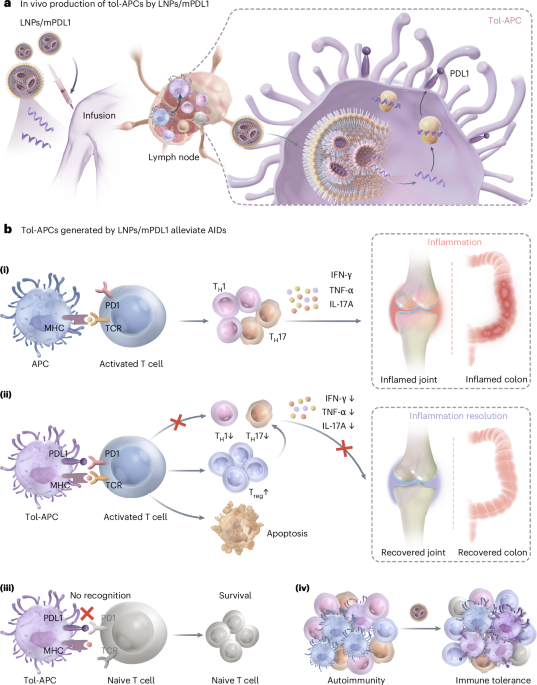
ABSTRACT Tolerogenic antigen-presenting cells (APCs) are promising as therapeutics for suppressing T cell activation in autoimmune diseases. However, the isolation and ex vivo manipulation
of autologous APCs is costly, and the process is customized for each patient. Here we show that tolerogenic APCs can be generated in vivo by delivering, via lipid nanoparticles, messenger
RNA coding for the inhibitory protein programmed death ligand 1. We optimized a lipid-nanoparticle formulation to minimize its immunogenicity by reducing the molar ratio of nitrogen atoms on
the ionizable lipid and the phosphate groups on the encapsulated mRNA. In mouse models of rheumatoid arthritis and ulcerative colitis, subcutaneous delivery of nanoparticles encapsulating
mRNA encoding programmed death ligand 1 reduced the fraction of activated T cells, promoted the induction of regulatory T cells and effectively prevented disease progression. The method may
allow for the engineering of APCs that target specific autoantigens or that integrate additional inhibitory molecules. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value
online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS A NANOPARTICLE VACCINE THAT TARGETS
NEOANTIGEN PEPTIDES TO LYMPHOID TISSUES ELICITS ROBUST ANTITUMOR T CELL RESPONSES Article Open access 12 November 2020 A NANOVACCINE FOR ANTIGEN SELF-PRESENTATION AND IMMUNOSUPPRESSION
REVERSAL AS A PERSONALIZED CANCER IMMUNOTHERAPY STRATEGY Article 11 April 2022 BIOMIMETIC NANOVACCINE-MEDIATED MULTIVALENT IL-15 SELF-TRANSPRESENTATION (MIST) FOR POTENT AND SAFE CANCER
IMMUNOTHERAPY Article Open access 24 October 2023 DATA AVAILABILITY The data supporting the results in this study are available within the paper and its Supplementary Information. The raw
and analysed datasets generated during the study are available for research purposes from the corresponding authors on reasonable request. Source data are provided with this paper.
REFERENCES * Fugger, L., Jensen, L. T. & Rossjohn, J. Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. _Cell_ 181, 63–80 (2020). CAS PubMed
Google Scholar * Conrad, N. et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of
22 million individuals in the UK. _Lancet_ 401, 1878–1890 (2023). PubMed Google Scholar * McKinney, E. F., Lee, J. C., Jayne, D. R., Lyons, P. A. & Smith, K. G. T-cell exhaustion,
co-stimulation and clinical outcome in autoimmunity and infection. _Nature_ 523, 612–616 (2015). CAS PubMed PubMed Central Google Scholar * Cully, M. T cell-regulating therapies for
autoimmune diseases take FDA rejection in stride. _Nat. Rev. Drug Discov._ 20, 655–657 (2021). CAS PubMed Google Scholar * Mullard, A. PD1 agonist antibody passes first phase II trial for
autoimmune disease. _Nat. Rev. Drug Discov._ 22, 526 (2023). PubMed Google Scholar * Zhang, B. et al. Site-specific PEGylation of interleukin-2 enhances immunosuppression via the
sustained activation of regulatory T cells. _Nat. Biomed. Eng._ 5, 1288–1305 (2021). PubMed Google Scholar * Edner, N. M., Carlesso, G., Rush, J. S. & Walker, L. S. K. Targeting
co-stimulatory molecules in autoimmune disease. _Nat. Rev. Drug Discov._ 19, 860–883 (2020). CAS PubMed Google Scholar * Herold, K. C. et al. Anti-CD3 monoclonal antibody in new-onset
type 1 diabetes mellitus. _N. Engl. J. Med._ 346, 1692–1698 (2002). CAS PubMed Google Scholar * Cifuentes-Rius, A., Desai, A., Yuen, D., Johnston, A. P. R. & Voelcker, N. H. Inducing
immune tolerance with dendritic cell-targeting nanomedicines. _Nat. Nanotechnol._ 16, 37–46 (2021). CAS PubMed Google Scholar * Audiger, C., Rahman, M. J., Yun, T. J., Tarbell, K. V.
& Lesage, S. The importance of dendritic cells in maintaining immune tolerance. _J. Immunol._ 198, 2223–2231 (2017). CAS PubMed Google Scholar * Brown, C. C. & Rudensky, A. Y.
Spatiotemporal regulation of peripheral T cell tolerance. _Science_ 380, 472–478 (2023). CAS PubMed Google Scholar * Kenison, J. E., Stevens, N. A. & Quintana, F. J. Therapeutic
induction of antigen-specific immune tolerance. _Nat. Rev. Immunol._ 24, 338–357 (2024). CAS PubMed Google Scholar * Sugiura, D. et al. Restriction of PD-1 function by _cis_-PD-L1/CD80
interactions is required for optimal T cell responses. _Science_ 364, 558–566 (2019). CAS PubMed Google Scholar * Oh, S. A. et al. PD-L1 expression by dendritic cells is a key regulator
of T-cell immunity in cancer. _Nat. Cancer_ 1, 681–691 (2020). CAS PubMed Google Scholar * Giannoukakis, N., Phillips, B., Finegold, D., Harnaha, J. & Trucco, M. Phase I (safety)
study of autologous tolerogenic dendritic cells in type 1 diabetic patients. _Diabetes Care_ 34, 2026–2032 (2011). PubMed PubMed Central Google Scholar * Morante-Palacios, O., Fondelli,
F., Ballestar, E. & Martínez-Cáceres, E. M. Tolerogenic dendritic cells in autoimmunity and inflammatory diseases. _Trends Immunol._ 42, 59–75 (2021). CAS PubMed Google Scholar *
Zubizarreta, I. et al. Immune tolerance in multiple sclerosis and neuromyelitis optica with peptide-loaded tolerogenic dendritic cells in a phase 1b trial. _Proc. Natl Acad. Sci. USA_ 116,
8463–8470 (2019). CAS PubMed PubMed Central Google Scholar * Benham, H. et al. Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis
patients. _Sci. Transl. Med._ 7, 290ra87 (2015). PubMed Google Scholar * Passeri, L., Marta, F., Bassi, V. & Gregori, S. Tolerogenic dendritic cell-based approaches in autoimmunity.
_Int. J. Mol. Sci._ 22, 8415 (2021). CAS PubMed PubMed Central Google Scholar * Rurik, J. G. et al. CAR T cells produced in vivo to treat cardiac injury. _Science_ 375, 91–96 (2022). CAS
PubMed PubMed Central Google Scholar * Sahin, U., Karikó, K. & Türeci, Ö. mRNA-based therapeutics—developing a new class of drugs. _Nat. Rev. Drug Discov._ 13, 759–780 (2014). CAS
PubMed Google Scholar * Hassett, K. J. et al. Impact of lipid nanoparticle size on mRNA vaccine immunogenicity. _J. Control. Release_ 335, 237–246 (2021). CAS PubMed Google Scholar *
Pardi, N. et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. _Nature_ 543, 248–251 (2017). CAS PubMed PubMed Central Google Scholar * Verbeke, R.,
Hogan, M. J., Loré, K. & Pardi, N. Innate immune mechanisms of mRNA vaccines. _Immunity_ 55, 1993–2005 (2022). CAS PubMed PubMed Central Google Scholar * Barbier, A. J., Jiang, A.
Y., Zhang, P., Wooster, R. & Anderson, D. G. The clinical progress of mRNA vaccines and immunotherapies. _Nat. Biotechnol._ 40, 840–854 (2022). CAS PubMed Google Scholar * Wang, C.,
Zhao, C., Wang, W., Liu, X. & Deng, H. Biomimetic noncationic lipid nanoparticles for mRNA delivery. _Proc. Natl Acad. Sci. USA_ 120, e2311276120 (2023). CAS PubMed PubMed Central
Google Scholar * Kenjo, E. et al. Low immunogenicity of LNP allows repeated administrations of CRISPR-Cas9 mRNA into skeletal muscle in mice. _Nat. Commun._ 12, 7101 (2021). CAS PubMed
PubMed Central Google Scholar * Krienke, C. et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. _Science_ 371, 145–153 (2021). CAS PubMed
Google Scholar * Wilson, E. et al. Efficacy and safety of an mRNA-based RSV PreF vaccine in older adults. _N. Engl. J. Med._ 389, 2233–2244 (2023). CAS PubMed Google Scholar * Kauffman,
K. J. et al. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. _Nano Lett._ 15, 7300–7306 (2015). CAS
PubMed Google Scholar * Zhao, P. et al. Depletion of PD-1-positive cells ameliorates autoimmune disease. _Nat. Biomed. Eng._ 3, 292–305 (2019). CAS PubMed PubMed Central Google Scholar
* Wu, Y. et al. Omicron-specific mRNA vaccine elicits potent immune responses in mice, hamsters, and nonhuman primates. _Cell Res._ 32, 949–952 (2022). CAS PubMed PubMed Central Google
Scholar * Peng, Q. et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. _Nat. Commun._ 11, 4835 (2020). CAS PubMed PubMed
Central Google Scholar * O’Shea, J. J., Laurence, A. & McInnes, I. B. Back to the future: oral targeted therapy for RA and other autoimmune diseases. _Nat. Rev. Rheumatol._ 9, 173–182
(2013). PubMed PubMed Central Google Scholar * Kingsmore, K. M., Grammer, A. C. & Lipsky, P. E. Drug repurposing to improve treatment of rheumatic autoimmune inflammatory diseases.
_Nat. Rev. Rheumatol._ 16, 32–52 (2020). CAS PubMed Google Scholar * Brand, D. D., Latham, K. A. & Rosloniec, E. F. Collagen-induced arthritis. _Nat. Protoc._ 2, 1269–1275 (2007). CAS
PubMed Google Scholar * Wu, J. et al. TNF antagonist sensitizes synovial fibroblasts to ferroptotic cell death in collagen-induced arthritis mouse models. _Nat. Commun._ 13, 676 (2022).
CAS PubMed PubMed Central Google Scholar * Wirtz, S. et al. Chemically induced mouse models of acute and chronic intestinal inflammation. _Nat. Protoc._ 12, 1295–1309 (2017). CAS PubMed
Google Scholar * Tang, C. et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing Treg cells through modification of the intestinal microbiota.
_Nat. Immunol._ 19, 755–765 (2018). CAS PubMed Google Scholar * Van Assche, G. et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe
ulcerative colitis. _Gastroenterology_ 125, 1025–1031 (2003). PubMed Google Scholar * Sharpe, A. H. & Pauken, K. E. The diverse functions of the PD1 inhibitory pathway. _Nat. Rev.
Immunol._ 18, 153–167 (2018). CAS PubMed Google Scholar * Breda, L. et al. In vivo hematopoietic stem cell modification by mRNA delivery. _Science_ 381, 436–443 (2023). CAS PubMed
PubMed Central Google Scholar * Kranz, L. M. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. _Nature_ 534, 396–401 (2016). PubMed
Google Scholar * Serra, P. & Santamaria, P. Antigen-specific therapeutic approaches for autoimmunity. _Nat. Biotechnol._ 37, 238–251 (2019). CAS PubMed Google Scholar * Miller, S.
D., Turley, D. M. & Podojil, J. R. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. _Nat. Rev. Immunol._ 7, 665–677 (2007). CAS PubMed
Google Scholar * Kurochkina, Y. et al. SAT0212 The safety and tolerability of intra-articular injection of tolerogenic dendritic cells in patients with rheumatoid arthritis: the preliminary
results. _Ann. Rheum. Dis._ 77, 966–967 (2018). Google Scholar * Jauregui-Amezaga, A. et al. Intraperitoneal administration of autologous tolerogenic dendritic cells for refractory Crohn’s
disease: a phase I study. _J. Crohns Colitis_ 9, 1071–1078 (2015). PubMed Google Scholar * Dong, S. et al. The effect of low-dose IL-2 and Treg adoptive cell therapy in patients with type
1 diabetes. _JCI Insight_ 6, e147474 (2021). PubMed PubMed Central Google Scholar * Raffin, C., Vo, L. T. & Bluestone, J. A. Treg cell-based therapies: challenges and perspectives.
_Nat. Rev. Immunol._ 20, 158–172 (2020). CAS PubMed Google Scholar * Hirai, T. et al. Selective expansion of regulatory T cells using an orthogonal IL-2/IL-2 receptor system facilitates
transplantation tolerance. _J. Clin. Invest._ 131, e139991 (2021). PubMed PubMed Central Google Scholar * Bluestone, J. A. & Tang, Q. Treg cells—the next frontier of cell therapy.
_Science_ 362, 154–155 (2018). CAS PubMed Google Scholar * Murray, J. A. et al. Safety and tolerability of KAN-101, a liver-targeted immune tolerance therapy, in patients with coeliac
disease (ACeD): a phase 1 trial. _Lancet Gastroenterol. Hepatol._ 8, 735–747 (2023). CAS PubMed Google Scholar * Tremain, A. C. et al. Synthetically glycosylated antigens for the
antigen-specific suppression of established immune responses. _Nat. Biomed. Eng._ 7, 1142–1155 (2023). CAS PubMed Google Scholar * Kelly, C. P. et al. TAK-101 nanoparticles induce
gluten-specific tolerance in celiac disease: a randomized, double-blind, placebo-controlled study. _Gastroenterology_ 161, 66–80.e8 (2021). CAS PubMed Google Scholar * Tsai, S. et al.
Reversal of autoimmunity by boosting memory-like autoregulatory T cells. _Immunity_ 32, 568–580 (2010). CAS PubMed Google Scholar * Singha, S. et al. Peptide-MHC-based nanomedicines for
autoimmunity function as T-cell receptor microclustering devices. _Nat. Nanotechnol._ 12, 701–710 (2017). CAS PubMed Google Scholar * Baden, L. R. et al. Efficacy and safety of the
mRNA-1273 SARS-CoV-2 vaccine. _N. Engl. J. Med._ 384, 403–416 (2021). CAS PubMed Google Scholar * Katakura, K. et al. Toll-like receptor 9–induced type I IFN protects mice from
experimental colitis. _J. Clin. Invest._ 115, 695–702 (2005). CAS PubMed PubMed Central Google Scholar * Moskowitz, R. W. Osteoarthritis cartilage histopathology: grading and staging.
_Osteoarthr. Cartil._ 14, 13–29 (2006). Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the National Natural Science Foundation of China (52025036 to Y.W.,
82173390 to M.L. and 52495014 to Y.W.), the National Key R&D Program of China (2020YFA0710700 and 2022YFC2303300 to Y.W.), the Strategic Priority Research Program of the Chinese Academy
of Sciences (XDB0490000 and XDB0940303 to Y.W.), the Anhui Provincial Key Research and Development Project (2023s07020019 to Y.W.), the Anhui Provincial Major Science and Technology Project
(202303a07020010 to Y.W.), the Anhui Provincial Natural Science Foundation (2408085J042 to M.L.), the project of collaborative innovation for colleges of Anhui province (GXXT-2022-063 to
M.L.) and the USTC Research Funds of the Double First-Class Initiative (YD9100002054 to Y.W. and YD9110002021 to M.L.). This work was partially carried out at the USTC Center for Micro and
Nanoscale Research and Fabrication. This work was partially carried out at the Instruments Center for Physical Science, University of Science and Technology of China. AUTHOR INFORMATION
Author notes * These authors contributed equally: Yang Liu, Qian Liu, Baowen Zhang. AUTHORS AND AFFILIATIONS * Department of Radiology, the First Affiliated Hospital of University of Science
and Technology of China, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China Yang Liu, Qian Liu, Shanshan Chen, Min Li & Yucai Wang *
National Key Laboratory of Immune Response and Immunotherapy, School of Basic Medical Sciences, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei,
China Yang Liu, Qian Liu, Baowen Zhang, Yanqiong Shen, Zhibin Li, Jiachen Zhang, Min Li & Yucai Wang * Institute of Health and Medicine, Hefei Comprehensive National Science Center,
Hefei, China Yanqiong Shen & Yucai Wang * RNAlfa Biotech, Hefei, China Yanqiong Shen, Yi Yang & Yucai Wang * Key Laboratory of Anhui Province for Emerging and Reemerging Infectious
Diseases, Hefei, China Min Li & Yucai Wang Authors * Yang Liu View author publications You can also search for this author inPubMed Google Scholar * Qian Liu View author publications You
can also search for this author inPubMed Google Scholar * Baowen Zhang View author publications You can also search for this author inPubMed Google Scholar * Shanshan Chen View author
publications You can also search for this author inPubMed Google Scholar * Yanqiong Shen View author publications You can also search for this author inPubMed Google Scholar * Zhibin Li View
author publications You can also search for this author inPubMed Google Scholar * Jiachen Zhang View author publications You can also search for this author inPubMed Google Scholar * Yi
Yang View author publications You can also search for this author inPubMed Google Scholar * Min Li View author publications You can also search for this author inPubMed Google Scholar *
Yucai Wang View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.W., M.L., Y.L. and Q.L. conceptualized and designed the research. Y.L., Q.L.,
B.Z., S.C., Y.S., Z.L., J.Z. and Y.Y. performed the experiments. S.C. provided help in designing LNP formulations. Y.L., Q.L. and B.Z. analysed the experimental data. Y.L., M.L., Q.L., B.Z.
and Y.W. prepared the figures and wrote the paper. Y.W. supervised the project. CORRESPONDING AUTHORS Correspondence to Min Li or Yucai Wang. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Biomedical Engineering_ thanks Jeffrey Hubbell, Tianmeng Sun and the other, anonymous, reviewer(s) for
their contribution to the peer review of this work. Peer reviewer reports are available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 IN VIVO-PRODUCED TOL-APCS INHIBIT RA PROGRESSION. A, Statistical data of OARSI score. B, C, The
percentage of IFN-γ+ (B) and TNF-α+ (C) area per FOV. D, Representative images of CD4, CD8, and Foxp3 staining from the knee joint of one mouse in a group of four. Scale bar = 200 µm. Arrows
refer to Foxp3+ cells. E-G, Number of CD4+ (E), CD8+ (F) and Foxp3+ (G) cells per FOV. RA mice were subcutaneously treated with PBS, LNPs, or LNPs/mPDL1 (5 μg mRNA) at the lower right back.
Mice treated with iTNF-α served as the positive control group. Normal group comprises healthy mice. _n_ = 4 biologically independent mice per group for data in A-C and E-G. Data are
expressed as the mean ± s.e.m. Statistical significances were determined using one-way ANOVA with Dunnett’s post hoc test. Comparisons were performed between the LNPs/mPDL1 group and each of
the other groups. N.S. is _P_ ≥ 0.05, and significant _P_ values are displayed. Source data EXTENDED DATA FIG. 2 IN VIVO-PRODUCED TOL-APCS MEDIATE POTENT THERAPEUTIC EFFECTS IN DSS-INDUCED
UC MICE. A, Representative images of CD8, Foxp3, IFN-γ, and TNF-α staining from the colon of one mouse in a group of four. Arrows refer to Foxp3+ cells. Scale bar = 200 µm. B-E, The number
of CD8+ (B) and Foxp3+ (C) cells and the percentage of IFN-γ+ (D) and TNF-α+ (E) area per FOV. Mice were treated with PBS, LNPs, or LNPs/mPDL1 (5 μg mRNA) via subcutaneous injection at the
lower right back. Mice treated with cyclosporine served as the positive control group. Normal group comprises healthy mice. _n_ = 4 biologically independent mice per group for data in B-E.
Data are expressed as the mean ± s.e.m. Statistical significances were determined using one-way ANOVA with Dunnett’s post hoc test. Comparisons were performed between the LNPs/mPDL1 group
and each of the other groups. N.S. is _P_ ≥ 0.05, and significant _P_ values are displayed. Source data SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary figures and tables.
REPORTING SUMMARY PEER REVIEW FILE SUPPLEMENTARY DATA Source data for the supplementary figures. SOURCE DATA SOURCE DATA FIGS. 2–7 AND EXTENDED DATA FIGS. 1 AND 2 Statistical source data.
RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other
rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Liu, Y., Liu, Q., Zhang, B. _et al._ Generation of tolerogenic antigen-presenting cells in vivo via the delivery of mRNA encoding PDL1 within
lipid nanoparticles. _Nat. Biomed. Eng_ (2025). https://doi.org/10.1038/s41551-025-01373-0 Download citation * Received: 08 January 2024 * Accepted: 27 February 2025 * Published: 28 March
2025 * DOI: https://doi.org/10.1038/s41551-025-01373-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
