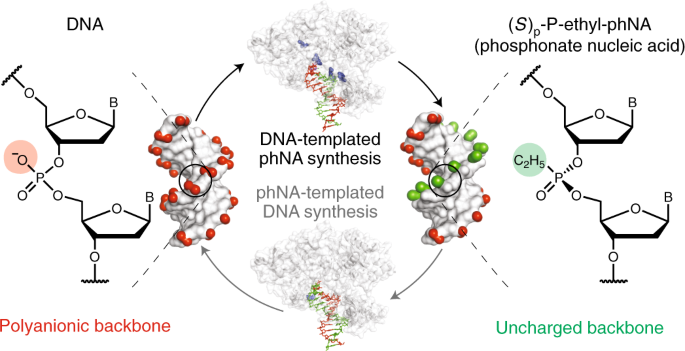
A synthetic genetic polymer with an uncharged backbone chemistry based on alkyl phosphonate nucleic acids
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The physicochemical properties of nucleic acids are dominated by their highly charged phosphodiester backbone chemistry. This polyelectrolyte structure decouples information content
(base sequence) from bulk properties, such as solubility, and has been proposed as a defining trait of all informational polymers. However, this conjecture has not been tested
experimentally. Here, we describe the encoded synthesis of a genetic polymer with an uncharged backbone chemistry: alkyl phosphonate nucleic acids (phNAs) in which the canonical, negatively
charged phosphodiester is replaced by an uncharged P-alkyl phosphonodiester backbone. Using synthetic chemistry and polymerase engineering, we describe the enzymatic, DNA-templated synthesis
of P-methyl and P-ethyl phNAs, and the directed evolution of specific streptavidin-binding phNA aptamer ligands directly from random-sequence mixed P-methyl/P-ethyl phNA repertoires. Our
results establish an example of the DNA-templated enzymatic synthesis and evolution of an uncharged genetic polymer and provide a foundational methodology for their exploration as a source
of novel functional molecules. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal
Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices
may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support
SIMILAR CONTENT BEING VIEWED BY OTHERS A MATING MECHANISM TO GENERATE DIVERSITY FOR THE DARWINIAN SELECTION OF DNA-ENCODED SYNTHETIC MOLECULES Article 06 December 2021 SYNTHETIC DNA
APPLICATIONS IN INFORMATION TECHNOLOGY Article Open access 17 January 2022 DIRECTED EVOLUTION AND SELECTION OF BIOSTABLE L-DNA APTAMERS WITH A MIRROR-IMAGE DNA POLYMERASE Article Open access
06 June 2022 DATA AVAILABILITY The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. The
molecular modelling data and related settings for computations that support the findings of this study are available in the Zenodo database (https://zenodo.org/) with the following record
2579703 (https://doi.org/10.5281/zenodo.2579703). REFERENCES * Westheimer, F. H. Why nature chose phosphates. _Science_ 235, 1173–1178 (1987). Article CAS Google Scholar * Benner, S. A.
Understanding nucleic acids using synthetic chemistry. _Acc. Chem. Res._ 37, 784–797 (2004). Article CAS Google Scholar * Benner, S. A. & Hutter, D. Phosphates, DNA, and the search
for nonterrean life: a second generation model for genetic molecules. _Bioorg. Chem._ 30, 62–80 (2002). Article CAS Google Scholar * Malyshev, D. A. & Romesberg, F. E. The expanded
genetic alphabet. _Angew. Chem. Int. Ed._ 54, 11930–11944 (2015). Article CAS Google Scholar * Pinheiro, V. B. & Holliger, P. The XNA world: progress towards replication and evolution
of synthetic genetic polymers. _Curr. Opin. Chem. Biol._ 16, 245–252 (2012). Article CAS Google Scholar * Pinheiro, V. B. et al. Synthetic genetic polymers capable of heredity and
evolution. _Science_ 336, 341–344 (2012). Article CAS Google Scholar * Taylor, A. I. et al. Catalysts from synthetic genetic polymers. _Nature_ 518, 427–430 (2015). Article CAS Google
Scholar * Malyshev, D. A. et al. A semi-synthetic organism with an expanded genetic alphabet. _Nature_ 509, 385–388 (2014). Article CAS Google Scholar * Liu, C. et al. Phosphonomethyl
oligonucleotides as backbone-modified artificial genetic polymers. _J. Am. Chem. Soc._ 140, 6690–6699 (2018). Article CAS Google Scholar * Zhang, S. L., Blain, J. C., Zielinska, D.,
Gryaznov, S. M. & Szostak, J. W. Fast and accurate nonenzymatic copying of an RNA-like synthetic genetic polymer. _Proc. Natl Acad. Sci. USA_ 110, 17732–17737 (2013). Article CAS
Google Scholar * Ghadessy, F. J. et al. Generic expansion of the substrate spectrum of a DNA polymerase by directed evolution. _Nat. Biotechnol._ 22, 755–759 (2004). Article CAS Google
Scholar * Shaw, B. R. et al. Reading, writing, and modulating genetic information with boranophosphate mimics of nucleotides, DNA, and RNA. _Ann. NY Acad. Sci._ 1002, 12–29 (2003). Article
CAS Google Scholar * King, D. J., Ventura, D. A., Brasier, A. R. & Gorenstein, D. G. Novel combinatorial selection of phosphorothioate oligonucleotide aptamers. _Biochemistry_ 37,
16489–16493 (1998). Article CAS Google Scholar * Meng, M. & Ducho, C. Oligonucleotide analogues with cationic backbone linkages. _Beilstein J. Org. Chem._ 14, 1293–1308 (2018).
Article Google Scholar * Nielsen, P. E. DNA analogues with nonphosphodiester backbones. _Annu. Rev. Biophys. Biomol. Struct._ 24, 167–183 (1995). Article CAS Google Scholar * Steinbeck,
C. & Richert, C. The role of ionic backbones in RNA structure: an unusually stable non-Watson–Crick duplex of a nonionic analog in an apolar medium. _J. Am. Chem. Soc._ 120, 11576–11580
(1998). Article CAS Google Scholar * Micklefield, J. Backbone modification of nucleic acids: synthesis, structure and therapeutic applications. _Curr. Med. Chem_. 8, 1157–1179 (2001).
Article CAS Google Scholar * Summerton, J. Morpholino antisense oligomers: the case for an RNase H-independent structural type. _Biochim. Biophys. Acta_ 1489, 141–158 (1999). Article CAS
Google Scholar * Nielsen, P. E. & Egholm, M. An introduction to peptide nucleic acid. _Curr. Issues Mol. Biol._ 1, 89–104 (1999). CAS PubMed Google Scholar * Dineva, M. A.,
Chakurov, S., Bratovanova, E. K., Devedjiev, I. & Petkov, D. D. Complete template-directed enzymatic synthesis of a potential antisense DNA containing 42 methylphosphonodiester bonds.
_Bioorgan. Med. Chem._ 1, 411–414 (1993). Article CAS Google Scholar * Higuchi, H., Endo, T. & Kaji, A. Enzymic synthesis of oligonucleotides containing methylphosphonate
internucleotide linkages. _Biochemistry_ 29, 8747–8753 (1990). Article CAS Google Scholar * Brudno, Y., Birnbaum, M. E., Kleiner, R. E. & Liu, D. R. An in vitro translation, selection
and amplification system for peptide nucleic acids. _Nat. Chem. Biol._ 6, 148–155 (2010). Article CAS Google Scholar * Murakami, A., Blake, K. R. & Miller, P. S. Characterization of
sequence-specific oligodeoxyribonucleoside methylphosphonates and their interaction with rabbit globin mRNA. _Biochemistry_ 24, 4041–4046 (1985). Article CAS Google Scholar * Arzumanov,
A. A. & Dyatkina, N. B. An alternative route for preparation of α-methylphosphonyl-β,γ-diphosphates of thymidine derivatives. _Nucleos. Nucleot._ 13, 1031–1037 (1994). Article CAS
Google Scholar * Burgers, P. M. J. & Eckstein, F. Stereochemistry of internucleotide bond formation by polynucleotide phosphorylase from _Micrococcus luteus_. _Biochemistry_ 18, 450–454
(1979). Article CAS Google Scholar * Xia, S. & Konigsberg, W. H. Mispairs with Watson–Crick base-pair geometry observed in ternary complexes of an RB69 DNA polymerase variant.
_Protein Sci._ 23, 508–513 (2014). Article CAS Google Scholar * Genna, V., Gaspari, R., Dal Peraro, M. & De Vivo, M. Cooperative motion of a key positively charged residue and metal
ions for DNA replication catalyzed by human DNA polymerase-η. _Nucleic Acids Res._ 44, 2827–2836 (2016). Article Google Scholar * Genna, V., Donati, E. & De Vivo, M. The catalytic
mechanism of DNA and RNA polymerases. _ACS Catal._ 8, 11103–11118 (2018). Article CAS Google Scholar * Genna, V., Carloni, P. & De Vivo, M. A strategically located Arg/Lys residue
promotes correct base paring during nucleic acid biosynthesis in polymerases. _J. Am. Chem. Soc._ 140, 3312–3321 (2018). Article CAS Google Scholar * Genna, V., Colombo, M., De Vivo, M.
& Marcia, M. Second-shell basic residues expand the two-metal-ion architecture of DNA and RNA processing enzymes. _Structure_ 26, 40–50.e2 (2018). Article CAS Google Scholar * Cozens,
C., Pinheiro, V. B., Vaisman, A., Woodgate, R. & Holliger, P. A short adaptive path from DNA to RNA polymerases. _Proc. Natl Acad. Sci. USA_ 109, 8067–8072 (2012). Article CAS Google
Scholar * Wynne, S. A., Pinheiro, V. B., Holliger, P. & Leslie, A. G. Structures of an apo and a binary complex of an evolved archeal B family DNA polymerase capable of synthesising
highly Cy-dye labelled DNA. _PLoS ONE_ 8, e70892 (2013). Article CAS Google Scholar * Bergen, K., Betz, K., Welte, W., Diederichs, K. & Marx, A. Structures of KOD and 9°N DNA
polymerases complexed with primer template duplex. _ChemBioChem_ 14, 1058–1062 (2013). Article CAS Google Scholar * Genna, V., Vidossich, P., Ippoliti, E., Carloni, P. & De Vivo, M. A
self-activated mechanism for nucleic acid polymerization catalyzed by DNA/RNA polymerases. _J. Am. Chem. Soc._ 138, 14592–14598 (2016). Article CAS Google Scholar * Nakamura, T., Zhao,
Y., Yamagata, Y., Hua, Y. J. & Yang, W. Watching DNA polymerase η make a phosphodiester bond. _Nature_ 487, 196–201 (2012). Article CAS Google Scholar * Pinheiro, V. B., Loakes, D.
& Holliger, P. Synthetic polymers and their potential as genetic materials. _BioEssays_ 35, 113–122 (2013). Article CAS Google Scholar * Dunn, M. R. & Chaput, J. C. Reverse
transcription of threose nucleic acid by a naturally occurring DNA polymerase. _ChemBioChem_ 17, 1804–1808 (2016). Article CAS Google Scholar * Thiviyanathan, V. et al. Structure of
hybrid backbone methylphosphonate DNA heteroduplexes: effect of _R_ and I stereochemistry. _Biochemistry_ 41, 827–238 (2002). Article CAS Google Scholar * Vyazovkina, E. V. et al.
Synthesis of specific diastereomers of a DNA methylphosphonate heptamer, d(CpCpApApApCpA), and stability of base pairing with the normal DNA octamer d(TpGpTpTpTpGpGpC). _Nucleic Acids Res._
22, 2404–2409 (1994). Article CAS Google Scholar * Tsai, C. H., Chen, J. & Szostak, J. W. Enzymatic synthesis of DNA on glycerol nucleic acid templates without stable duplex formation
between product and template. _Proc. Natl Acad. Sci. USA_ 104, 14598–14603 (2007). Article CAS Google Scholar * Burmeister, P. E. et al. Direct in vitro selection of a 2′-_O_-methyl
aptamer to VEGF. _Chem. Biol._ 12, 25–33 (2005). Article CAS Google Scholar * Alves Ferreira-Bravo, I., Cozens, C., Holliger, P. & DeStefano, J. J. Selection of
2′-deoxy-2′-fluoroarabinonucleotide (FANA) aptamers that bind HIV-1 reverse transcriptase with picomolar affinity. _Nucleic Acids Res._ 43, 9587–9599 (2015). PubMed PubMed Central Google
Scholar * Yu, H., Zhang, S. & Chaput, J. C. Darwinian evolution of an alternative genetic system provides support for TNA as an RNA progenitor. _Nat. Chem._ 4, 183–187 (2012). Article
CAS Google Scholar * Rangel, A. E., Chen, Z., Ayele, T. M. & Heemstra, J. M. In vitro selection of an XNA aptamer capable of small-molecule recognition. _Nucleic Acids Res_. 46,
8057–8068 (2018). Article CAS Google Scholar * Lee, E. J., Lim, H. K., Cho, Y. S. & Hah, S. S. Peptide nucleic acids are an additional class of aptamers. _RSC Adv._ 3, 5828–5831
(2013). Article CAS Google Scholar * Ichida, J. K. et al. An in vitro selection system for TNA. _J. Am. Chem. Soc._ 127, 2802–2803 (2005). Article CAS Google Scholar * Bing, T., Yang,
X. J., Mei, H. C., Cao, Z. H. & Shangguan, D. H. Conservative secondary structure motif of streptavidin-binding aptamers generated by different laboratories. _Bioorg. Med. Chem._ 18,
1798–1805 (2010). Article CAS Google Scholar * Weber, P. C., Ohlendorf, D. H., Wendoloski, J. J. & Salemme, F. R. Structural origins of high-affinity biotin binding to streptavidin.
_Science_ 243, 85–88 (1989). Article CAS Google Scholar * Freitag, S., LeTrong, I., Klumb, L., Stayton, P. S. & Stenkamp, R. E. Structural studies of the streptavidin binding loop.
_Protein Sci_. 6, 1157–1166 (1997). Article CAS Google Scholar * Houlihan, G., Arangundy-Franklin, S. & Holliger, P. Engineering and application of polymerases for synthetic genetics.
_Curr. Opin. Biotechnol._ 48, 168–179 (2017). Article CAS Google Scholar * Krishna, H. & Caruthers, M. H. Alkynyl phosphonate DNA: a versatile ‘click’able backbone for DNA-based
biological applications. _J. Am. Chem. Soc._ 134, 11618–11631 (2012). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by Trinity College Cambridge
(S.A.,-F.), by the Medical Research Council (S.A.-F. A.I.T., S.P.-C., P.H., program no. MC_U105178804), by the Biotechnology and Biological Sciences Research Council (B.T.P., BBSRC grant no
BB/N01023x/1), by the NICHD/ NIH Intramural Research Program (A.V. and R.W.) and by a European Molecular Biology Organization (EMBO) Long-Term Fellowship (V.G., ALTF 103-2018). AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * MRC Laboratory of Molecular Biology, Francis Crick Avenue, Cambridge Biomedical Campus, Cambridge, UK Sebastian Arangundy-Franklin, Alexander I.
Taylor, Benjamin T. Porebski, Sew Peak-Chew & Philipp Holliger * Institute for Research in Biomedicine (IRB Barcelona), The Barcelona Institute of Science and Technology, Barcelona,
Spain Vito Genna & Modesto Orozco * Section on DNA Replication, Repair and Mutagenesis, Bethesda, MD, USA Alexandra Vaisman & Roger Woodgate * Department of Biochemistry and
Biomedicine, University of Barcelona, Barcelona, Spain Modesto Orozco Authors * Sebastian Arangundy-Franklin View author publications You can also search for this author inPubMed Google
Scholar * Alexander I. Taylor View author publications You can also search for this author inPubMed Google Scholar * Benjamin T. Porebski View author publications You can also search for
this author inPubMed Google Scholar * Vito Genna View author publications You can also search for this author inPubMed Google Scholar * Sew Peak-Chew View author publications You can also
search for this author inPubMed Google Scholar * Alexandra Vaisman View author publications You can also search for this author inPubMed Google Scholar * Roger Woodgate View author
publications You can also search for this author inPubMed Google Scholar * Modesto Orozco View author publications You can also search for this author inPubMed Google Scholar * Philipp
Holliger View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.A.-F. and P.H. conceived and designed the experiments. S.A.-F. performed all the
experiments except the SPR measurements (A.T. and B.T.P.), MS (S.P.-C.) and steady-state kinetics (A.V. and R.W.) and Modelling and MD simulations (V.G. and M.O.). All the authors discussed
the results, and jointly wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Philipp Holliger. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION Supplementary Methods, Supplementary figures, Supplementary tables and Supplementary references. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Arangundy-Franklin, S., Taylor, A.I., Porebski, B.T. _et al._ A synthetic genetic polymer with an uncharged backbone chemistry based on alkyl phosphonate nucleic acids.
_Nat. Chem._ 11, 533–542 (2019). https://doi.org/10.1038/s41557-019-0255-4 Download citation * Received: 09 March 2018 * Accepted: 15 March 2019 * Published: 22 April 2019 * Issue Date: June
2019 * DOI: https://doi.org/10.1038/s41557-019-0255-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
