Bacterial c-di-gmp has a key role in establishing host–microbe symbiosis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Most microbes evolve faster than their hosts and should therefore drive evolution of host–microbe interactions. However, relatively little is known about the characteristics that
define the adaptive path of microbes to host association. Here we identified microbial traits that mediate adaptation to hosts by experimentally evolving the free-living bacterium
_Pseudomonas lurida_ with the nematode _Caenorhabditis elegans_ as its host. After ten passages, we repeatedly observed the evolution of beneficial host-specialist bacteria, with improved
persistence in the nematode being associated with increased biofilm formation. Whole-genome sequencing revealed mutations that uniformly upregulate the bacterial second messenger, cyclic
diguanylate (c-di-GMP). We subsequently generated mutants with upregulated c-di-GMP in different _Pseudomonas_ strains and species, which consistently increased host association. Comparison
of pseudomonad genomes from various environments revealed that c-di-GMP underlies adaptation to a variety of hosts, from plants to humans. This study indicates that c-di-GMP is fundamental
for establishing host association. SIMILAR CONTENT BEING VIEWED BY OTHERS GLOBAL ANALYSIS OF BIOSYNTHETIC GENE CLUSTERS REVEALS CONSERVED AND UNIQUE NATURAL PRODUCTS IN ENTOMOPATHOGENIC
NEMATODE-SYMBIOTIC BACTERIA Article Open access 25 April 2022 BACTERIAL–FUNGAL INTERACTIONS PROMOTE PARALLEL EVOLUTION OF GLOBAL TRANSCRIPTIONAL REGULATORS IN A WIDESPREAD _STAPHYLOCOCCUS_
SPECIES Article 31 July 2023 THE SACCHARIBACTERIUM TM7X ELICITS DIFFERENTIAL RESPONSES ACROSS ITS HOST RANGE Article Open access 24 August 2020 MAIN Host-associated microorganisms have
important effects on the physiological functioning and fitness of their plant and animal hosts1,2,3. These host–microbiota interactions are often studied using a host-centric view, with a
focus on microbiota-mediated host functions. This view neglects the important fact that most microbes evolve faster than their hosts due to their shorter generation times and higher mutation
rates, and thus that fitness improvements for the microbes may disproportionately drive the associations4. An important step in the evolution of a host–microbe association is the emergence
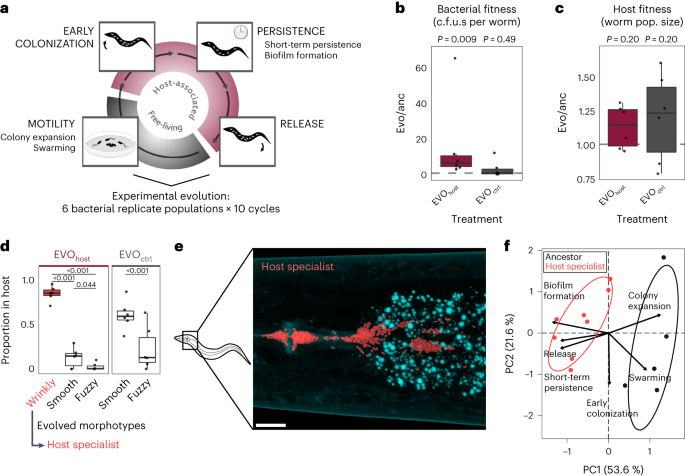
of a more specialized interaction that allows free-living bacteria to reliably enter the host, persist and finally be released into the environment to colonize new hosts (Fig. 1a)4. Thus
far, little is known about the traits and molecular processes that determine how bacteria adapt to such an association with the host. RESULTS EVOLUTION OF HOST-SPECIALIST BACTERIA We studied
the evolutionary transition from free-living to host association through controlled experimental evolution, using the bacterium _Pseudomonas lurida_ and the nematode host _Caenorhabditis
elegans_ as a model. This bacterium is occasionally found in the natural microbiota of _C. elegans_5,6. Under laboratory conditions, the presence of _P. lurida_ is associated with increased
population growth rates of _C. elegans_ and can provide protection against pathogens, yet both host and bacterium can proliferate without each other and thus do not depend upon one
another5,7,8. To select host-adapted bacteria, we serially passaged 6 _P. lurida_ populations either with or without a host-associated phase (Fig. 1a; EVOhost or EVOctrl, respectively). All
populations were inoculated from the same clonal ancestor. After 10 passages through hosts, the bacteria reached on average 5–10 times higher bacterial load in the host than their ancestor,
a significant change not observed for the control that evolved without exposure to hosts but otherwise had identical conditions (Fig. 1b and Extended Data Fig. 1). The increased bacterial
fitness did not come at a cost to the host, as nematode population growth (used as a proxy for nematode fitness5) did not change significantly, but rather increased in the presence of the
adapted bacteria (Fig. 1c). As a result of passaging, bacterial populations diversified in colony morphology. At the end of our experiment a ‘wrinkly’ morphotype was dominant in all
host-associated experimental replicate populations and absent in the controls, whereas ‘fuzzy’ and ‘smooth’ (ancestral) morphotypes were present across treatments (Fig. 1d and Supplementary
Table 1). Despite their significant advantage in hosts, the wrinkly morphotypes declined during growth on agar, while smooth and fuzzy types increased in abundance (Extended Data Fig. 1 and
Supplementary Table 2). As the wrinkly types were unique to and reached very high abundance in worm-adapted bacteria, we considered them host specialists. These specialists can be found in
clusters within the intestinal tract of the nematode, especially in the anterior and posterior parts (Fig. 1e and Extended Data Fig. 2). Notably, the evolved wrinkly morphotype is similar to
wrinkly _P. fluorescens_ that emerge at the air–liquid interface in static microcosms9 and to rugose variants of various pathogenic bacteria10,11,12. Our experiments suggest that this
morphological change also occurs in beneficial bacteria adapting to host association. For a further characterization of these adaptations, we focused on 47 clones of the distinct and
genetically stable morphotypes (Supplementary Table 3) isolated from the final populations of our evolution experiment. HOST SPECIALISTS HAVE A DISTINCT LIFESTYLE An analysis of trait
changes across the distinct stages of host association revealed specific adaptations of wrinkly morphotypes to the interaction with _C. elegans_. In detail, we characterized two traits of
importance for the free-living stage and four traits for host association (as listed in Fig. 1a). We found that the wrinkly isolate profiles were significantly distinct from the ancestral
trait profile (Fig. 1f and Supplementary Table 4). This was mainly due to significant increases in short-term persistence, release from the host and in vitro biofilm formation (Fig. 1f,
Extended Data Fig. 3 and Supplementary Tables 4–6)—all traits that define late-phase interactions with the host. The overall pattern of improved host association was also recovered by
analysing the genetically diverse populations from the end of the evolution experiment, where the host-associated populations similarly increased in persistence and release (Extended Data
Fig. 4 and Supplementary Tables 7 and 8). In detail, biofilm formation can enable persistent contact with the host and increase stress tolerance13,14, as exemplified by many pathogens15,
thereby improving survival in the nematode’s digestive tract. As a consequence of increased biofilm formation, aggregated cells may be expelled more easily16, thereby explaining the observed
increase in release. Such shedding also enhances the chance for transmission to other hosts4, which restarts the cycle of host association. Notably, wrinkly isolates did not differ from
ancestors in early colonization, yet showed a significant decrease in colony expansion and swarming on plates (Fig. 1f, Extended Data Fig. 3 and Supplementary Tables 4 and 5). The latter
result is consistent with a decrease in motility described for _E. coli_ that evolved to become a mutualist in stinkbugs17, but contrasts with findings that sufficient swarming is required
for colonization initiation of zebrafish and bobtail squid18,19. These contrasts are probably due to differences in symbiont recruitment between the host systems, defined by either aquatic
environments for zebrafish and squid, or terrestrial environments for _C. elegans_ and stinkbug. Moreover, our observations of increased biofilm formation and reduced motility may indicate
an evolved life-history trade-off between the traits defining host association and the free-living stage. We conclude that experimental evolution in the presence of the nematode host leads
to the emergence and spread of a host-specialist type. We next asked whether the improved host association has a common genetic basis. C-DI-GMP DETERMINES HOST SPECIALIZATION Whole-genome
sequencing of the isolated morphotypes and the ancestor revealed several independent mutations in wrinkly host specialists that affect the bacterial second messenger cyclic diguanylate
(c-di-GMP). In particular, a comparison of non-silent genomic variation identified variant genes specific to wrinkly host specialists (Fig. 2a and Supplementary Table 9). Two of the genes,
_wspE_ and _wspF_, code for a hybrid sensor histidine kinase and a methylesterase in the wrinkly spreader (_wsp_) operon, respectively20. These genes are part of a two-component system that
regulates c-di-GMP levels (Fig. 2g) and wrinkly formation in beta- and gamma-proteobacteria, including pseudomonads20,21,22,23. We found additional mutations unique to the host specialists
in the gene _rph_, encoding RNase PH that has not been linked to c-di-GMP signalling previously. Using both a fluorescence-based c-di-GMP sensor and liquid chromatography–mass spectrometry
(LC–MS), we found a roughly twofold c-di-GMP increase in three wrinkly isolates, each with a single mutation in either _wspE_, _wspF_ or _rph_, when compared with the ancestor (Fig. 2b,
Extended Data Fig. 5 and Supplementary Table 10). This points to a loss-of-function mutation in _wspF_ (which downregulates c-di-GMP) and alterations in active sites of WspE and Rph that all
converge at upregulating c-di-GMP. We aligned evolved and ancestral amino acid sequences (Extended Data Fig. 6) and confirmed a disruption in WspF functional domains, as well as a disrupted
receiver domain in WspE that probably prevents its de-autophosphorylation and thus constantly activates downstream WspR24. Amino acid substitutions in the exoribonuclease domain of Rph
further link its ribonuclease activity to c-di-GMP metabolism. As we observed similar increases in c-di-GMP levels in other wrinkly, but not in smooth or fuzzy mutants (Extended Data Fig.
5), we subsequently asked whether the wrinkly-specific mutations indeed cause improved host association. A functional genetic analysis of _wspE_, _wspF_ and _rph_ demonstrated their direct
involvement in host adaptation. For this analysis, we assessed the competitive fitness of mutants relative to the ancestor during host colonization. First, we re-assessed the three selected
wrinkly mutants and found them to be significantly more competitive than the ancestor (Fig. 2c, left panel, and Supplementary Table 11), alongside increased biofilm formation and decreased
swarming in vitro (Extended Data Fig. 7 and Supplementary Table 12). Thereafter, we rescued these mutants with the corresponding ancestral alleles, which indeed abolished the mutants’
fitness increase (Fig. 2c, middle panel, and Supplementary Table 11). Thirdly, an experimental introduction of each mutation into the ancestral background resulted in a significantly higher
competitiveness, at least for the _wspF_ and _rph_ mutations (Fig. 2c, right panel, and Supplementary Table 11). A similar fitness advantage was observed for the _wspE_ and _wspF_ mutants
when either was subjected to quartet competition with the ancestor and the two other morphotypes (Extended Data Fig. 8 and Supplementary Table 13). Notably, fitness advantages of evolved
mutants were consistently observed in a non-native host strain (the _C. elegans_ laboratory strain N2) (Fig. 2d and Supplementary Data Table 14). While two of these genes are components of
the Wsp system, which regulates c-di-GMP during surface sensing in other pseudomonads25,26, _Pl__MYb11 in theory possesses a variety of c-di-GMP modifying enzymes. This includes 34 genes
coding for GGDEF and 22 coding for EAL domains with putative diguanylate cyclase (DGC) and c-di-GMP-specific phosphodiesterase (PDE) functions, respectively. We validated the role of the Wsp
system’s cognate DGC in host adaptation using _wspR_ knockouts in our evolved host-specialist mutants. This change abolished the mutants’ competitive advantage in the host (Fig. 2e) and
caused a change from wrinkly to smooth colony morphology (Extended Data Fig. 7 and Supplementary Table 15), thus linking the DGC _wspR_ to _wspE_ and _wspF_ (as expected) and _rph_
(previously unknown). In addition, we directly manipulated c-di-GMP levels by heterologous expression of a PDE and a DGC from _P. aeruginosa_23,27, which respectively resulted in either
decreased or improved persistence in _C. elegans_, as expected (Fig. 2f, Extended Data Fig. 7 and Supplementary Table 16). We thus conclude that changes in _wspE_, _wspF_ and _rph_ that
converge on increasing c-di-GMP levels via the Wsp system enhance bacterial fitness in the host (Fig. 2g). As upregulation of this second messenger mediates a fundamental life-history
switch13, we next investigated whether it more generally mediates host association across pseudomonads. C-DI-GMP GENERALLY PROMOTES SYMBIOSIS Genetic manipulation of _wspF_ and a
bioinformatic analysis of _Pseudomonas_ genomes revealed a general involvement of _wsp_ genes in host association. For the former, we generated _wspF_ deletion mutants for _P. lurida_ strain
MYb193 and the distantly related _P. alkylphenolia_ MYb187 (both naturally associated with _C. elegans_), and further obtained mutant and wildtype _P. fluorescens_ strain SBW25, a model for
wrinkly formation21. We found that the mutants had significantly higher competitive fitness in the _C. elegans_ host than their respective wildtypes (Fig. 3a and Supplementary Table 17).
Furthermore, we correlated the presence of _wsp_ and _rph_ genes in 1,359 whole _Pseudomonas_ genomes from NCBI with the bacterial isolation source, a proxy for lifestyle (Extended Data Fig.
9 and Supplementary Table 18). _Pseudomonas_ isolates containing any of the _wsp_ genes or the complete, highly syntenic (Supplementary Table 19)20 _wsp_ operon were significantly more
often isolated from a host than isolates lacking these genes (Fig. 3b and Supplementary Table 19). These findings may seem surprising for genes with opposite regulatory effects (for example,
_wspE_ versus _wspF_), yet are probably explained by the syntenic inheritance of the entire operon with its set of interacting genes (Supplementary Table 19; see also ref. 20). Further,
_rph_ was more prevalent in isolates from healthy/undiagnosed hosts than from diseased hosts. Across lifestyles, we additionally detected signatures of negative selection for _wspE, wspF_
and _rph_, which additionally suggest that they are functionally stabilized by selection when present (Supplementary Table 20). We propose that the presence of these genes allows the
finetuned regulation of c-di-GMP and thereby, adjustment to a host-associated lifestyle. DISCUSSION Together, our study demonstrates that bacteria can improve their association with a host
by shifting their life history from a motile to a sessile, persisting lifestyle. This lifestyle shift results from correlated changes in a suite of life-history traits (Fig. 1f), which
together represent a transition in life-history strategy. One way to interpret this transition is as a shift along the r–K life-history continuum, from an r-like strategy characterized by
high reproductive rates to a K-like strategy characterized by persistence under high density conditions28,29. To demonstrate whether such a transition would generally lead to increased host
association, we used an extension of a previously published mathematical model of microbial evolution towards host association30. Exploration of a broad parameter space with this model
confirmed that increased within-host persistence is often the optimal strategy for microbial adaptation to hosts (Extended Data Fig. 10 and Supplementary Discussion), suggesting that the
results from our study may be generally applicable. In our experiments, the lifestyle shift from primarily free-living to host-associated is mediated by the Wsp system and subsequently,
activity of the bacterial second messenger c-di-GMP. C-di-GMP is well known to regulate key physiological functions in bacteria, including the regulation of virulence in bacterial
pathogens22,31. Our work demonstrates that this regulatory system promotes the adaptation of pseudomonads to diverse host systems, from plants to humans, not only in pathogens but extending
to beneficial host–bacterial relationships. Given the importance of beneficial microorganisms in the functioning of their hosts, understanding the mechanisms that mediate non-pathogenic
associations is crucial. Our study suggests that c-di-GMP plays an essential role in many such associations. METHODS HOST AND BACTERIAL STRAINS We performed evolution experiments with _P.
lurida_ strain MYb11 (_Pl__MYb11) and its natural host _C. elegans_ strain MY316 (_Ce__MY316) (ref. 5). In preparation for all experiments, we thawed frozen worm stocks (−80 °C) and raised
worms on nematode growth medium agar (NGM32) seeded with _E. coli_ OP50. In additional persistence colonization experiments, we used the standard laboratory strain _C. elegans_ N2 as a
non-native host for the evolved bacteria. A standard bleaching protocol was used to collect sterile and synchronized L1 larvae, which were then raised to L4 stage on _E. coli_ OP50 (20 °C),
unless stated otherwise. _P. lurida_ strains MYb11 and MYb193, and _P. alkylphenolia_ MYb187 were isolated from _Ce__MY316 (ref. 5), and _P. fluorescens_ SBW25 from sugar beet leaves9.
Bacteria were cultured on tryptic soy agar (20 °C, 48 h) and tryptic soy broth (28 °C, 150 r.p.m., overnight) unless stated otherwise. EVOLUTION EXPERIMENT Bacterial populations originating
from a clone of _Pl__MYb11 were serially passaged on NGM in the presence of _Ce__MY316 (host treatment, 6 replicates) or without worms (negative control, 6 replicates). For each replicate, a
lawn of _Pl__MYb11 was seeded onto NGM and cultured for 3.5 d. For each cycle of the host treatment, 10 _C. elegans_ L4 larvae were added per plate and incubated until the worms reached the
F1 generation (3.5 d). In the negative controls, bacteria were maintained on NGM without worms. At the end of every cycle, bacteria were collected from either worms or plates in the
host-associated and control treatments, respectively, 10% of the population (bottleneck) was transferred to the next cycle and a sample frozen (−80 °C). A similar number of colony-forming
units (c.f.u.) was used to bottleneck the negative control. A total of 10 cycles were performed. Frozen bacteria from cycle 10 were recovered and before further experiments were conducted,
these were subjected to one more cycle of the evolution experiment to minimize any potential selective effects of freezing/thawing. To focus on evolved differences between populations of the
host treatment and the negative control, rather than physiological responses to recent host exposure, bacteria were grown on NGM for 2 d as a common garden treatment and then used in
subsequent assays. BACTERIAL COLONIZATION OF INDIVIDUAL WORMS Bacterial fitness during host association was quantified as c.f.u.s per worm. In preparation, bacterial lawns (125 µl, optical
density (OD)600 = 2) were seeded on NGM and 5 synchronized L4 _Ce__MY316 added. After 3.5 d at 20 °C, worms were collected with M9 buffer containing 0.025% Triton-100 and 25 mM of the
paralyzing antihelminthic tetramisole. The worms were washed in buffer using a custom-made filter tip washing system33 and collected in M9 with Triton-100. Worm-free supernatant was
collected as a background sample. Following homogenization by bead beating, serial dilution and plating were used to quantify c.f.u.s. C.f.u.s per worm was calculated as the difference in
c.f.u. between worm and supernatant samples, divided by the number of worms per population. For diversified populations, colony morphologies were scored as smooth, fuzzy or wrinkly. WORM
POPULATION GROWTH Worm population growth resulting from 5 L4 larvae over 3.5 d was quantified as a proxy for host fitness. Bacteria and worms were prepared as for colonization assays and
washed worms frozen in 48-well plates. Photographs of worms were automatically scored in ImageJ2 (ref. 34): worms were detected as particles, approximated by ellipses, and those fitting _C.
elegans_-like dimensions (major axis 0.18–1.3 mm, minor axis ≤0.1 mm (ref. 35)) were counted. Detection quality was validated by correlating automatic worm counts with counts of two
independent experimenters (_r_(58) = 0.736, _P_ = 2.106 × 10−11). EARLY COLONIZATION, PERSISTENCE AND RELEASE IN WORMS To quantify early colonization, persistence and release from L4 stage
worms, bacterial lawns were prepared from ancestral _Pl__MYb11 and evolved populations (post common garden) or clonal morphotypes (overnight cultures). In early colonization assays, we
quantified bacteria that entered L4 _Ce__MY316 that were previously raised on non-colonizing _E. coli_ O50. Colonization levels were then assayed as above resulting in c.f.u.s per worm as a
measure of early colonization. For persistence and release assays, worms were raised on the respective assay bacteria (from L1 until L4 stage), mimicking the development of worms in the F1
generation of the evolution experiment. Worms were then collected, washed using the filter tip washing system and samples divided into supernatant (supernatant 1) and worm sample (100 µl
each). Worms were then suspended in 200 µl M9 and incubated for 1 h, after which 100 µl supernatant containing released bacteria (supernatant 2) was collected. The c.f.u.s released per worm
were determined by the difference in c.f.u.s between supernatant 2 and supernatant 1. Along with this, we quantified c.f.u.s maintained in worms of this sample as a measure of persistence.
BACTERIAL GROWTH, COLONY EXPANSION AND SWARMING To measure bacterial growth, bacterial populations (common garden treatment or overnight cultures) were adjusted to OD600 = 0.1 and 50 µl
spotted on NGM. After incubation (24 h or 3 d at 20 °C), lawns were scraped off, homogenized and c.f.u.s determined by serial dilution. Colony expansion and swarming were assayed on NGM
containing 0.5% or 3.4% agar, respectively. In either case, 0.5 µl of cell suspension (OD600 = 1) was spotted on surface-dried agar plates. Colony diameter was measured after 24 h, 3 and 7
d. BIOFILM FORMATION In vitro biofilm formation was assayed in microtitre plates as described previously36. Notably, assays were performed in a randomized layout in Nunclon Delta
surface-treated plates. Staining was performed after 48 h of incubation (20 °C, orbital shaking at 180 r.p.m.). Absorption of dyed biofilm solutions was measured at 550 nm using Gen5
microplate reader and Imager software (Biotek, v.3.08.01). To illustrate biofilm formation in liquid, glass test tubes were filled with 2 ml tryptic soy broth, inoculated with single
colonies of ancestral _Pl__MYb11 or evolved host-specialist mutants (_wspE_, _wspF_, _rph_) and incubated at 20 °C for 48 h until photographing. ISOLATION OF MORPHOTYPES Representative
colonies with visually distinct morphologies were isolated from evolved cycle 10 populations. The evolved populations were thawed, serially diluted and plated (48 h, 20 °C). Unique
morphotypes from all evolved populations were re-streaked and archived as frozen stocks (Supplementary Table 3). All morphotypes were thawed and re-streaked once, and showed stable colony
morphology during 2 d of incubation. GROWTH OF MACROCOLONIES Macrocolonies of _Pl__MYb11 morphotypes and mutants were prepared as described previously37. Briefly, 5 µl of overnight culture
were spotted on tryptic soy agar plates supplemented with 40 μg ml−1 Congo Red and incubated at 20 °C. After 24 h or 48 h, photographs were taken using a Leica fluorescence dissecting scope
(LEICA M205 FA). FLUORESCENT LABELLING OF WRINKLY MORPHOTYPE MT12 AND IN VIVO MICROSCOPY The wrinkly morphotype MT12 was labelled with red fluorescent dTomato (dT) using Tn7 transposon-based
chromosomal insertion as previously described38,39. Insertion of the label did not affect the wrinkly morphology of the colonies. Fluorescently labelled MT12 was used to localize
colonization in _Ce__MY316 using confocal laser scanning microscopy (ZEISS LSM 880). For this, synchronized L1 stage larvae were exposed to labelled bacteria for 72 h (20 °C), then collected
using gravity washing and mounted for microscopy as previously described39. Overviews of complete worms were created using a ×25 LD LCI Plan-Apochromat multi-immersion objective (numerical
aperture (NA) = 0.8) and details imaged using a ×40 C-Apochromat water immersion objective (NA = 1.2), in both cases using Immersol W (2010) with a refractive index of 1.334. Bacterial
fluorescence and worm autofluorescence were sequentially excited (561 nm and 488 nm) and detected with an Airyscan detector (R-S sensitivity mode; longpass filter ≥570 nm; bandpass filter
495–550 nm). Data were processed with the automatic Airyscan processing function of ZEISS Efficient Navigation 2. For a list of the genetically modified bacteria used in this study, see
Supplementary Table 21. After looking at the colonization of >10 worms in at least 3 biological replicate populations of consecutive weeks of experiments, a representative worm was imaged
for Fig. 1e and Extended Data Fig. 2. GENOME SEQUENCING AND ANALYSIS Total DNA was isolated using a cetyl-trimethylammonium-bromid-based protocol40. For Illumina MiSeq (paired-end, 300 bp)
sequencing, libraries were prepared using the Nextera DNA Flex kit. Read quality was inspected using FastQC (v.0.11.8) (ref. 41) and reads trimmed using Trimmomatic (v.0.3.9) (ref. 42).
Paired reads were aligned to the _Pl__MYb11 reference genome (RefSeq: GCF_002966835.1; Bowtie2 v.2.3.3 (ref. 43)) and duplicate regions removed using Picardtools (v.2.22.2) (ref. 44).
Variants were called using BCFtools (v.1.10.2) (ref. 45) and VarScan (v.2.3.9) (ref. 46), and then annotated (snpEff47,48). We filtered for non-synonymous variants not present in the
ancestral control in R49,50. Gene ontology was inferred using Pseudomonas.com51. To infer genes coding for enzymes with putative DGC or PDE activity, we searched for proteins with GGDEF and
EAL domains using the InterProScan of the conserved domains database (CDD) via Pseudomonas.com51. AMINO ACID SEQUENCE ALIGNMENTS To prepare amino acid sequence alignments of ancestral and
mutated WspE, WspF and RPH, nucleotide sequences were translated using EMBOSS Transeq52 (frame 1; bacterial codon table; forward for _wspE_ and _wspF_, reverse for _rph_) and resulting amino
acid sequences aligned using Clustal Omega52 (v.1.2.4; ClustalW with character counts and standard settings). For annotation and visualization of protein domains, domain predictions of the
respective sequences were collected from Pfam/InterPro (sourced from Pseudomonas.com51) and visually highlighted in protein visualizations prepared with DOG (v.2.0)53. QUANTIFICATION OF
RELATIVE C-DI-GMP ABUNDANCES USING A BIOSENSOR To quantify intracellular concentrations of c-di-GMP in ancestral _Pl__MYb11 and evolved wrinkly isolates (MT12: _wspF_EVO, MT14: _wspE_EVO and
MT22: _rph_EVO), we used an established plasmid-based biosensor54. Bacterial strains carrying the plasmid were grown on gentamicin-selective plates (70 h, 20 °C). For microscopy, single
colonies were resuspended in 1X PBS, spotted on 2% agarose patches on microscopy slides and sealed. Bacterial fluorescence was visualized using confocal laser scanning microscopy (ZEISS LSM
700 with ×40 Plan-Apochromat oil immersion objective (NA = 1.4) and Immersol 518F with a refractive index of 1.518). Fluorescence of the sensor and normalizer were sequentially excited (555
nm and 488 nm) and detected with a photomultiplier tube detector and a variable secondary dichroic transmitting light with wavelengths ≤630 nm and ≤550 nm, respectively. The excitation and
detection settings were kept identical across all measurements. Fluorescence intensity per cell was measured in Image J34: all cells and five background areas were identified as regions of
interest, and area, integrated density and mean grey values were measured. Data from the untransformed images were used to calculate the corrected total cell fluorescence55. In addition to
single-cell measurements, we quantified c-di-GMP at the population level. For this, colonies of evolved wrinkly (MT12, MT21, MT25, MT26), smooth (MT13, MT33) and fuzzy (MT11) isolates were
grown as described above, resuspended in 1X PBS and adjusted to OD600 = 0.1. Cell suspensions (200 µl) were then transferred to black, flat-bottomed 96-well plates with transparent bottoms
(Greiner Bio-One CELLSTAR 96-well, cell culture-treated) in triplicate. After shaking (10 s, orbital shaking at 1 mm amplitude), fluorescence was sequentially excited (454 nm and 460 nm,
bandwidth 9 nm, 10 flashes) and emission detected (585 nm and 510 nm, bandwidth 20 nm; optimal gain, 20 µs integration) in a plate reader (Tecan, Infinite M200Pro), with 1X PBS serving as
the background control. To infer c-di-GMP concentration, we calculated the relative fluorescence intensity, or the ratio between TurboRFP and AmCyan fluorescence intensities, as previously
described54, and compared average relative fluorescence intensities between ancestral _Pl__MYb11 and evolved wrinkly, smooth and fuzzy morphotypes. For the images used in Fig. 2, linear LUT
was used at full range. Brightness and contrast were applied equally to all images. QUANTIFICATION OF C-DI-GMP USING PARALLEL REACTION MONITORING LC–MS/MS To quantify intracellular c-di-GMP
using LC–MS in parallel reaction monitoring mode, ancestral and evolved _Pl__MYb11 (MT12, MT14 and MT22) were grown in LB medium to an OD600 of 1.8 and pelleted by centrifugation. After
washing with salt-free LB medium, pelleted cells were snap frozen and stored (−80 °C). Cells were mixed with 10 pmol of internal standard (cyclic-di-GMP-13C20,15N10, Toronto Research
Chemicals) in 60 μl of water. Extraction of c-di-GMP was performed as previously described56 with the following modifications: extraction solution (240 μl of 1:1 acetonitrile (ACN)/methanol
(MeOH)) was added and samples were vigorously vortexed. Following incubation on ice (15 min) and centrifugation (20,800 × _g_, 4 °C, 2 min), extract supernatant was collected and solvent
extraction repeated twice (200 μl of 2:2:1 ACN/MeOH/water). Pooled extracts were dried, resuspended in 50 μl of water and centrifuged to remove insoluble compounds. Concentrations of
solubilized protein precipitates were determined using the Pierce BCA protein assay kit (Thermo Fisher). For LC–MS/MS, 1 μl extract was injected onto an EASY-nLC 1000 UHPLC (Thermo Fisher)
and separated on a 15-cm ReproSil-Pur C18-AQ nano LC column (0.1 mm i.d., 1.9 μm, 120 Å, Altmann Analytik) at 400 nl min−1. Eluent A was 10 mM NH4OAc with 0.1% HAc, eluent B was 100% MeOH.
Chromatographic conditions were 5% eluent B (5 min), followed by a linear gradient from 5% to 20% B (15 min) and an increase to 70% B (1 min), followed by 70% B (5 min) and 5% B (5 min);
higher-energy collisional dissociation of the _m_/_z_ 691.1021 and _m_/_z_ 721.0714 precursors was performed on a Q Exactive HF Orbitrap MS (Thermo Fisher). Peak areas for the qualifying57
product ions _m_/_z_ 248.0778 (light) and _m_/_z_ 263.0965 (heavy) determined in Skyline (v.21.1.0.146.3, MacCoss Lab software)58 were used to calculate total c-di-GMP amounts, which were
normalized to total protein amount as obtained by the BCA assay. MUTANT GENERATION A two-step allelic replacement method based on previously described protocols21,59 was used to introduce
the evolved mutant alleles into an ancestral background and also to revert mutations by introducing ancestral alleles in the mutant background. We applied the following modifications: ~700
bp long PCR amplicons surrounding each mutation were cloned into pUISacB allowing for sucrose selection. The constructs were transformed into competent _E. coli_ cells and transferred to
_Pseudomonas_ isolates via conjugative mating with an _E. coli_ helper strain containing pRK2013 (ref. 60). Primers (see Supplementary Table 22) were designed using NCBI’s BLAST tool61 and
NCBI Primer-BLAST62, NEBuilder v.2.3.0 (New England Biolabs) and Oligo Analyse Tool (Eurofins Genomics). BLASTn and alignments with Clustal Omega63 were performed using default settings.
HETEROLOGOUS EXPRESSION OF PHOSPHODIESTERASE AND DIGUANYLATE CYCLASE To manipulate intracellular c-di-GMP levels and study the consequences for host association and colony morphology, we
expressed a heterologous PDE and a heterologous DGC in our evolved host-specialist mutants (_wspE, wspF_ and _rph_). The PDE PA2133 from _P. aeruginosa_ was expressed from plasmid pJN2133
(ref. 23). A constitutively active GCN4-WspR fusion construct27 was synthesized (Eurofins) and then cloned into pJStrep to generate a C-terminal StrepII-tagged GCN4-WspR construct. Empty
pJStrep (a modified pJN105 (ref. 64) vector containing the StrepII tag coding sequence) and empty pJN105 plasmids were used as controls. All plasmids were introduced into _Pl__MYb11 and
evolved mutants using a previously described electroporation protocol65. IN VIVO COMPETITION ASSAYS Competition experiments were performed as described for the short-term persistence assays.
Co-inoculated bacteria were OD-adjusted and mixed in equal volumes before seeding as lawns on NGM agar. A _Pl__MYb11 labelled with dTomato39 was used, which is equivalent to the ancestral
_Pl__MYb11, as no differences were observed in short-term persistence (analysis of variance (ANOVA), _F_ value = 0.99, d.f. = 1, _P_ = 0.35). C.f.u.s per worm were determined by subtracting
c.f.u.s in supernatants from those in worm samples. A competitive index was calculated as the ratio of c.f.u.s per worm of evolved or constructed mutants to c.f.u.s per worm of the ancestor.
CORRELATION OF _WSP_ AND _RPH_ GENE PRESENCE WITH ISOLATE SOURCE ACROSS PSEUDOMONADS Whole-genome sequences from NCBI were mined for c-di-GMP modulating genes (focus: _wsp_ operon, _rph_)
with bacterial lifestyle in members of the genus _Pseudomonas_. First, candidate genomes were obtained (NCBI Nucleotide’s command line search tool; size: 5–8 million bp). This retrieved
2,279 sequences, for which sample information from NCBI’s Biosample database was collected. When available, host, host disease status, isolation source and sample type were used to manually
classify genomes as originating from free-living or host-associated isolates with or without/unknown disease (Supplementary Table 18). Next, we downloaded all available _Pseudomonas_
reference sequences for _rph_ and _wsp_ genes from pseudomonas.com51. These were used to identify candidate sequences of _rph_, _wspA_, _wspB_, _wspC_, _wspD_, _wspE_, _wspF_ and _wspR_.
These target gene candidates were found in the selected genomes using BLAST (R package ‘rBLAST’) and filtered on the basis of sequence lengths and percent identities of the BLAST hits
(Extended Data Fig. 9). Percent identity and sequence length were selected to maximize the chance that genes were correctly identified (red rectangles in Extended Data Fig. 9). If at least
one candidate gene was identified during BLAST searches with the reference genes as query, this gene was considered present in the respective genome. We then used _χ_2 goodness-of-fit tests
to infer whether isolates with and without the target genes differed in the relative proportions of host-associated lifestyles (Supplementary Table 19). DETECTION OF SIGNATURES OF SELECTION
To assess whether our focal host-specialist genes (_wspE, wspF, rph_) were experiencing positive or purifying selection in the genus _Pseudomonas_, we performed MUSCLE codon-based multiple
sequence alignments of nucleotide sequences (see dataset described above) using MEGA11 (ref. 66; default settings). Subsequently, we performed codon-based _z_-tests (default settings) to
test for significant deviations from neutral selection. In addition, we analysed signatures of selection in Blast hits for a set of three _Pseudomonas_ core genes (_gyrB:_ PA0004, _rpoD:_
PA0576 and a 16S rRNA methyltransferase: PA0419; see also ref. 67) in the set of genomes studied for _wsp_ and _rph_ presence/absence, also using multiple sequence alignments and codon-based
tests of neutrality. STATISTICAL ANALYSES Before data collection, no statistical methods were used to pre-determine samples sizes, but our sample sizes are similar to those reported in
previous publications. In all experiments, treatments and samples were blinded and randomized. Before data analysis, assumptions of parametric models (normality, homogeneity of variances)
were checked by visual inspection (box-/qqplots) and with Shapiro–Wilk and Levene tests. When these were not met, non-parametric tests were applied. Boxplots show median (centre line),
upper/lower quartiles (box limits) and 1.5× interquartile ranges (whiskers). To check whether evolved populations differed from the ancestor in c.f.u.s per worm, we compared the shift in the
evolved phenotype (ratio of c.f.u.s per worm of evolved populations to those of ancestral _Pl__MYb11) to the ancestral phenotype using one-sample _t_-tests (alpha = 0.05, mu = 1) with false
discovery rate (FDR68) correction for multiple testing. We applied this approach to analyse: bacterial colonization of individual worms, worm population growth, early colonization,
persistence and release, colony expansion and swarming. To infer overall phenotypic shifts according to evolutionary treatment, a principal component analysis (PCA) including the assayed
phenotypes was performed. We performed permutational analysis of variances (PERMANOVA, 1,000 permutations) followed by pairwise comparisons of groups (FDR-corrected) to test for differences
in phenotype sets of ancestral and evolved groups, and plotted confidence ellipses (one standard deviation). Packages used included ggbiplot69, missMDA70, vegan71 and pairwise.adonis72.
Differences in proportions of the different colony morphologies (wrinkly, smooth and fuzzy) within worms were identified using generalized linear models (GLM; quasinormal distribution) with
Tukey post hoc tests (using lme4 (ref. 73), lmtest74 and multcomp75). Changes in morphotype proportions over time were tested using beta-regressions (using gamlss76). Differences between
morphotype phenotypes were detected using ANOVA or GLMs, followed by Tukey or Dunnett post hoc tests. To infer functional specializations across phenotypes, we used PCA and PERMANOVA.
Differences in biofilm formation and motility between evolved wrinkly host specialists (_wspE_, _wspF_ and _rph_ mutants) and ancestral _Pl__MYb11 were analysed using nested ANOVAs followed
by Tukey post hoc tests. When no batch effects (evolved population of origin) were detected, mutants were compared across populations. In the case of swarming diameters, however, the _rph_
mutant was compared to the co-analysed ancestral _Pl__MYb11 using a _t_-test. Differences in c-di-GMP concentrations between evolved isolates were inferred using Welch’s ANOVA, nested ANOVA
or ANOVA with Games–Howell or Dunnett post hoc comparisons. We tested for differences in c.f.u.s per worm between morphotypes or mutants using GLMs and linear mixed models (LMMs), and
Dunnett or Tukey post hoc comparisons. All analyses and plotting were performed in R49,50,77,78. MATHEMATICAL MODEL We built a model to assess the selection gradient experienced by bacteria
during the evolution experiment (Extended Data Fig. 10). We focused on the phase when bacteria are in contact with worms and considered a homogeneous population. The dynamics of the number
of bacteria living in (any) host association _n(t)_ can be described by the equation
$$\frac{{dn}(t)}{{dt}}=f\,W\left(t\right)+r\,n\left(t\right)\left(1-\frac{n\left(t\right)}{K\,W\left(t\right)}\right)-\delta n(t),$$ (1) where _W(t)_ denotes the biomass of worms on the
plate at time _t_. We consider that growing to saturation, bacteria on the plate are always in excess so that only the number of worms and the rate _f_ at which they feed on bacteria limit
the immigration of free-living bacteria to the host. We assume logistic growth of the bacterial population within the worms, with maximal rate _r_ and a carrying capacity proportional to the
biomass of worms _W_(_t_) and the per unit of worm biomass carrying capacity _K_. Finally, a fraction of the host-associated bacterial population is removed from the host at a rate _δ_,
which encompasses bacterial death and expulsion to the environment. As in the evolution experiment, we assume that only host-associated bacteria are selected and continue to the next cycle,
ignoring on-plate dynamics. We assume linear growth for the worm biomass, _W_(_t_) = _g t_ + _W__0_, encompassing both reproduction and development. We neglect the potential evolution of
beneficial effects on worm growth and fix the parameters _W_0 = 10 and _g_ = 711 d−1 to experimentally observed values. We studied how the final number of host-associated bacteria, _n_f, is
affected by changes in the parameters that describe the bacterial life cycle (_r, δ, f, K_). We defined a range of biologically plausible values for each of these parameters (that is, the
trait space) that are informed by experimental data where possible: * 10−1 d−1 < _r_ < 101.25 d−1, that is, between a small fraction and around twice the maximum on-plate growth rate
(~7 d−1). * 10−0.5 d−1 < _δ_ < 104 d−1, as the typical time for a worm to lose 50% of its microbiome (in the absence of feeding and replication) should range between seconds and days.
* 104 < _K_ < 106.25, given the orders of magnitude from the maximal number of bacteria per worm measured experimentally (~105). * 103 d−1 < _f_ < 107.5 d−1, as the typical time
for an empty worm to be colonized at 10% of its carrying capacity (_K_ = 105) should vary between seconds and days, neglecting bacterial release and within-host replication. For each point
of the trait space, we numerically solved equation (1) to compute the expected final number of bacteria at _t_f = 3.5 d, _n_f = _n(t_f). Finally, we assessed the elasticity of _n_f along
each direction of the trait space, which measures the expected relative change in _n_f with respect to a small relative change in one of the traits. We interpreted the vector of the
elasticities as the selection gradient on the phenotypic traits79 and used the dominant element of this vector to define an ‘optimal evolution strategy’30 for each point of the trait space.
REPORTING SUMMARY Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY Raw sequencing data are available at
NCBI under Bioproject PRJNA862108. All other data are accessible at https://github.com/nobeng/c-di-GMP_host-association. CODE AVAILABILITY Custom code and associated data are available at
https://github.com/nobeng/c-di-GMP_host-association. REFERENCES * McFall-Ngai, M. et al. Animals in a bacterial world, a new imperative for the life sciences. _Proc. Natl Acad. Sci. USA_
110, 3229–3236 (2013). Article CAS PubMed PubMed Central Google Scholar * Toft, C. & Andersson, S. G. E. Evolutionary microbial genomics: insights into bacterial host adaptation.
_Nat. Rev. Genet._ 11, 465–475 (2010). Article CAS PubMed Google Scholar * Douglas, A. E. _Fundamentals of Microbiome Science: How Microbes Shape Animal Biology_ (Princeton Univ. Press,
2018). * Obeng, N., Bansept, F., Sieber, M., Traulsen, A. & Schulenburg, H. Evolution of microbiota–host associations: the microbe’s perspective. _Trends Microbiol_. 29, 779–787 (2021).
Article CAS PubMed Google Scholar * Dirksen, P. et al. The native microbiome of the nematode _Caenorhabditis elegans_: gateway to a new host-microbiome model. _BMC Biol._ 14, 38 (2016).
Article PubMed PubMed Central Google Scholar * Johnke, J., Dirksen, P. & Schulenburg, H. Community assembly of the native _C. elegans_ microbiome is influenced by time, substrate and
individual bacterial taxa. _Environ. Microbiol._ 22, 1265–1279 (2020). Article CAS PubMed Google Scholar * Kissoyan, K. A. B. et al. Natural _C. elegans_ microbiota protects against
infection via production of a cyclic lipopeptide of the viscosin group. _Curr. Biol._ 29, 1030–1037.e5 (2019). Article CAS PubMed Google Scholar * Dirksen, P. et al. CeMbio—the _C.
elegans_ microbiome resource. _G3_ 10, 3025–3039 (2020). Article CAS PubMed PubMed Central Google Scholar * Rainey, P. B. & Travisano, M. Adaptive radiation in a heterogeneous
environment. _Nature_ 394, 69–72 (1998). Article CAS PubMed Google Scholar * Starkey, M. et al. _Pseudomonas aeruginosa_ rugose small-colony variants have adaptations that likely promote
persistence in the cystic fibrosis lung. _J. Bacteriol._ 191, 3492–3503 (2009). Article CAS PubMed PubMed Central Google Scholar * Anriany, Y. A., Weiner, R. M., Johnson, J. A.,
Rezende, C. E. D. & Joseph, S. W. _Salmonella enterica_ serovar Typhimurium DT104 displays a rugose phenotype. _Appl. Environ. Microbiol._ 67, 4048–4056 (2001). Article CAS PubMed
PubMed Central Google Scholar * Yildiz, F. H. & Schoolnik, G. K. _Vibrio cholerae_ O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide
production, chlorine resistance, and biofilm formation. _Proc. Natl Acad. Sci. USA_ 96, 4028–4033 (1999). Article CAS PubMed PubMed Central Google Scholar * Hengge, R. Linking bacterial
growth, survival, and multicellularity—small signaling molecules as triggers and drivers. _Curr. Opin. Microbiol._ 55, 57–66 (2020). Article CAS PubMed Google Scholar * Pankey, M. S. et
al. Host-selected mutations converging on a global regulator drive an adaptive leap towards symbiosis in bacteria. _eLife_ 6, e24414 (2017). Article Google Scholar * Hall-Stoodley, L.,
Costerton, J. W. & Stoodley, P. Bacterial biofilms: from the natural environment to infectious diseases. _Nat. Rev. Microbiol._ 2, 95–108 (2004). Article CAS PubMed Google Scholar *
Schlomann, B. H., Wiles, T. J., Wall, E. S., Guillemin, K. & Parthasarathy, R. Sublethal antibiotics collapse gut bacterial populations by enhancing aggregation and expulsion. _Proc.
Natl Acad. Sci. USA_ 116, 21392–21400 (2019). Article CAS PubMed PubMed Central Google Scholar * Koga, R. et al. Single mutation makes _Escherichia coli_ an insect mutualist. _Nat.
Microbiol._ https://doi.org/10.1038/s41564-022-01179-9 (2022). * Robinson, C. D. et al. Host-emitted amino acid cues regulate bacterial chemokinesis to enhance colonization. _Cell Host
Microbe_ 29, 1221–1234.e8 (2021). Article CAS PubMed PubMed Central Google Scholar * Isenberg, R. Y., Christensen, D. G., Visick, K. L. & Mandel, M. J. High levels of cyclic
diguanylate interfere with beneficial bacterial colonization. _mBio_ 0, e01671-22 (2022). Google Scholar * Kessler, C., Mhatre, E., Cooper, V. & Kim, W. Evolutionary divergence of the
Wsp signal transduction systems in Beta- and Gammaproteobacteria. _Appl. Environ. Microbiol._ https://doi.org/10.1128/AEM.01306-21 (2021). Article PubMed PubMed Central Google Scholar *
Bantinaki, E. et al. Adaptive divergence in experimental populations of _Pseudomonas fluorescens_. III. Mutational origins of wrinkly spreader diversity. _Genetics_ 176, 441–453 (2007).
Article CAS PubMed PubMed Central Google Scholar * Jenal, U., Reinders, A. & Lori, C. Cyclic di-GMP: second messenger extraordinaire. _Nat. Rev. Microbiol._ 15, 271–284 (2017).
Article CAS PubMed Google Scholar * Hickman, J. W., Tifrea, D. F. & Harwood, C. S. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate
levels. _Proc. Natl Acad. Sci. USA_ 102, 14422–14427 (2005). Article CAS PubMed PubMed Central Google Scholar * Bourret, R. B. Receiver domain structure and function in response
regulator proteins. _Curr. Opin. Microbiol._ 13, 142–149 (2010). Article CAS PubMed PubMed Central Google Scholar * Laventie, B.-J. & Jenal, U. Surface sensing and adaptation in
bacteria. _Annu. Rev. Microbiol._ 74, 735–760 (2020). Article CAS PubMed Google Scholar * O’Neal, L. et al. The Wsp system of _Pseudomonas aeruginosa_ links surface sensing and cell
envelope stress. _Proc. Natl Acad. Sci. USA_ 119, e2117633119 (2022). Article PubMed PubMed Central Google Scholar * De, N., Navarro, M. V. A. S., Raghavan, R. V. & Sondermann, H.
Determinants for the activation and autoinhibition of the diguanylate cyclase response regulator WspR. _J. Mol. Biol._ 393, 619–633 (2009). Article CAS PubMed PubMed Central Google
Scholar * Pianka, E. R. On r- and K-selection. _Am. Nat._ 104, 592–597 (1970). Article Google Scholar * Andrews, J. H. _Comparative Ecology of Microorganisms and Macroorganisms_
(Springer, 2017). * Bansept, F., Obeng, N., Schulenburg, H. & Traulsen, A. Modeling host-associating microbes under selection. _ISME J._ 15, 3648–3656 (2021). Article PubMed PubMed
Central Google Scholar * Valentini, M. & Filloux, A. Multiple roles of c-di-GMP signaling in bacterial pathogenesis. _Annu. Rev. Microbiol._ 73, 387–406 (2019). Article CAS PubMed
Google Scholar * Stiernagle, T. Maintenance of C. elegans. _WormBook_ https://doi.org/10.1895/wormbook.1.101.1 (2006). * Papkou, A. et al. The genomic basis of Red Queen dynamics during
rapid reciprocal host–pathogen coevolution. _Proc. Natl Acad. Sci. USA_ 116, 923–928 (2019). Article CAS PubMed Google Scholar * Rueden, C. T. et al. ImageJ2: ImageJ for the next
generation of scientific image data. _BMC Bioinformatics_ 18, 529 (2017). Article PubMed PubMed Central Google Scholar * Mörck, C. & Pilon, M. _C. elegans_ feeding defective mutants
have shorter body lengths and increased autophagy. _BMC Dev. Biol._ 6, 39 (2006). Article PubMed PubMed Central Google Scholar * O’Toole, G. A. Microtiter dish biofilm formation assay.
_J. Vis. Exp._ https://doi.org/10.3791/2437 (2011). Article PubMed PubMed Central Google Scholar * Serra, D. O., Richter, A. M. & Hengge, R. Cellulose as an architectural element in
spatially structured _Escherichia coli_ biofilms. _J. Bacteriol._ 195, 5540–5554 (2013). Article CAS PubMed PubMed Central Google Scholar * Wiles, T. J. et al. Modernized tools for
streamlined genetic manipulation and comparative study of wild and diverse proteobacterial lineages. _mBio_ 9, e01877-18 (2018). Article PubMed PubMed Central Google Scholar * Kissoyan,
K. A. B. et al. Exploring effects of _C. elegans_ protective natural microbiota on host physiology. _Front. Cell. Infect. Microbiol._ 12, 775728 (2022). Article CAS PubMed PubMed Central
Google Scholar * Schulenburg, V. D. et al. Extreme length and length variation in the first ribosomal internal transcribed spacer of ladybird beetles (Coleoptera: Coccinellidae). _Mol.
Biol. Evol._ 18, 648–660 (2001). Article PubMed Google Scholar * Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data (Babraham Institute, 2010). * Bolger, A. M.,
Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. _Bioinformatics_ 30, 2114–2120 (2014). Article CAS PubMed PubMed Central Google Scholar *
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. _Nat. Methods_ 9, 357–359 (2012). Article CAS PubMed PubMed Central Google Scholar * Picard Toolkit (Broad
Institute, 2019). * Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data.
_Bioinformatics_ 27, 2987–2993 (2011). Article CAS PubMed PubMed Central Google Scholar * Koboldt, D. C. et al. VarScan 2: somatic mutation and copy number alteration discovery in
cancer by exome sequencing. _Genome Res._ 22, 568–576 (2012). Article CAS PubMed PubMed Central Google Scholar * Cingolani, P. et al. A program for annotating and predicting the effects
of single nucleotide polymorphisms, SnpEff. _Fly_ 6, 80–92 (2012). Article CAS PubMed PubMed Central Google Scholar * Cingolani, P. et al. Using _Drosophila melanogaster_ as a model
for genotoxic chemical mutational studies with a new program, SnpSift. _Front. Genet_. 3, 35 (2012). * RStudio Team. RStudio: Integrated Development for R (RStudio Inc., 2015). * R Core
Team. _R: A Language and Environment for Statistical Computing_ (R Foundation for Statistical Computing, 2016). * Winsor, G. L. et al. Enhanced annotations and features for comparing
thousands of _Pseudomonas_ genomes in the _Pseudomonas_ genome database. _Nucleic Acids Res._ 44, D646–D653 (2016). Article CAS PubMed Google Scholar * Madeira, F. et al. Search and
sequence analysis tools services from EMBL-EBI in 2022. _Nucleic Acids Res._ 50, W276–W279 (2022). Article CAS PubMed PubMed Central Google Scholar * Ren, J. et al. DOG 1.0: illustrator
of protein domain structures. _Cell Res._ 19, 271–273 (2009). Article CAS PubMed Google Scholar * Zamorano-Sánchez, D. et al. Functional specialization in _Vibrio cholerae_ diguanylate
cyclases: distinct modes of motility suppression and c-di-GMP production. _mBio_ 10, e00670-19 (2019). Article PubMed PubMed Central Google Scholar * _Measuring Cell Fluorescence Using
ImageJ_ (The Open Lab Book, 2014). * Bähre, H. & Kaever, V. in _c-di-GMP Signaling: Methods and Protocols_ (ed. Sauer, K.) 45–58 (Springer, 2017). * Gao, X. et al. Functional
characterization of core components of the _Bacillus subtilis_ cyclic-di-GMP signaling pathway. _J. Bacteriol._ 195, 4782–4792 (2013). Article CAS PubMed PubMed Central Google Scholar *
Adams, K. J. et al. Skyline for small molecules: a unifying software package for quantitative metabolomics. _J. Proteome Res._ 19, 1447–1458 (2020). Article CAS PubMed PubMed Central
Google Scholar * Hmelo, L. R. et al. Precision-engineering the _Pseudomonas aeruginosa_ genome with two-step allelic exchange. _Nat. Protoc._ 10, 1820–1841 (2015). Article CAS PubMed
PubMed Central Google Scholar * Figurski, D. H. & Helinski, D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in _trans_.
_Proc. Natl Acad. Sci. USA_ 76, 1648–1652 (1979). Article CAS PubMed PubMed Central Google Scholar * Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local
alignment search tool. _J. Mol. Biol._ 215, 403–410 (1990). Article CAS PubMed Google Scholar * Ye, J. et al. Primer-BLAST: a tool to design target-specific primers for polymerase chain
reaction. _BMC Bioinformatics_ 13, 134 (2012). Article CAS PubMed PubMed Central Google Scholar * Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence
alignments using Clustal Omega. _Mol. Syst. Biol._ 7, 539 (2011). Article PubMed PubMed Central Google Scholar * Newman, J. R. & Fuqua, C. Broad-host-range expression vectors that
carry the l-arabinose-inducible _Escherichia coli_ araBAD promoter and the _araC_ regulator. _Gene_ 227, 197–203 (1999). Article CAS PubMed Google Scholar * Choi, K.-H., Kumar, A. &
Schweizer, H. P. A 10-min method for preparation of highly electrocompetent _Pseudomonas aeruginosa_ cells: application for DNA fragment transfer between chromosomes and plasmid
transformation. _J. Microbiol. Methods_ 64, 391–397 (2006). Article CAS PubMed Google Scholar * Tamura, K., Stecher, G. & Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis
version 11. _Mol. Biol. Evol._ 38, 3022–3027 (2021). Article CAS PubMed PubMed Central Google Scholar * Hesse, C. et al. Genome-based evolutionary history of _Pseudomonas_ spp.
_Environ. Microbiol._ 20, 2142–2159 (2018). Article CAS PubMed Google Scholar * Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach
to multiple testing. _J. R. Stat. Soc. B_ 57, 289–300 (1995). Google Scholar * Vu, V. et al. vqv/ggbiplot: A biplot based on gplot2. _Github_ www.github.com/vqv/ggbiplot (2021). * Josse,
J., & Husson, F. missMDA: a package for handling missing values in multivariate data analysis. _J. Stat. Softw_. https://doi.org/10.18637/jss.v070.i01 (2016). * Oksanen, J. et al. vegan:
Community Ecology Package. _Github_ https://github.com/vegandevs/vegan (2022). * Martinez Arbizu, P. pairwiseAdonis: Pairwise multilevel comparison using adonis. R package version 0.4
_Github_ https://github.com/pmartinezarbizu/pairwiseAdonis (2020). * Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. _J. Stat. Softw._ 67,
1–48 (2015). Article Google Scholar * Zeileis, A. & Hothorn, T. Diagnostic checking in regression relationships. _R News_ 2, 7–10 (2002). * Hothorn, T., Bretz, F. & Westfall, P.
Simultaneous inference in general parametric models. _Biom. J._ 50, 346–363 (2008). Article PubMed Google Scholar * Rigby, R. A. & Stasinopoulos, D. M. Generalized additive models for
location, scale and shape. _J. R. Stat. Soc. C_ 54, 507–554 (2005). Article Google Scholar * Wikham, H. _ggplot: Elegant Graphics for Data Analysis_ (Springer, 2016). * Kassambara, A.
ggpubr: ‘ggplot2’ based publication ready plots. R package version 0.6.0. https://rpkgs.datanovia.com/ggpubr/ (2023). * Caswell, H. _Matrix Population Models_ (Sinauer, 2001). Download
references ACKNOWLEDGEMENTS We thank E. Stukenbrock (University of Kiel, Germany) for access to the LSM 880; K. Guillemin (University of Oregon, Eugene, United States), P. Rainey (Max-Planck
Institute for Evolutionary Biology, Ploen, Germany), H. Schweizer (Northern Arizona University, United States), F. Yildiz (University of California Santa Cruz, United States) and G. O’Toole
(Dartmouth Medical School, United States) for providing bacterial strains or plasmids; D. Rogers, J. Summers (both Max-Planck Institute for Evolutionary Biology, Ploen, Germany) for
guidance in allelic exchange; J. Zimmermann (Schulenburg group, University of Kiel, Germany) for bioinformatic support; B. Pees (Schulenburg group, University of Kiel, Germany) for
illustration support; S. Joel, J. Hofmann, J. Löwenstrom, J. Lorenzen, H. Griem-Krey, L. Bluhm and L. Rheindorf (all Schulenburg group, University of Kiel, Germany) for lab support; the Kiel
BiMo/LMB for access to their core facilities; the Schulenburg lab for project feedback; and B. Bohannan (University of Oregon, Eugene, United States), R. Knight (University of California
San Diego, United States) and P. Engel (Université de Lausanne, Switzerland) for advice on the manuscript. Funding was provided by the Deutsche Forschungsgemeinschaft (DFG, German Research
Foundation), Project-ID 261376515 – SFB 1182, Projects A4 and Z3 (N.O., A.C., F.B., J.L., A. Tholey, A. Traulsen, H. Schulenburg); the DFG Research Infrastructure NGS_CC project 407495230
(J.F.) as part of the Next Generation Sequencing Competence Network project 423957469; the International Max-Planck Research School for Evolutionary Biology (N.O., A.C.); the Max-Planck
Society (Fellowship to H. Schulenburg); and NIH project R01AI168017 (M.J.G.G., H. Sondermann). FUNDING Open access funding provided by Christian-Albrechts-Universität zu Kiel. AUTHOR
INFORMATION Author notes * Thekla Schultheiß Present address: Institute of Toxicology and Pharmacology, University of Kiel, Kiel, Germany AUTHORS AND AFFILIATIONS * Department of
Evolutionary Ecology and Genetics, University of Kiel, Kiel, Germany Nancy Obeng, Anna Czerwinski, Daniel Schütz, Jan Michels, Thekla Schultheiß, Melinda Kemlein & Hinrich Schulenburg *
Department of Systematic Proteome Research and Bioanalytics, University of Kiel, Kiel, Germany Jan Leipert & Andreas Tholey * Max Planck Institute for Evolutionary Biology, Plön, Germany
Florence Bansept, Arne Traulsen & Hinrich Schulenburg * CSSB Centre for Structural Systems Biology, Deutsches Elektronen-Synchrotron DESY, Hamburg, Germany María J. García García &
Holger Sondermann * Institute of Clinical Molecular Biology, University of Kiel, Kiel, Germany Janina Fuß * Section of Biology, University of Kiel, Kiel, Germany Holger Sondermann Authors *
Nancy Obeng View author publications You can also search for this author inPubMed Google Scholar * Anna Czerwinski View author publications You can also search for this author inPubMed
Google Scholar * Daniel Schütz View author publications You can also search for this author inPubMed Google Scholar * Jan Michels View author publications You can also search for this author
inPubMed Google Scholar * Jan Leipert View author publications You can also search for this author inPubMed Google Scholar * Florence Bansept View author publications You can also search
for this author inPubMed Google Scholar * María J. García García View author publications You can also search for this author inPubMed Google Scholar * Thekla Schultheiß View author
publications You can also search for this author inPubMed Google Scholar * Melinda Kemlein View author publications You can also search for this author inPubMed Google Scholar * Janina Fuß
View author publications You can also search for this author inPubMed Google Scholar * Andreas Tholey View author publications You can also search for this author inPubMed Google Scholar *
Arne Traulsen View author publications You can also search for this author inPubMed Google Scholar * Holger Sondermann View author publications You can also search for this author inPubMed
Google Scholar * Hinrich Schulenburg View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS N.O., A.C., F.B., A. Traulsen and H. Schulenburg
conceptualized the project. N.O., A.C., M.J.G.G. and H. Sondermann developed the methodology. N.O., A.C., J.M., J.L., T.S., M.K. and J.F. conducted investigations. N.O., A.C., D.S. and F.B.
analysed data. N.O., A.C., D.S., J.M., J.L., F.B., M.J.G.G., T.S., M.K., J.F., A. Tholey, A. Traulsen, H. Sondermann and H. Schulenburg contributed to the writing of the manuscript. N.O., A.
Tholey, A. Traulsen, H. Sondermann and H. Schulenburg supervised the project. F.B. performed modelling. CORRESPONDING AUTHOR Correspondence to Hinrich Schulenburg. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Microbiology_ thanks Hassan Salem and the other, anonymous, reviewer(s) for their
contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 BACTERIAL FITNESS DURING AND RESULTING FROM EXPERIMENTAL EVOLUTION. A, B, BACTERIAL FITNESS DURING THE EVOLUTION EXPERIMENT. a, Bacterial
fitness in host across cycles of the evolution experiment measured as colony forming units (CFU) per worm population after 3.5 days of exposure to _Ce__MY316. b, In the negative control,
bacterial fitness was assessed on nematode growth agar in absence of the host. For each data point, bacteria were collected at the bottleneck time point of the noted cycle. Replicate
populations (n = 6) are shown as separate thin lines, with the mean shown as a thick line. C, Mean CFU per individual host in a worm population for the evolved bacterial populations of cycle
10. Five L4 _C. elegans_ larvae proliferated on evolved or ancestral bacterial lawns for 3.5 days (reaching F1 generation) and CFUs were extracted from the whole worm population. CFUs per
population were divided by the number of worms in the population. Overall, results are shown as boxplots, with boxes indicating 25% above and below the median, which is given as the thick
line within boxes; replicate populations (n=6) are indicated as individual data points. D, E, Dynamic changes in morphotype composition during the free-living phase of the host-associated
life cycle for bacterial populations from the end of the evolution experiment. c, Results for the replicate populations from the host-associated evolution treatment. Box plots show median
(center line), upper and lower quartiles (box limits) and the interquartile range (whiskers). d, Results for the replicate populations from the control treatment. Proportions of the
different colony morphotypes (see graphical legend) is shown across time of the host-associated life cycle. Time point 0 is at the end of the host-associated phase, when bacteria are
transferred to the free-living phase, which itself lasts 168 hours. Fdr-corrected beta-regressions were used to predict proportions and test for a change in proportions over time (see
Supplementary Table 2). EXTENDED DATA FIG. 2 COLONIZATION OF THE _C. ELEGANS_ INTESTINE BY WRINKLY HOST SPECIALISTS. Confocal laser scanning micrographs (upper panel: longitudinal optical
section; lower panels: maximum intensity projections showing longitudinal optical sections) revealing intact bacterial cells (red) within the intestinal system of a young adult _Ce__MY316
(cyan). The upper micrograph shows an overview of the complete worm, and the lower micrographs show detailed views of the worm sections indicated by the dashed frames above. These include
the posterior pharynx with the worm grinder and the first intestinal ring (left), a central intestinal (middle) and the anal region (right). The bottom left panel is identical to the
micrograph shown in the main text (Fig. 1e). Scale bars = 50 µm (overview) and 10 µm (detailed views). EXTENDED DATA FIG. 3 WRINKLY ISOLATES FROM THE END OF THE HOST-ASSOCIATED EVOLUTION
TREATMENT EVOLVE A HOST-ASSOCIATED LIFESTYLE. Phenotypes of morphotype clones isolated from independent host-evolved populations (left) and control populations (right), including smooth,
fuzzy and wrinkly morphotypes, are shown. Results for each morphology are summarized as boxplots (1 < n < 4). Box plots show median (center line), upper and lower quartiles (box
limits) and the interquartile range (whiskers). Dashed lines and grey shaded areas indicate the mean and standard error of ancestral traits, respectively. Differences between evolved
morphologies and the ancestor were assessed with generalized linear models and fdr-corrected Tukey post-hoc tests. Letters indicate statistical differences between morphologies, asterisks
indicate deviation from the ancestor (see Supplementary Table 6). EXTENDED DATA FIG. 4 EVOLUTION OF A HOST-INTERACTION LIFE-STYLE IN THE POPULATIONS FROM THE END OF THE HOST-ASSOCIATED
EVOLUTION TREATMENTS. A, Principal component analysis on characteristic stages of host-association for ancestral, host evolved and control evolved bacterial populations. Individual data
points refer to replicate populations colored according to evolution treatment (Supplementary Table 5). B–F, Shifts in phenotypes from the bacterial ancestor in the evolved populations for
(B) early colonization, determined by CFU extracted from L4 larvae exposed to bacteria for 1.5 hour; (C) persistence in L4 larvae kept in M9 buffer for 1h (raised on bacteria); (D) CFU of
bacteria released from L4 larvae into buffer within 1h (previously raised on bacteria from L1 to L4), (E) swarming distance on 0.5% agar within 24; and (F) colony expansion on 3.4% agar
within 72h. All panels show ratios of evolved over ancestral populations for five replicates shown as individual data points. The dashed line indicates the mean values obtained for the
ancestral population. The difference between evolved and ancestral phenotypes were assessed using one-sided t-tests (fdr-corrected; Supplementary Table 8). In all box plots, median (center
line), upper and lower quartiles (box limits) and the interquartile range (whiskers) are shown. EXTENDED DATA FIG. 5 INCREASED INTRACELLULAR C-DI-GMP CONCENTRATIONS IN WRINKLY ISOLATES. A,
Amount of intracellular c-di-GMP measured with a fluorescence sensor. Raw fluorescence intensity (RFI) is the ratio of TurboRFP (c-di-GMP-dependent) and AmCyan (plasmid copy
number-dependent) and, thus normalized for copy number of the sensor plasmid. B, Total c-di-GMP determined by isotope dilution PRM analysis. C, C-di-GMP determined by isotope dilution PRM
analysis and normalized by total protein amount. In A, B and C, c-di-GMPs levels are studied for the ancestor and three wrinkly isolates from the end of the evolution experiment, each with a
single mutation in either _wspF_, _wspE_, or _rph_. We compared c-di-GMP levels replicate cell populations (n = 5). D, Intracellular c-di-GMP of isolates from end the of the evolution
experiment with wrinkly, smooth, and fuzzy colony morphology measured with a fluorescence sensor (nested ANOVA and fdr-corrected Dunnett post hoc test; n = 5). E, RFI of the different
isolates normalized by ancestral RFI to correct for replication-dependent effects (ANOVA and fdr-corrected Dunnett post hoc test; n = 5; Supplementary Table 10. For respective mutations see
Supplementary Table 9). Box plots show median (center line), upper and lower quartiles (box limits) and the interquartile range (whiskers). EXTENDED DATA FIG. 6 AMINO ACID SEQUENCE
ALIGNMENTS OF ANCESTRAL AND EVOLVED C-DI-GMP REGULATING ENZYMES. On top, illustrations of protein domains show the organization of WspF, WspE and ribonuclease PH with sites affected by
_Pl__MYb11 mutation highlighted by red arrows. Below, protein sequence alignments of ancestral and evolved proteins are shown with domains highlighted in the same colors as above. EXTENDED
DATA FIG. 7 BIOFILM FORMATION AND MOTILITY OF FOCAL WRINKLY HOST SPECIALISTS AND MACROCOLONIES OF _PL__MYB11, EVOLVED ISOLATES, Δ_WSPR_ MUTANTS AND PDE/DGC EXPRESSING DERIVATIVES. A, Biofilm
formation of _wspF_, _wspE_, and _rph_ mutants compared to _Pl__MYb11 (ancestral median = dashed line) after two days shaking incubation microtiter plates. Illustration of biofilms with
photographs of biofilms from test tubes for after 48h incubation. Scale bars = 4mm. B, Swarming motility and C, colony expansion of isolates _wspF_, _wspE_, and _rph_ mutants were compared
to _Pl__MYb11 (ancestral median = dashed line) after 24 and 72h, respectively. b-c, Scale bars = 0.5 cm. a-c, Experiments were performed with min. 3 replicates/treatment and analyzed with
ANOVA and fdr-corrected Dunnett post-hoc tests or a one-sided t-test. Box plots show median (center line), upper and lower quartiles (box limits) and the interquartile range (whiskers). D,
Macrocolonies of ancestral _Pl__MYb11 and three wrinkly isolates from the end of the evolution experiment (with single mutations in either _wspF_, _wspE_, or _rph_). E, Macrocolonies of
Δ_wspR_ mutants in _Pl__MYb11 _wspF_, _wspE_, or _rph_ background. F, Macrocolonies of _wspF_, _wspE_, or _rph_ isolates expressing the phosphodiesterase PA2133 from plasmid, _Pl__ MYb11
expressing the constitutively active diguanylate cyclase GCN4-WspR from plasmid, and empty vector controls. d-f, Macrocolonies were grown on tryptic soy agar supplemented with Congo Red for
one (d,e) to three (f) days. Scale bars = 1mm. EXTENDED DATA FIG. 8 INCREASED COMPETITIVE FITNESS OF WRINKLY ISOLATES IN BACTERIAL MIXTURES OF FOUR STRAINS (QUARTETS). Three co-evolved
morphotypes isolated from host-evolved replicate population T3 were paired with the ancestor. In one quartet the wrinkly _wspF_ mutant (MT12) was present and in the other the wrinkly _wspE_
mutant (MT14). Data points represent independent replicates, min. 3 replicates per treatment. Differences between morphotypes and ancestor were assessed using a linear mixed model and
subsequent fdr-corrected Dunnett post-hoc comparisons; see Supplementary Table 13. Box plots show median (center line), upper and lower quartiles (box limits) and the interquartile range
(whiskers). EXTENDED DATA FIG. 9 FILTERS FOR THE IDENTIFICATION OF _RPH_ AND _WSP_ GENE CANDIDATES. Distribution of sequence lengths and percent identities of the BLAST results for
individual genes. The proportion of BLAST results belonging to particular sequence length and percent identity classes are shown as blue shades with varying intensity (cf. scale). Red
rectangles show the areas for which the presence of the considered gene is assumed, and were set to include the largest BLAST hit values at both maximum sequence length and percent
identities. EXTENDED DATA FIG. 10 MODEL ASSESSING THE SELECTION GRADIENT ON BACTERIA FOLLOWING A HOST-ASSOCIATED LIFE CYCLE. A, Definition of the rates for the model of a microbial lineage
being taken up by, replicating within and being expulsed from worms on a plate. B, Distribution of the optimal strategies across the whole traits space. C, Projection of the trait space over
the axes (_r, δ_). For each point of the map, the color represents the proportions of times each of the 4 possible strategies are optimal, integrating over the values of _f_ and _K_. The
color scheme uses the CMYK color code: a purely cyan (respectively magenta, yellow) pixel indicates that the only optimal strategy for the considered values (_r, δ_) is ↓ δ (respectively ↑
_f_, ↑_K_). A color of a darker shade indicates that ↑ _r_ is also optimal in a small proportion at that point, as shown on the additional color scales for each edge. SUPPLEMENTARY
INFORMATION REPORTING SUMMARY SUPPLEMENTARY TABLES Supplementary Tables 1–22. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Obeng, N., Czerwinski, A., Schütz, D. _et al._ Bacterial c-di-GMP has a key role in
establishing host–microbe symbiosis. _Nat Microbiol_ 8, 1809–1819 (2023). https://doi.org/10.1038/s41564-023-01468-x Download citation * Received: 21 September 2022 * Accepted: 10 August
2023 * Published: 31 August 2023 * Issue Date: October 2023 * DOI: https://doi.org/10.1038/s41564-023-01468-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
